ABSTRACT
Introduction
The study estimated the extent to which drug innovations over the past 30 years may have improved outcomes for six diseases.
Areas covered
We analyzed six diseases (ischemic heart disease, lung cancer, breast cancer, human immunodeficiency virus [HIV] infection, type 2 diabetes mellitus, and rheumatoid arthritis [RA]) with significant mortality or morbidity for which there have been major drug innovations over the past 30 years. We used U.S. data from the Global Burden of Disease (GBD) database and a patient registry to perform counterfactual time-series analyses predicting the improved health outcomes that may have been associated with major drug innovations. For 5 conditions using data from the GBD study, years of life lost per individual with the condition could have been higher by 17.1% (breast cancer) to 660.6% (HIV infection) in 2017 had the major drug innovations not been introduced. For RA, using patient registry data, patients’ functional status could have been 11.5% worse had biological therapies not been introduced.
Expert opinion
Policies targeting drug prices should be broadened to consider the price and value of all health-care services. The societal importance of the pharmaceutical industry’s ability to respond rapidly to emerging diseases should be recognized.
1. Introduction
Health-care spending in the United States (US) has grown significantly over the past several decades despite efforts that have been made to control the growth of health spending at the local, state, and federal levels. Health-care spending as a percentage of gross domestic product, as reported by the Centers for Medicare and Medicaid Services (CMS), increased from 5.0% in 1960 to 12.1% in 1990 to 17.7% in 2019 [Citation1]. These increases in medical care spending over the past 6 decades have been accompanied by life expectancy gains and morbidity decreases in the U.S. population. For example, life expectancy from birth increased by 10 years between 1960 (69.7 years) and 2015 (79.4 years) [Citation2].
In response to rising health-care spending, a variety of policies focusing on reducing health-care spending overall have been proposed [Citation3–5]. As part of this effort, policymakers are currently considering policies that aim to reduce spending for drugs, including several pieces of legislation that allow the government to directly set drug prices [Citation6]. Potential legislation is likely to incorporate elements of H.R. 3, the Lower Drug Costs Now Act (2019–2020) [Citation7], which was passed in the House of Representatives to allow the Health and Human Services Secretary to directly set prices on the top 250 drugs with the greatest total cost to Medicare and the entire U.S. health system [Citation8]. Significant uncertainty surrounds the potential impact of H.R. 3 on pharmaceutical innovation, with varying 10-year estimates of the impact on new drug approvals ranging from 30 to 56 fewer drugs [Citation9,Citation10].
Important for consideration in drug pricing policies is that many studies have also shown a linkage between innovative pharmaceuticals and significant health improvements in the United States with several studies by Lichtenberg using econometric fixed effects models showing a relationship between the number of new drug launches each year and population-level mortality, longevity, or years of life lost (YLLs) from different diseases [Citation11–17] as well as the impact of pharmaceutical innovation, defined as the percentage of drugs prescribed that were approved after 1990 on total health care spending between 1990 and 2003 [Citation12]. Other studies looking at the impact of pharmaceuticals on health outcomes and medical expenditures include a study using cause-deletion analysis indicating a significant impact of the use of pharmaceuticals on key population health outcomes, such as improved life expectancy [Citation18] and a study using simulated benefits of specific drug classes showing the impact of pharmaceuticals in slowing the growth in Medicare spending for cardiovascular events [Citation19].
What has not been as well characterized at the U.S. population level is how a reduction in the availability of specific drug classes would have impacted patient-level outcomes in the treated conditions. This question was examined in our study by identifying specific drug classes or other innovative medical treatments introduced between the years 1990 and 2017 that clinicians indicated had major impacts on disease outcomes for six serious diseases. For these conditions, we identified observational database studies of the changes in outcomes over time and performed a counterfactual time-series analysis of the U.S. data from either the Global Burden of Disease (GBD) database or a patient registry to provide indicative estimates of the impact of these specific drug classes or other medical innovations on disease outcomes.
2. Patients and methods
In this study, we estimated the magnitude of the change in health outcomes in the United States that may be associated with specific new drug classes or other medical treatments being introduced into general practice. We focused our analysis on six medical conditions with significant mortality or morbidity (as reflected in the GBD database) for which there have been major medical innovations over the past 30 years. We interviewed physicians specializing in the treatment of each of the six conditions, asking them to review a list of innovative pharmaceutical or other medical interventions that were approved for use during this time period and to indicate those they believed had a major impact on patient outcomes. Next, we performed a targeted review of published longitudinal observational data studies in the conditions to identify changes in disease outcomes and the timing of these changes. We then used the U.S. data from either the GBD database or a patient registry for those diagnosed with the six conditions to perform a counterfactual time-series analysis predicting the trajectory of health outcomes that may have occurred had the major medical innovations identified by the specialist physicians not been introduced into general practice.
