ABSTRACT
Introduction
While essential for cost-effectiveness analyses, there are no current resource use and cost data available for advanced hepatocellular carcinoma (HCC) and selective internal radiation therapy (SIRT). The study aims to assess current resource use and costs in HCC and for SIRT compared to historical survey data.
Areas covered
To address this data gap, resource use was elicited via surveys and interviews with medical professionals experienced with HCC and SIRT in the United Kingdom. Unit costs were from publicly available databases. Resource use and costs were estimated and compared to prior surveys.
Expert opinion
From eleven responses, pre-progression costs for SIRT and systemic therapy were £256.77 and £292.27/month, respectively. One-off progression and post-progression costs were £209.98 and £522.84/month. Monthly costs were 54%-79% lower than in previous surveys, due to reduction in hospitalizations and funded social care. Furthermore, substantial differences in resource use associated with SIRT between clinical practice and clinical trials were found. In conclusion, increased availability and familiarity with systemic treatments has led to important changes in HCC care and SIRT administration. The uncertainty from the use of expert opinion and the limited number of hospitals with SIRT experience can be addressed with future research using large databases, registries.
1. Introduction
Hepatocellular carcinoma (HCC) poses a significant health burden to society. In the United Kingdom (UK) there were an estimated 7,618 new cases of liver cancer in 2018, with HCC as the predominant histological subtype of primary liver cancer, accounting for 70–85% of all cases [Citation1–3]. The incidence and mortality of HCC have both increased in recent decades, and this trend is projected to continue over the next 20 years [Citation2,Citation4,Citation5].
The presentation, and management of HCC is complex because of the interaction between the impaired liver function and the liver cancer. The majority of HCC cases occur in patients with underlying liver disease (cirrhosis), mostly as a result of hepatitis B or C virus infection, alcohol abuse or nonalcoholic fatty liver disease [Citation6,Citation7]. This underlying disease places a significant physical burden on the individual, independent of the increased mortality risk induced by the progression of liver cancer.
The increase in HCC incidence has led to a greater demand for diagnosis, management, and treatment. Clinical trials are designed to capture data on the efficacy of treatments and are considered the gold standard for providing evidence of their effectiveness and safety. However, economic evaluations are often needed to position treatments in the clinical pathway. These evaluations, which aim to quantify the impact of new technologies on current care in terms of costs and health benefits, require the estimation and costing of resource use.
Trials however do not routinely collect data on resource use, and therefore further data collection outside of a clinical trial setting is often required. The information on resource use available from clinical trials is usually limited both in scope and in generalizability as it may often reflect protocol-mandated care, and might not represent real-life treatment practice.
Data collection on resource use and costs has additional challenges for medical technologies, because of learning effects which can drive both patient selection for treatment, and changes in the implementation of the technology in clinical practice [Citation8–11].
There have been efforts to describe clinical practice and resource use in the treatment of HCC in the UK for cost-effectiveness analyses of systemic first line (sorafenib [Citation12,Citation13] and lenvatinib [Citation14]) and second line treatments (regorafenib [Citation15]). All the above analyses were based on a survey of four UK clinicians conducted in 2007, to which results of a survey of three additional clinicians were added in 2015. The questionnaires covered treatment patterns, medical staff contacts, acute care, and laboratory and radiological tests in ‘pre-progression’ and ‘post-progression’ health states in advanced HCC. Information was also obtained for patients at time of progression for laboratory and radiological tests, and for adverse events.
Medical knowledge and practice is an evolutionary process, and both experience in the management of HCC and in the range of available treatments have advanced in HCC in the past five years [Citation16]. A prospective study from Italy [Citation17] and a retrospective study from France [Citation18] showed, that as experience in the use of sorafenib improved, treatment duration and even potentially overall survival have increased. As a result, it is likely that resource utilization will have also been subject to some change since 2007, and even 2015. Additionally, new loco-regional therapies, such as selective internal radiation therapy (SIRT), are now being used in advanced HCC. These can modify treatment practice pre-progression, as existing systemic therapies require continuous treatment until progression or adverse events, whereas SIRT with Y-90 resin microspheres is performed as a one-off procedure after work-up for most patients [Citation19,Citation20]. The treatment pathway can also differ post-progression, due to the potentially different subsequent treatments for systemic and loco-regional therapies.