2.1. Selection of conditions and innovations
The conditions selected for inclusion in our study were identified through a three-step process that included (1) identifying a list of candidate conditions with significant disease burden, (2) checking the condition-specific disease burden trends from the 2017 GBD database [Citation20], and (3) including conditions reflecting a diversity of therapeutic groups and disease characteristics (see Table A-1 for additional details). Based on these criteria, the six conditions selected for the analysis were ischemic heart disease (IHD), lung cancer, breast cancer, human immunodeficiency virus (HIV) infection, type 2 diabetes mellitus (T2DM), and rheumatoid arthritis (RA).
For each condition, we reviewed publicly available information (e.g. regulatory approvals, clinical practice guidelines) on new drugs (or drug classes) entering the market in the last three decades. We then conducted interviews with two clinical specialists per condition to (1) prioritize the drug innovations they believed had a major impact on condition-specific outcomes and (2) determine when those innovations became part of general clinical practice. Drug innovations identified by clinical specialists as having a significant impact on outcomes were selected for inclusion in the time-series analysis. The selected innovations for each condition and the years when they became part of clinical practice are presented in through 6. A summary of the specialists’ clinical experience, the materials presented to the specialists, the findings from the interviews, and the innovations excluded from the analysis is presented in Table A-2.
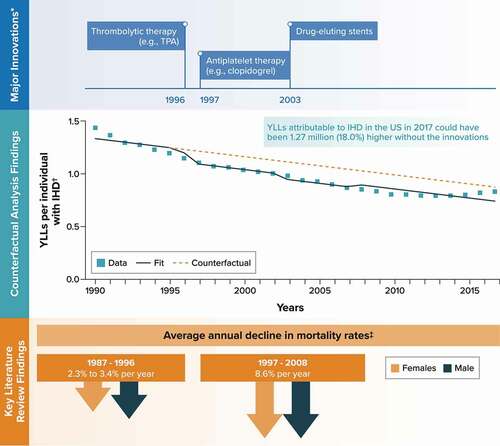
Figure 1. Ischemic heart disease.
2.2. Literature review of observational studies
For each condition selected for inclusion in the study, a targeted review of longitudinal observational outcome studies using databases other than the GBD was undertaken. The studies were identified in a search of the MEDLINE database using search terms for the condition and for key disease outcomes, such as mortality, life expectancy, and disease severity or functional status. The specific outcomes used to compute life expectancy losses and disability weights for the GBD database [Citation21] were included in the search strategy for each condition (see Tables B-1 and B-2). Studies were included only if they presented outcomes data for at least some period covered by the GBD database used for this study (1990 to 2017). Using the findings from the targeted review, the timing of changes in key disease-specific outcomes were compared with the timing of major innovations between 1990 and 2017 within each condition that were identified by specialist physicians and compared with the results from the time-series analysis.
2.3. Time series counterfactual analysis
The approach for the time series counterfactual analysis was based on the interrupted time-series methodology [Citation22], which has been used previously in the evaluation of population-level health policy interventions and health improvement initiatives [Citation23–27]. This methodology is designed to evaluate the effect of an intervention using longitudinal, population-level observational data for which there is no natural control group available while controlling for secular trends in the outcome of interest [Citation22,Citation23]. Examples of its use from the literature include evaluations of the impacts of an early statin administration initiative on stroke outcomes [Citation24], a rotavirus vaccination program on type 1 diabetes incidence in children [Citation25], physical distancing policies on the incidence of COVID-19 [Citation26], and eliminating prescription fees on hospital admissions [Citation27]. In our study, we consider the introduction of an innovative drug or drug class to general practice to be a population-level health intervention that is hypothesized to have had an impact on observable patient outcomes in the condition in question.
The population-level outcomes (i.e. dependent variables) included in the time-series analysis were drawn from the GBD 2017 study database [Citation20] and, for RA, published longitudinal observational registry data. Specifically, for IHD, lung cancer, breast cancer, HIV infection, and T2DM (conditions with significant disease-related mortality), the selected outcome was condition-specific, discounted (at 3% annually) YLLs per individual with the condition each year between 1990 and 2017. The YLL component of disability-adjusted life-years (DALYs) was selected because it reflects a combination of condition-specific deaths and remaining life expectancy for similarly aged healthy individuals at the ages of these deaths. The years lived with disability component of DALYs was not used due to concerns about changes in the disability weight methodology over time [Citation21,Citation28]. To account for changes in epidemiology over time, the estimates for YLLs per 100,000 U.S. population from GBD were divided by condition-specific prevalence estimates to yield estimates of YLLs per individual with the condition. For RA (a condition with limited disease-related mortality during the period of concern), the outcome for the analysis was mean score on the Health Assessment Questionnaire (HAQ) (a condition-specific functional measure) between 1983 and 2006 from an analysis of Arthritis, Rheumatism and Aging Medical Information System data identified in the targeted literature review [Citation29]. Detailed tables of the longitudinal health outcomes used in the analysis are presented on condition-specific worksheets in Appendix C.
The covariates (i.e. independent variables) included in the time-series analysis for each condition included a continuous time variable and a set of condition-specific binary indicator variables corresponding to the entry of major innovations into (and in some instances their exit from) clinical practice. The continuous time variable in each analysis was interpreted as a proxy for the changes in the population, public health, medical practice, or health system landscape (e.g. socioeconomic and public health factors, such as smoking, obesity, employment status, health insurance coverage, and education; clinical practice factors, such as diagnosis, surgical procedures, and non-pharmaceutical interventions; and health insurance reform or other health system organizational changes) that may have contributed to trends in the outcomes. In scenario analyses, the continuous time variable was replaced with a covariate for a measure of public health relevant to each condition (severe obesity rates for IHD, T2DM, and RA; smoking for lung cancer; mammogram rates for breast cancer; and undiagnosed proportions for HIV infection). Due to the high correlation between these public health measures and the time variable, analyses with both covariates were not considered. In addition to capturing when innovations entered clinical practice, the binary indicator variables for the innovations accounted for instances where the clinical specialists indicated that an innovation’s impact was replaced by subsequent innovations. Due to the long-term progressive nature of T2DM, the innovation covariates for this condition reflected a lag between the introduction of innovations and their anticipated impact. Such a lag between drug introduction and clinical impact has also been used in the Lichtenberg econometric analyses where considered appropriate [Citation30]. Detailed tables of the binary innovation covariates, along with the public health covariates considered in scenario analyses, are presented in Appendix C.
The results of the time-series analyses were used to perform a counterfactual analysis for each condition to predict the health outcomes that might have occurred had the innovations not been introduced. The counterfactuals were estimated by evaluating the time-series regressions with all binary innovation covariates set to 0 while maintaining the continuous time covariates. This allowed the counterfactuals to reflect changes in outcomes attributable to population, public health, medical practice, and health system trends that would have continued in the absence of the innovations. The difference between the counterfactual predictions and the original time-series predictions (i.e. with innovation covariates) was used to estimate differences in health outcomes, which we interpret as being indicative of the impact of the major medical innovations in each condition.
All statistical analyses were conducted using R Project for Statistical Computing, version 4.1.1. The interrupted time-series models were fit using linear least-squares regression. Significance testing was assessed for each model fit using the F statistic, emphasizing the overall impact of all the innovations considered in each condition over the impacts of individual innovations. To assess the significance of including innovations in the models for each condition, the goodness-of-fit for models with innovation covariates was compared with that for models without the innovation covariates (i.e. controlling for time or public health covariates only) using the likelihood ratio test. For all fitted model and counterfactual outcomes estimated using the time-series models, 95% confidence intervals (CIs) are reported around the mean predictions.
3. Results
The major medical innovations included in the time-series analyses for each condition and the timing of their introduction to clinical practice confirmed by the physician interviews are shown in through 6 along with key findings from the observational data studies and the results from the time-series counterfactual analyses. A total of 44 studies (7 for IHD, 3 for lung cancer, 3 for breast cancer, 15 for HIV, 10 for T2DM, and 6 for RA) were identified for inclusion in the literature review (see Appendix B for study references and summaries of key findings). In each condition, findings from these observational studies indicated an improvement in outcomes over time, with instances where a trend in outcomes either accelerated or switched to a positive trajectory at timepoints aligned with the introduction of physician-confirmed major innovations. The time-series analyses also suggested that the introduction of these major innovations was associated with additional improvements in health outcomes, with the counterfactual analyses predicting how much worse outcomes might have been had the innovations not been introduced. For all base-case analyses, as well as in the scenario analyses using public health covariates in place of time, the inclusion of innovation covariates in the time-series regression significantly improved the model fits. With the exception of IHD, the 95% CIs around the mean estimates for the fitted model and counterfactual predictions were nonoverlapping. More detailed information on the GBD (for 5 of 6 conditions) and registry data (for RA) outcomes and the time-series analysis results (data tables, regression equations, goodness-of-fit statistics, and fitted and predictive values [including 95% CIs] for all base-case and scenario analyses) are presented in Appendix C.
3.1. IHD
The major medical innovations identified for IHD and included as binary covariates in the time-series analysis were thrombolytic therapy (e.g. tissue plasminogen activator) in 1996, antiplatelet therapy (e.g. clopidogrel) in 1997, and drug-eluting stents in 2003. The binary innovation covariate for tissue plasminogen activator was switched off in 2008 based on feedback from the clinical specialists that it ceased to have a significant impact on outcomes in IHD after the introduction of second-generation drug-eluting stents.
Among the seven studies identified in the literature review, a published analysis of longitudinal data from the Atherosclerosis Risk in Communities study found that the average annual decline in age-adjusted mortality rates for coronary heart disease was 2.3% (females) and 3.4% (males) between 1987 and 1996 and subsequently increased to an annual decline of 8.6% (both females and males) between 1997 and 2008 [Citation31] ().