Additionally, in 2007, when the majority of the information on resource use was elicited, there was limited experience even with sorafenib in the advanced HCC population, and no experience with regorafenib, lenvatinib or SIRT in the UK. Using previously elicited resource use, even if updated with new unit costs, may then be misleading for current and future economic evaluations. Therefore, additional data collection to obtain information on resource use in advanced HCC and with SIRT in UK clinical practice is required for a UK-based economic evaluation.
This study aimed to assess current treatment patterns and costs in HCC from the UK National Health Service (NHS) and Personal and Social Services perspective according to the UK requirements for technology appraisal [Citation21] and to compare it to historical data reported in previous publications. This includes data collection on treatment administration patterns for SIRT, and resource use and costs in the treatment of advanced HCC according to progression status. The implications of these changes are assessed for patients receiving SIRT and systemic therapy for advanced HCC in the UK.
2. Patients and methods
Various methods exist to collect resource use data. Prospective studies, chart reviews or database analyses can provide the most robust results, these methods however can only elicit resource use for technologies that have been in use for a sufficiently long period of time for the consequences of their use to appear in the databases. As SIRT is currently not routinely used to treat HCC in the UK due to lack of routine funding, retrospective data collection or chart review is not possible.
Resource use was, therefore, elicited via two questionnaires from experienced UK-based medical professionals involved in the care of HCC patients:
•a short survey which obtained information on the administration of SIRT, and
•a longer survey which elicited information on resource use patterns in advanced HCC.
Both surveys aimed to elicit data for the same population of adults with unresectable HCC, who are refractory, intolerant of, or contraindicated for transarterial chemoembolization (TACE), who have preserved liver function (defined as a Child-Pugh A without ascites) and an Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2. This reflects a population with predominantly advanced HCC, classified as stage C in the Barcelona Clinic Liver Cancer (BCLC) staging system. However, in clinical practice this population may also include some patients with intermediate-stage HCC (BCLC stage B). Neither of the surveys differentiated between glass and resin microspheres.
Participants were clinical experts with experience with SIRT and HCC in the UK, including hepatologists, oncologists, interventional radiologists, and specialist nurses who are involved in the care of unresectable HCC patients. Clinical experts were not blinded to the sponsors of the study.
The short survey questionnaire elicited information through e-mail on:
the proportion of patients undergoing work-up for SIRT and who proceed to receive the SIRT procedure,
the number of work-ups and treatments required, and
the average length of stay after work-up and treatment.
Mean results were compared to historical data from the SARAH trial, a multicenter, open-label, randomized, controlled, investigator-initiated, phase 3 trial comparing SIRT with yttrium-90 resin microspheres with sorafenib in locally advanced and inoperable HCC [Citation28] and the ENRY study [Citation19], a large, multicenter retrospective analysis of consecutive SIRT patients with unresectable HCC. Both studies were conducted in one or multiple European countries: this facilitates comparisons with the present survey as treatment patterns in HCC are known to vary across geographies [Citation22].
The longer survey questionnaire elicited information through face-to-face or telephone interviews lasting 60–90 minutes. Background information of each interviewee’s experience with HCC and SIRT was obtained. The questionnaire focused on the ‘pre-progression’, ‘at progression’, and ‘post-progression’ health states as they represent a typical patient pathway and align with health states commonly used in economic evaluations. A distinction was made between pre-progression after treatment with SIRT and pre-progression whilst being treated with a systemic therapy. Monthly resource use was captured for the pre- and post-progression health states and as a one-off event for the ‘at progression’ transition. Only direct medical costs were of interest (i.e. societal costs or travel costs were excluded). Subsequent drug costs were not included in the monthly health state costs due to the different treatment lengths. The proportion of patients receiving and frequency of use for each of the following resources were elicited ():
Table 1. Resources included
medical staff contacts (secondary and primary care),
diagnostic procedures (imaging and laboratory tests),
monitoring,
hospitalization and
personal and social care.
The questionnaire was developed based on the previously published survey in HCC in the technology appraisal (TA) 189 of sorafenib by the National Institute for Health and Care Excellence (NICE) [Citation12], clinical practice guidelines (by the European Association for the Study of the Liver (EASL) in 2018 [Citation23] and the European Society for Medical Oncology (ESMO) in 2018 [Citation24]) to confirm the treatment pathway and recommended medical staff contacts, laboratory and radiological tests. The questionnaire was validated by a medical oncologist with experience in treating patients both with SIRT and systemic therapy.