The outcome for the time-series analysis, YLLs per individual with IHD from the GBD database, decreased from 1.43 to 0.83 over the period between 1990 and 2017 (). The counterfactual analysis predicted that YLLs per individual with IHD in 2017 could have been 18.0% higher without these innovations (0.87 [95% CI, 0.73–1.02] vs. 0.74 [95% CI, 0.70–0.78]) ( and Appendix C). Across the 9.51 million individuals in the United States with IHD in 2017 [Citation20], this translates to an estimated 1.27 million additional YLLs had the innovations not been introduced into clinical practice. In a scenario analysis controlling for rates of severe obesity instead of time, the counterfactual analysis predicted that YLLs per individual with IHD in 2017 could have been 37.9% higher without the innovations, translating to an estimated 2.61 million additional YLLs overall in the United States (Appendix C).
3.2. Lung cancer
The major medical innovations identified for lung cancer and included as binary covariates in the time-series analysis were chemotherapies (e.g. paclitaxel- and docetaxel-based regimens) in 1995 and epidermal growth factor receptor inhibitors (e.g. gefitinib and erlotinib) in 2003.
Among the three studies identified in the literature review, an analysis of lung cancer statistics from the Surveillance, Epidemiology, and End Results registry found that the 5-year relative survival rate for newly diagnosed lung cancer increased from 12.2% to 14.3% between 1975–1977 and 1993–1995 and then increased from 14.6% to 20.6% between 1996–1998 and 2009–2015 [Citation32] ().
Figure 2. Lung cancer.
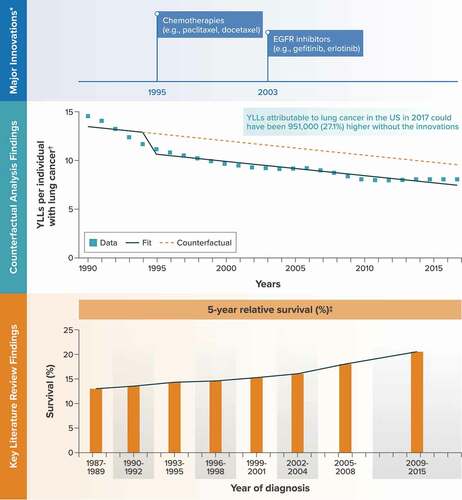
The outcome for the time-series analysis, YLLs per individual with lung cancer from the GBD database, decreased from 14.6 to 8.2 over the period from 1990 to 2017 (). The counterfactual analysis predicted that YLLs per individual with lung cancer in 2017 could have been 27.1% higher without these innovations (9.67 [95% CI, 8.27–11.06] vs. 7.61 [95% CI, 7.16–8.05]) ( and Appendix C). Across the roughly 462,000 individuals in the United States with lung cancer in 2017 [Citation20], this translates to an estimated 951,000 additional YLLs had the innovations not been introduced into clinical practice. In a scenario analysis controlling for smoking rates instead of time, the counterfactual analysis predicted that YLLs per individual with lung cancer in 2017 could have been 42.3% higher without the innovations, translating to an estimated 1.49 million additional YLLs overall in the United States (Appendix C).
3.3. Breast cancer
The major medical innovations identified for breast cancer and included as binary covariates in the time-series analysis were chemotherapy (e.g. paclitaxel) in 1994, aromatase inhibitors (e.g. anastrozole) in 1995, and human epidermal growth factor receptor 2 inhibitors (e.g. trastuzumab) in 1998.
The literature review identified three studies for breast cancer. Among these, a meta-regression of survival found that the median survival for recurrent metastatic breast cancer increased by 5.0% (20.2 months to 21.2 months) in the 1980s, 23.6% (21.2 months to 26.2 months) in the 1990s, and 45.0% (26.2 months to 38.0 months) in the 2000s. A similar increase was found in median survival for de novo metastatic breast cancer (18.9 months in 1980, 19.5 months in 1990, 23.1 months in 2000, 31.0 months in 2010) [Citation33] ().
Figure 3. Breast cancer.