The resource use data was combined with publicly available unit costs from the National Health Service (NHS) National Schedule of Reference Costs 2019–2020 [Citation25] and the Personal Social Services Research Unit (PSSRU) 2020 report [Citation26] to estimate mean health state costs. (Unit costs used are available in the Supplementary Table.) Standard deviations were estimated from the mean costs for each medical expert.
The mean health state resource use and cost estimates were compared to historical data, the 2007 survey results reported in the NICE TA189 and the pooled and updated results from the 2007 and 2015 surveys reported in the 2018 NICE TA551 [Citation14]. The 2015 data is not available separately, only as merged costs with the 2007 results.
Eleven experts responded in total: two oncologists, one hepatologist, three specialist nurses took part in the interview with the long survey, and an additional five interventional radiologists filled out the short survey.
Results and conclusions from both surveys were validated through discussions with clinicians in a face-to-face advisory board.
3. Results
For the administration of SIRT, most patients receive one work-up (95%) and one procedure (81%), with an average 1.05 work-ups and 1.20 treatments, respectively. Only 4% of patients require two work-ups, and 19% two procedures. Less than 1% of patients receive more than two work-ups or procedures. The mean number of nights in hospital for work-ups was less than one (0.69). 93% of patients will undergo SIRT after receiving work-up and will require a 1-night stay in hospital following the procedure (mean number of nights in hospital: 1.19).
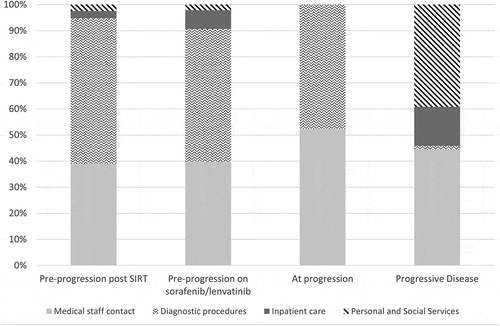
Pre-progression health state costs for SIRT and systemic therapy were £256.77 and £292.27 per month, respectively. The one-off cost at progression was estimated to be £209.98, and post-progression palliative care was £522.84 per month (). Standard deviations were large, especially for the one-off resource use at the confirmation of progression and as a result, there was no statistically significant difference between the estimates for the different health states. In the ‘progression-free’ health state, costs were driven by diagnostic procedures (51–56% of costs), closely followed by medical staff contacts (39–40% of costs). At the time of progression and the post-progression costs were driven by medical staff contacts (52% and 45% of costs respectively), with a large proportion of costs due to personal and social services after progression (39%) (). Pre-progression costs were 14% lower after SIRT compared to systemic therapies. This difference was driven by medical staff contacts and inpatient care, which may potentially be due to the continuous therapy with a systemic treatment compared to a one-off intervention with the SIRT procedure.
Table 2. Health state costs
Subsequent active treatments after SIRT were mainly comprised of sorafenib (42%), potentially curative treatments (liver transplant, resection, and percutaneous tumor ablation, 6%) and limited use of regorafenib (2%). After sorafenib active treatments included regorafenib (19%) and lenvatinib (1%). After lenvatinib, the majority of patients received regorafenib (32%), with a small proportion of patients on sorafenib (7%). The remaining patients have received best supportive care or took part in clinical trials.
These health states costs were substantially lower (by 54% and 79% pre- and post-progression respectively) than the costs reported in the literature for patients treated with a systemic therapy based on the 2007 and the pooled 2007/2015 surveys results (NICE TA551, 2018). This difference mainly comes from the lower hospitalization and hospital follow-up visit costs and the social care costs (, , ). The large decrease in costs is partly due to different unit costs used. The NICE TA551 used the NHS Reference costs to determine the cost of outpatient consultations, which varied from £42 for an appointment with a clinical nurse to £216.66 for an appointment with a hepatologist [Citation14,Citation25]. This approach assumes, that each medical staff contact was within a separate consultation and at a separate visit (e.g. a nurse was seen at a separate visit to the consultation with a specialist). The current study unit costs were taken from the PSSRU for medical staff contacts, and ranged from £25.00 for a specialist nurse phone call lasting 30 minutes, to £59.32 for a consultant appointment lasting 30 minutes [Citation26]. This source allowed us not to make any assumptions for the timing of the visits.
Table 3. Comparison of current (2019) health state costs and costs from NICE TA551 (2007 and 2015) [Citation14]
Table 4. Comparison of hospitalizations and follow-up visits between current and 2007 survey
Figure 2. Comparison of pre- and post-progression health state costs as a proportion of total costs for sorafenib/lenvatinib from current (2019) and previous surveys (2007 and 2015).
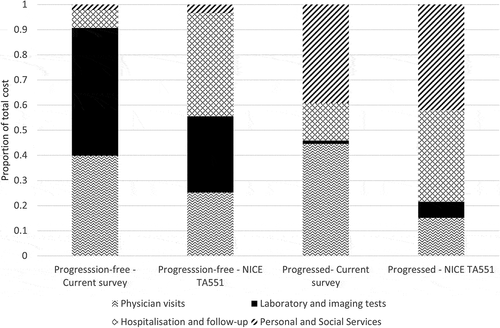
Beside the unit costs, an important shift has been seen in the resource use. In the current study, the main cost drivers pre- and post-progression for systemic treatments were diagnostic procedures (51%) and medical staff contacts (40%), whereas in the previous surveys (TA189, 2007), hospitalizations (41%) and social care (42%) were the biggest cost drivers (). These differences reflect changes in the clinical pathway for patients with HCC: the proportion of patients hospitalized post-progression almost halved in the current survey compared to 2007 (29% vs. 48% of patients in the current and the 2007 surveys respectively) and the number of hospitalization reduced by more than two third (1.4 vs. 4.8 hospitalizations annually in the current and 2007 surveys respectively). There is also a shift in the burden of care, as the current survey found that fewer patients receive funded care: residential post-progression care was only funded in approximately half of the cases.
4. Discussion
Due to lack of recent published data, and to provide up to date information on treatment delivery, resource use and cost information that incorporate recent clinical practice, two resource use surveys were conducted among clinicians with experience with SIRT and HCC in the UK. These provided an update to previous studies informing HCC resource use and cost information, and report on real-world clinical experience with SIRT outside the confines of a clinical trial protocol.
Results show important changes in both SIRT administration and HCC disease management. The SARAH trial, which started recruitment in 2011, found that 22% of patients randomized to SIRT with Y-90 resin microspheres did not receive treatment. However, some of the patients who failed to receive SIRT with Y-90 resin microspheres may have been poor candidates for this therapy [Citation28]. This survey found that, in current UK clinical practice, 93% of patients receiving a work-up for SIRT receive SIRT as clinicians may now have more experience selecting patients for SIRT. This is supported by the findings of Palmer et al (2020) showing that improved patient selection can reduce the proportion of patients dropping out of SIRT treatment after their work-up, therefore leading to better patient outcomes [Citation28].
Clinicians in the short survey also reported other items indicative of changes in SIRT treatment over time. Fewer SIRT procedures (with 81% of patients receiving a single treatment) were reported to be required by patients compared with that observed in the SARAH trial (average number of treatments 1.43 with 63% receiving a single treatment). The SARAH trial mandated in the protocol that, for patients with bi-lobar HCC, the contralateral liver lobe should be treated during a separate hospital admission, 30–60 days after the first lobe [Citation29]. However, SIRT with Y-90 resin microspheres can be administered to both lobes of the liver during a single treatment session, with multiple infusions of the same source vial being performed selectively and in different arteries during the same procedure. Additionally, the use of SIRT for bilobar disease may be less prevalent than for unilobar disease. This may contribute to UK clinicians reporting a lower number of SIRT treatments per patient. The figure reported by UK clinicians is also similar to the findings of the ENRY study, that reported an average of 1.08 procedures per patient (93% receiving a single treatment) [Citation19]. Additionally, the survey reported a move toward providing SIRT work-up in an outpatient setting and SIRT treatment with a 1-night stay.
This has highlighted the importance of assessing changes in clinical practice over time and the differences between protocol-mandated resource use and real word resource use. Economic evaluations of SIRT relying only on historic trial data might result in inaccurate estimates of the cost of SIRT.