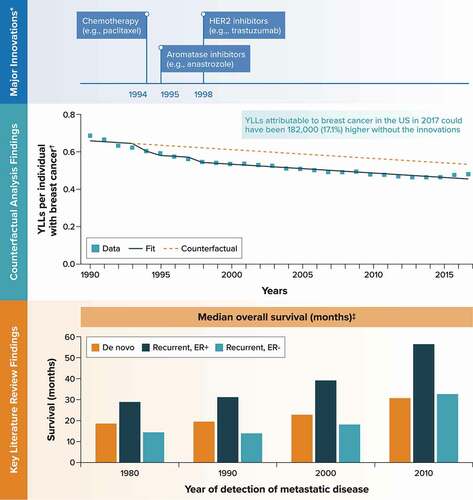
The outcome for the time-series analysis, YLLs per individual with breast cancer, decreased from 0.69 to 0.48 over this same period (). The counterfactual analysis predicted that YLLs per individual with breast cancer in 2017 could have been 17.1% higher without these innovations (0.53 [95% CI, 0.51–0.56] vs. 0.45 [95% CI, 0.44–0.47]) ( and Appendix C). Across the 2.33 million individuals in the United States with breast cancer in 2017 [Citation20], this translates to an estimated 182,000 additional YLLs had the innovations not been introduced into clinical practice. In a scenario analysis controlling for mammogram rates instead of time, the counterfactual analysis predicted that YLLs per individual with breast cancer in 2017 could have been 42.7% higher without the innovations, translating to an estimated 483,000 additional YLLs overall in the United States (Appendix C).
3.4. HIV infection
The major medical innovations identified for HIV infection and included as binary covariates in the time-series analysis were highly active antiretroviral combination regimens (e.g. protease inhibitors plus reverse transcriptase inhibitors) in 1996, fixed-dose combination therapies (e.g. lamivudine/zidovudine) in 1997, and integrase inhibitors (e.g. raltegravir) in 2007.
Among the 15 studies identified in the literature review, an analysis of a long-term cohort of patients in large, integrated health systems found that the age-adjusted mortality rates for individuals with HIV infection decreased by 85% between 1996–1997 and 2008–2011, with roughly two-thirds of the decline occurring by 1998–1999; the comparable decline for individuals without HIV infection was 13.2% [Citation34] ().
Figure 4. Human immunodeficiency virus.
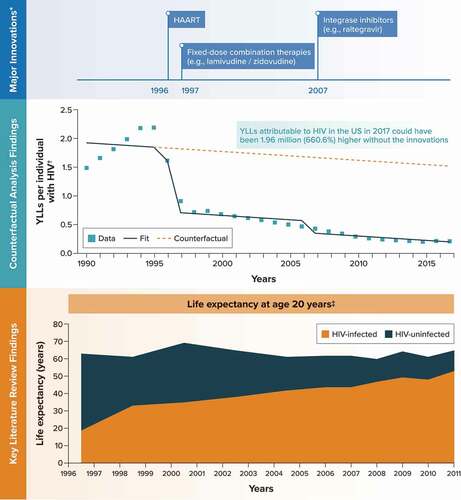
The outcome variable for the time-series analysis, YLLs per individual with HIV infection from the GBD database, increased from 1.5 in 1990 to 2.2 in 1995 before decreasing to 0.2 in 2017 (). The counterfactual analysis predicted that YLLs per individual with HIV infection in 2017 could have been 660.6% higher without these innovations (1.52 [95% CI, 0.95–2.08] vs. 0.20 [95% CI, 0.05–0.35]) ( and Appendix C). Across the 1.49 million individuals in the United States with HIV in 2017 [Citation20], this translates to an estimated 1.96 million additional YLLs had the innovations not been introduced into clinical practice. In a scenario analysis controlling for rates of undiagnosed HIV instead of time, the counterfactual analysis predicted that YLLs per individual with HIV infection in 2017 could have been 625.4% higher without the innovations, translating to an estimated 2.94 million additional YLLs overall in the United States (Appendix C).
3.5. T2DM
The major medical innovations identified for T2DM and included as binary covariates in the time-series analysis were metformin in 1994, thiazolidinediones (e.g. troglitazone) in 1996, and glucagon-like peptide-1 receptor agonists (e.g. exenatide) in 2005. The innovation covariate for thiazolidinediones was switched off in 2005 based on feedback from the clinical specialists that its impact on outcomes in T2DM lessened after the introduction of glucagon-like peptide-1 receptor agonists.
The literature review identified 10 studies for T2DM. Among these, published analyses of data from the U.S. Renal Data System found that the age-adjusted incidence of diabetes-related end-stage renal disease increased by an average of 5.8% per year between 1990 and 1996 and then decreased by an average of 2.8–2.9% per year between 1996 and 2014 [Citation35,Citation36] ().
Figure 5. Type 2 diabetes mellitus.