This study has found that disease management costs (excluding drug costs for subsequent systemic therapy) of patients with advanced HCC varied between £209.98 and £522.84 per month, depending on whether the patient was pre- or post-progression, and whether they were treated with SIRT or systemic therapy. The disease management cost for systemic therapy is 14% higher per month compared to SIRT as it requires continuous treatment which can result in higher monitoring and hospitalization costs, whereas SIRT is a one-off procedure for most patients. This is in line with the resource use reported from a focus group in an Italian budget impact analysis, where more intensive follow-up was found after sorafenib that with transarterial radioembolization [Citation30]. Similarly to the UK findings, monthly follow-up with medical staff visit and laboratory tests and three monthly imaging were required after sorafenib, and a less frequent follow-up with 3 monthly visits, tests and imaging after transarterial radio-embolization. This was even less than seen in the UK (visits and tests an average every 1.5 months) [Citation30].
While the differences in pre-progression costs would affect the cost-effectiveness of treatments, this effect is more pronounced when there is a significant difference in the duration of the pre-progression period. The two large published RCTs (SARAH and SIRveNIB), did not show a significant difference in progression-free survival (PFS) between sorafenib and SIRT [Citation29,Citation31], though subsequent post-hoc analysis of the SARAH trial data suggested survival benefit with SIRT for patients with good liver function and low tumor burden [Citation28].
Progression has a substantial effect, increasing costs by more than 100% for SIRT-treated patients and by 79% for patients on systemic treatment, which is driven by increased hospitalizations and increased used of personal and social services. Post-progression health state costs were an important driver in determining the economic value of systemic treatments too, therefore understanding their magnitude and composition is pivotal for optimizing the HCC treatment pathway [Citation14]. The large standard deviations were probably due to variations between patients characteristics in each center and the potential variations in clinical practice, or, for the one-off resource use at the confirmation of progression, the difference in scheduling procedures for the confirmation as part of the routine follow-up or as separate visits.
Disease management costs are also subject to change over time even when assuming the same unit costs. Our results suggest that disease management costs have decreased compared to those elicited in 2007 and 2015, driven by reduced hospitalizations and reduced use of funded social care. In the subsequent discussions, clinical experts suggested, that the changes could be due to clinicians being less familiar with sorafenib and how to treat people progressing on sorafenib in 2007. This echoes the experience seen in Italy and France, where the increased experience and better management of adverse events with sorafenib resulted in better clinical outcomes [Citation17,Citation18].
Patients in 2007 were also typically managed by hepatologists, while now patients treated with sorafenib or other systemic therapies are managed by oncologists. Additionally, currently most of the post-progression palliative care has been seen to shift to community palliative care services including charities and home care. Increased familiarity with treatments may have resulted in less frequent follow-up and reduced hospitalizations. Similarly, improvements in the prognosis of HCC might have also affected patients’ quality of life pre- and post-progression and as a result, reduced the intensity of follow-up required.
The key limitations associated with this study include the high degree of uncertainty in the estimates provided by the clinical experts as they are not based on prospective or retrospective data. Both surveys were also based on small sample sizes due to the limited number of hospitals which currently have sufficient experience with SIRT. Due to the limited sample size and the differences in treatment practice, there was no statistically significant difference between the estimates for the different health states. Despite the inclusion of different specialties, the variation seen in resource use is not between the different specialist groups, but between the different hospitals. It is also important to note the limitation of comparing across surveys. When making comparison between our survey and the 2007/2015 surveys, it was not possible to control for all differences such as questionnaire designs and interview techniques. While there are also differences in costing assumptions, the effect of these can be assessed. Additionally, new systemic therapies, e.g. atezolizumab plus bevacizumab have recently been approved by NICE, and its use could potentially influence future resource use in HCC.
Neither of the surveys differentiated between the different SIRT technologies, assuming that resource use and disease management costs associated with them are comparable. Published data is scarce, mainly reliant on two published large RCTs with SIRT with yttrium-90 resin microspheres. However, SIRT technologies differ in the isotope used (e.g. yttrium-90 vs. holmium-166), in the material (e.g. glass vs. resin), or radioactivity per micro-sphere at calibration time or number of microspheres in a 3 GBq total dose. Additionally, there are differences in the administration with regards to the use of imaging (e.g. X-ray, MRI, single-photon emission computerized tomography [SPECT], magnetic resonance imaging [MRI]), the flexibility of dosing and the treatment strategy for bi-lobar disease. While these differences can potentially impact the resource use in the administration of SIRT, due to the low patient numbers and the paucity of published data, this comparison would require additional research.