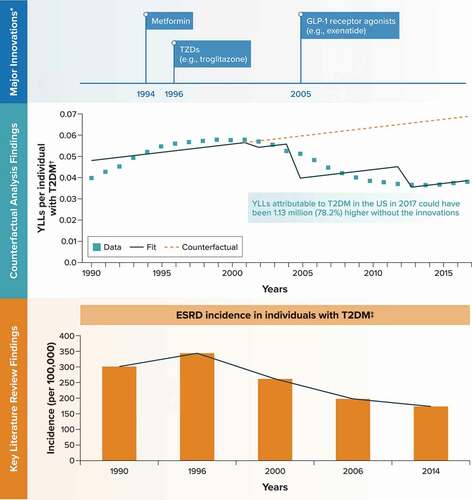
The outcome variable for the time-series analysis, YLLs per individual with type 2 diabetes from the GBD database, increased from 0.040 in 1990 to 0.058 in 1999–2001 before decreasing to 0.038 in 2017 (). In the analysis, the time period between starting a treatment, such as metformin, and the occurrence of long-term outcomes that impact mortality (e.g. end-stage renal disease) was captured by adding a lag of 8 years between the introduction of the innovations and their impact on YLLs. The counterfactual analysis predicted that YLLs per individual with T2DM in 2017 could have been 78.2% higher without these innovations (0.069 [95% CI, 0.053–0.084] vs. 0.039 [95% CI, 0.034–0.043]) ( and Appendix C). Across the 37.39 million individuals in the United States with T2DM in 2017 [Citation20], this translates to an estimated 1.13 million additional YLLs had the innovations not been introduced into clinical practice. In a scenario analysis controlling for severe obesity rates instead of time, the counterfactual analysis predicted that YLLs per individual with T2DM in 2017 could have been 83.1% higher without the innovations, translating to an estimated 1.33 million additional YLLs overall in the United States (Appendix C).
3.6. RA
The major medical innovation identified for RA and included as a binary covariate in the time-series analysis was tumor necrosis factor inhibitor therapy (e.g. etanercept) starting in 1998.
Among the six studies identified in the literature review, a longitudinal analysis of statewide inpatient hospitalizations in California reported that age-adjusted rates of joint surgery (knee, hip, ankle, wrist) among patients with RA increased between 1983–1987 and 1993–1997 and then decreased between 1993–1997 and 2003–2007 [Citation37] ().
Figure 6. Rheumatoid arthritis.
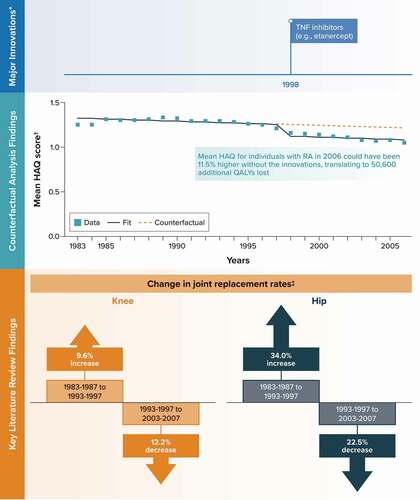
Because of the limited condition-related mortality associated with RA, the mean HAQ score from a published, longitudinal study of registry data was selected as the outcome variable for the time-series analysis [Citation29]. In this study, the mean HAQ score among individuals with RA increased by 6.3% (from 1.26 to 1.34) from 1983 to 1989 before decreasing by 21.6% (to 1.05) in 2006 (). The counterfactual analysis predicted that the mean HAQ score for individuals with RA in 2006 could have been 11.5% higher without these innovations (1.21 [95% CI, 1.15–1.27] vs. 1.09 [95% CI, 1.06–1.11]) ( and Appendix C). Combined with the 1.44 million individuals in the United States with RA in 2006 [Citation20] and evidence linking HAQ scores to quality of life [Citation38], this translates to an estimated 50,600 additional quality-adjusted life-years lost due to RA had the innovation not been introduced into clinical practice. In a scenario analysis controlling for severe obesity rates instead of time, the counterfactual analysis predicted that the mean HAQ score for individuals with RA in 2006 could have been 11.2% higher without the innovations, translating to an estimated 48,800 additional quality-adjusted life-years lost overall in the United States (Appendix C).
4. Discussion
This study used a time-series counterfactual analysis of longitudinal data to simulate the impacts on population-level health outcomes of specific major pharmaceutical innovations in six medical conditions with significant mortality or morbidity. The literature review of observational database studies provided supporting evidence from alternative data sources for the impact of the major pharmaceutical innovations on disease outcomes. For the five conditions utilizing data from the GBD study in the time-series analysis, the counterfactual results predicted that YLLs per prevalent individual (i.e. per individual with the condition) could have been 17.1% (breast cancer) to 660.6% (HIV infection) higher in 2017 had the major innovations not been introduced. When prevalence estimates for the five conditions in the United States in 2017 were applied to these results, the additional YLLs predicted for the U.S. population had the innovations not been introduced ranged from 181,000 (breast cancer) to 1,962,000 (HIV infection). For the sixth condition, the counterfactual analysis predicted that the mean HAQ score among individuals with RA could have been 11.5% higher (i.e. worse functional status) with an associated impact on individuals’ quality of life had biological therapies not been introduced.