Future research should aim to reduce the uncertainty inherent in surveys, using patient level information from large databases or registries. Prospective data collection would also provide more current and useful for future economic evaluations.
5. Conclusions
In current UK clinical practice patients receive less SIRT procedures, with shorter inpatient stay for both work-up and procedure than seen in clinical trials. Pre-progression health state costs for SIRT and systemic therapy and the post-progression palliative care costs were £256.77, £292.27 and £522.84 per month, respectively. The one-off cost at progression was £209.98. Monthly costs were lower than in previous studies (by 54% and 79% pre- and post-progression respectively), mainly due to reduced hospitalizations and reduced use of funded social care. The results of this study suggest that increased familiarity with systemic treatments has led to important changes in clinical practice in HCC care since 2007, especially post-progression. Furthermore, substantial differences in resource use associated with SIRT between real life clinical practice and clinical trials were found. This study has highlighted the importance of updating resource use as treatment practice changes, and that relying on existing data sources may misinform economic evaluations. Further research would be beneficial using patient level information from large databases and registries to confirm results of these surveys.
6. Expert Opinion
Economic evaluations are important tools in determining the value of different healthcare interventions. However, for these evaluations to be informative, they need to rely on resource use and cost data, that reflect current clinical practice. Clinical practice can change with the introduction of new therapies and with increased familiarity with older treatments. Similarly, resource use described in clinical trials can differ from real-world use, as clinical trial protocols often mandate additional or more stringent tests than are used in routine care. The present study has shown how resource use and costs for selective internal radiation therapies and for systemic treatments in advanced hepatocellular carcinoma in the United Kingdom have changed over time. The substantial changes found have highlighted the importance of the collection of current and relevant data, as the reliance of historical and trial-based resource use data can misinform economic evaluations.
Author Contributions
NM has led the conception, design, collection and interpretation of data and was involved in the analysis of data and the drafting of the paper. She is responsible for the final approval of the version to be published and agrees to be accountable for all aspects of the work.
RE has led the analyses of data and the drafting of the paper and was involved in the design, collection, and interpretation of data. She agrees to be accountable for all aspects of the work.
ER co-led the conception of the study, was involved in the design, interpretation of data and the drafting of the paper. She agrees to be accountable for all aspects of the work.
VB was involved in the conception, design, interpretation of data and the drafting of the paper. She agrees to be accountable for all aspects of the work.
FC was involved in the conception, design, interpretation of data and the drafting of the paper. He agrees to be accountable for all aspects of the work.
SS was involved in the conception, design, collection and interpretation of data and the drafting of the paper. She agrees to be accountable for all aspects of the work.
PR was involved in the design, collection and interpretation of data and the revising of the paper critically for intellectual content. He agrees to be accountable for all aspects of the work.
DM was involved in the design, collection and interpretation of data and the revising of the paper critically for intellectual content. He agrees to be accountable for all aspects of the work.
Data availability
The data that support the findings of this study are available from the corresponding author, NM, upon reasonable request.
Declaration of interest
NM, ER and RE are partners/employees of Visible Analytics Ltd, which conducted this survey and received consultancy fees and expenses from Sirtex Medical Ltd. VKB, FC and SS are employees of Sirtex Medical Ltd. PR and DM has no financial interest or benefit that has arisen from the direct applications of this research. They have received consulting fees from Sirtex Medical Ltd but have not received honoraria for this manuscript.
Previous presentations
Part of this study was presented as a poster at ISPOR - The Professional Society for Health Economics and Outcomes Research European Meeting 2-6 November 2019, Copenhagen, Denmark
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Review of Pharmacoeconomics and Outcomes Research for their review work. A reviewer on this manuscript has served as a consultant for Bayer, Ipsen and EISAI. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (20.6 KB)Supplemental Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- World Health Organization. CANCER TODAY: estimated number of new cases in 2018, worldwide, both sexes, all ages [internet]. 2020. [ cited 2020 Feb 6]. Available from: http://gco.iarc.fr/today/home.