Alternative specifications of the counterfactual analyses with condition-specific public health covariates in place of the continuous time covariates predicted differences in outcomes that were consistent with, and in some instances larger than, the primary analysis (see Appendix C). These scenario results suggest that our use of a linear, continuous time variable to account for secular health system trends in the base-case analyses may be conservative. Across all six conditions, the estimates identified in the targeted literature review also showed that a broader set of health outcomes examined in these studies had also improved over time periods aligned with the introduction of the medical innovations deemed to have had a major impact by clinician specialists and included in the counterfactual analysis.
The results of this study are generally consistent with previous studies on the association of pharmaceutical innovations with improved survival outcomes. In particular, the Lichtenberg studies of the impact of pharmaceutical innovation on mortality or longevity in the United States showed that the number of new drug approvals had a major impact on these disease outcomes for HIV infection [Citation11], cancer [Citation13], and rare diseases [Citation16] as well as on population life expectancy [Citation12,Citation14,Citation15]. Lichtenberg has also shown similar results in other countries, including Australia, Germany, France, Ireland, Canada, and other European countries. Additionally, Lichtenberg [Citation12,Citation39] estimated that between 1990 and 2003 the impact of new drugs in the United States on medical expenditure was negative because of offsetting cost reductions in hospitals and nursing homes in addition to reducing the number of YLLs before age 75 years [Citation12]. More recently, Lichtenberg estimated the incremental cost per life-year saved in 2013 for expenditure before age 85 years on drugs launched post-1981 was $2,837, or 8% of the U.S. gross domestic product per capita [Citation17]. Our study, based on an alternative regression approach and counterfactual time-series analysis, used data between 1990 and 2017 to extend the findings from these studies, showing how the introduction of a number of major pharmaceutical innovations into clinical practice for six serious diseases resulted in changes in the trajectories of disease outcomes for individuals diagnosed with these conditions.
Other studies have estimated the impact of pharmaceuticals on disease outcomes and health-care spending. For example, Buxbaum and colleagues [Citation18] estimated that 35% of the 3.3 years gain in life expectancy in the United States between 1990 and 2015 was attributable to pharmaceuticals, while 44% was attributable to public health changes and 13% to other medical care. They also reported that changes in incidence and outcomes of IHD were the largest contributor to this increase in life expectancy. Additionally, Cutler and colleagues [Citation19] estimated that half of the observed slowing of Medicare spending after 2005 was attributable to reduced spending on cardiovascular disease and that roughly half of this impact on health spending was attributable to reduced major cardiovascular events because of the use of drugs that reduced cardiovascular risk factors.
Although the U.S. observational database analyses and our counterfactual estimates for the United States presented in this study suggest that the introduction of drug innovations to clinical practice for six serious diseases has resulted in improved clinical outcomes, it should be noted that adverse events may also be experienced by those taking these drugs. Such adverse events may include renal disorders [Citation40] or drug-induced liver injury resulting from the use of many drugs and identified with the use of the RUCAM (Roussel Uclaf Causality Assessment Method) diagnostic injury algorithm [Citation41]. Information about such potential adverse effects might not be available when the drug is first introduced into clinical practice but would be added to the product label over time. Our study looked only at clinical outcomes following drug innovations introduced at least 5 years before 2017 that were still considered by the specialists interviewed in 2020 to have had a major impact on disease outcomes (except for tissue plasminogen activator in IHD and thiazolidinediones in T2DM). Thus, drugs for which a high incidence of serious adverse effects was observed in the years following launch are unlikely to have been included in our analysis. Nevertheless, the risks and benefits for any innovative drug used to treat a serious medical condition are important to review over time in individual patients and in the total treated population to ensure that the long-term benefits outweigh any long-term risks.
These findings have significant ramifications for policymakers considering implementation of policies narrowly focused on drug spending and not the value associated with specific drugs, such as H.R. 3 passed by the House of Representatives. Because it is not known which innovations will be the most significant, especially early in the development process, policies like the aforementioned ones may unintentionally disincentivize research into therapies that can change the trajectory of patient health like the ones analyzed in this study.
A strength of this study includes the use of counterfactual time-series regression analyses to provide indicative quantitative estimates of the impact of specific innovative drugs or drug classes that clinical specialists indicated had a major impact on patient outcomes. The predicted changes in health outcomes from the counterfactual analyses using the GBD or registry data were also shown to be consistent with the analyses of other observational databases identified in a targeted literature search that looked at the disease-specific health outcomes during this same time period. Our results were also consistent with other published regression estimates of the impact of innovative drugs on disease outcomes as well as with analyses of the individual drugs based on clinical trial evidence.