- Cancer Research UK. Liver cancer incidence statistics [internet]. Cancer Research UK. 2015. [ cited 2020 Feb 6]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/liver-cancer/incidence.
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127.
- Konfortion J, Coupland VH, Kocher HM, et al. Time and deprivation trends in incidence of primary liver cancer subtypes in England. J Eval Clin Pract. 2014;20:498–504.
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462.
- Anstee QM, Reeves HL, Kotsiliti E, et al. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–428.
- Kuznietsova V, Woodward RS. Estimating the learning curve of a novel medical device: bipolar sealer use in unilateral total knee arthroplasties. Value Health. 2018;21(3):283–294.
- Ramsay CR, Grant AM, Wallace SA, et al. Statistical assessment of the learning curves of health technologies. Health Technol Assess. 2001;5(12):1–79.
- Sorenson C, Tarricone R, Siebert M, et al. Applying health economics for policy decision making: do devices differ from drugs?. Europace. 2011;13(Suppl 2):ii54–58.
- Subramonian K, Muir G. The “learning curve” in surgery: what is it, how do we measure it and can we influence it?. BJU Int. 2004;93:1173–1174.
- National Institute for Health and Care Excellence (NICE). TA189 sorafenib for the treatment of advanced hepatocellular carcinoma [internet]. 2010. [ cited 2020 Feb 6]. Available from: https://www.nice.org.uk/guidance/ta189.
- National Institute for Health and Care Excellence (NICE). TA474 sorafenib for treating advanced hepatocellular carcinoma [internet]. 2017. [ cited 2020 Feb 6]. Available from: https://www.nice.org.uk/guidance/TA474.
- National Institute for Health and Care Excellence (NICE). TA551 lenvatinib for untreated advanced hepatocellular carcinoma [internet]. [ cited 2020 Feb 6]. Available from: https://www.nice.org.uk/guidance/TA551.
- National Institute for Health and Care Excellence (NICE). TA514 regorafenib for previously treated advanced hepatocellular carcinoma [internet]. [ cited 2020 Feb 6]. Available from: https://www.nice.org.uk/guidance/ta514.
- Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;S0168-8278(21):6–01903.
- Tovoli F, Ielasi L, Casadei-Gardini A, et al. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol. 2019;71(6):1175–1183.
- Raoul J-L, Adhoute X, Penaranda G, et al. Sorafenib: experience and better manage-ment of side effects improve overall survival in hepatocellular carcinoma patients: a real-life retrospective analysis. Liver Cancer. 2019;8:457–467.
- Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868–878.
- National Institute for Health and Care Excellence (NICE). MIB63 SIR-spheres for treating inoperable hepatocellular carcinoma [internet]. 2016. [ cited 2020 Jun 9]. Available from: https://www.nice.org.uk/advice/MIB63.
- National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal [internet]. 2013. [ cited 2019 Apr 9]. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword.
- Park J-W, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166.
- European Association for the Study of Liver (EASL). EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69(1):182-236.
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2018;29:iv238–iv255.
- National Health Service. National schedule of reference costs 2019-2020 [internet]. 2021. [cited 2021 Aug 20] Available from: https://improvement.nhs.uk/resources/reference-costs/.
- Personal Social Services Research Unit. Unit costs of health and social care 2020 [internet]. [cited 2021 Aug 20] 2021. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/.
- National Institute for Health and Care Excellence (NICE). Multiple Technology Appraisal. Selective internal radiation therapies (SIRT) for treating hepatocellular carcinoma [ID1276] - Committee papers [Internet]. (2019). Available from: https://www.nice.org.uk/guidance/gid-ta10381/documents/committee-papers.
- Palmer DH, Hawkins NS, Vilgrain V, et al. Tumor burden and liver function in HCC patient selection for selective internal radiation therapy: SARAH post-hoc study. Future Oncol. 2020;16(1):4315–4325.
- Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636.
- Rognoni C, Ciani O, Sommariva S, et al. Trans-arterial radioembolization for intermediate-advanced hepatocellular carcinoma: a budget impact analysis. BMC Cancer. 2018;18(1):715.
- Chow PKH, Gandhi M, Tan S-B, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. JCO. 2018;36:1913–1921.