Limitations of the study include the short time horizon for which data were available and the use of aggregate rather than individual-level data. In the time-series counterfactual analysis, these limitations impacted the ability to fully control for public health and other variables due to high correlations between these variables and time. In particular, the differences between our base-case and scenario analyses emphasize the limitations of using a single continuous time (or public health) variable as a proxy for complex, secular trends in population, public health, non-pharmaceuticcal interventions, medical practice, or health system factors. In addition, we were not able to include pharmaceuticals introduced to practice in recent years (e.g. immunotherapy for lung cancer) because of the limited time to observe their impact on clinical outcomes in the 2017 GBD database. We also focused only on the health benefits of treatment, primarily measured as YLL or functional status since the GBD data and observational data reviewed did not provide information on any specific short- or long-term drug-related adverse effects. While our analysis accounted for the uncertainty inherent in the time-series regression predictions, we did not incorporate the uncertainty around the aggregated outcomes reported in the GBD or patient registry data sources. Finally, we did not attempt to estimate expenditures for the new drug classes or use them to derive their cost-effectiveness, but such economic evaluation studies have been published elsewhere for the drug classes that we included in our analyses as well as in aggregate by Lichtenberg [Citation17].
5. Conclusion
This study presents quantitative and qualitative evidence from physician interviews, a targeted literature review of longitudinal observational studies, and a counterfactual time-series analysis that together suggest that major pharmaceutical innovations over the past 30 years have improved condition-related outcomes for six serious medical conditions. Rather than exclusively focusing on reducing all drug prices, drug pricing policies also need to consider the value that specific drug expenditures produce and continue to incentivize innovation that has the potential to provide important health-care benefits for patients. The result will be an increase in health system efficiency that may indirectly reduce health-care spending.
6. Expert opinion
The findings presented in our study suggest improved health outcomes for six serious conditions associated with the introduction of several new drug classes into practice over the past 30 years. Our results, based on counterfactual time-series analyses and a targeted literature review, add to the findings in several econometric studies by Lichtenberg estimating the extent to which all new drug introductions or new drug introductions for specific diseases impact life-years lost in the population.
The improvements estimated in our study were not necessarily predictable at the time that testing of these new drug classes was initiated, which occurred many years before regulatory approval and integration into the treatment pathway. Although research and development efforts target specific diseases and are based on sound scientific principles, because of the complexities of human physiology and of disease processes, it is not possible to accurately predict which scientific advances will make the most difference for a specific condition and which will be safe for human use. A good example of this was seen in the development of drug treatments for HIV infection, a newly identified infection in 1985. The first drug with activity against this disease, zidovudine, had limited benefits when used alone because resistance developed rapidly. However, once other antiretrovirals that targeted the same or different cellular mechanisms were developed and combination treatments introduced, rapid development of resistance could be avoided and the disease controlled. There have also been spillover effects from HIV antiretroviral research. Thus, drug classes similar to those used to treat HIV are now used successfully to treat chronic hepatitis B and to cure chronic hepatitis C.
A more recent example is that of COVID-19. Society was able to develop vaccines so quickly only because several innovative vaccine technologies were quite advanced in terms of research and development, although commercial application of these technologies had not yet been widespread. The technologies to deliver a vaccine for a new virus existed, allowing industry to focus on how to add the right information about the new virus to be effective for the new disease. In addition, many years of research into antiviral treatments, including HIV infection, are beginning to pay off in the development of drugs to treat COVID-19 cases. Thus, we need to design policies not only to incentivize research and development to ensure innovation in all known disease areas but also to allow society to pivot quickly as new risks emerge.
Over the next 5 years, concerns about high drug prices in the United States (US) will continue to be an area of focus for policymakers. However, a focus on lowering all drug prices without consideration of the value obtained from specific drugs and the uncertainty that exists in the early stages of drug development creates the risk that significant health improvement gains will not continue. An alternative approach would be to develop pricing policies that focus on rewarding value in a manner that recognizes the inherent uncertainty in the drug development process to ensure that the United States continues to incentivize the type of innovation that provides important health-care benefits for patients. In parallel, efforts should be made to advance the science around the early identification of which therapies are likely to have the greatest impact on patients.
Recently, the concept of a “value-based price’ has been introduced, which focuses on the need for new drugs to be priced according to their value as measured by their impact on patient health and broader value elements. Over the next five years, this debate on the use of ‘value-based pricing’ is likely to continue, especially, but not only, in the United States. We believe it is critical for this debate to be carried out within the context of ‘value-based prices’ in other parts of the health system, including surgical procedures and inpatient and outpatient care. In addition, consideration of the structural aspects of national health systems is critical to control health-care costs by encouraging innovative care patterns, such as greater use of telehealth or primary and some secondary care by paraprofessionals where this can be delivered safely. Such care patterns could make health care more accessible to those in rural or underserved areas, reducing health inequities and improving outcomes of many conditions.
Article Highlights
We selected six diseases with significant morbidity and mortality for which there have been major medical innovations over the past 30 years — ischemic heart disease, lung cancer, breast cancer, human immunodeficiency virus infection, type 2 diabetes mellitus, and rheumatoid arthritis.
For each of these conditions, clinicians identified the specific drug treatments that they perceived to have had a major impact on disease outcomes during this period.
Counterfactual time-series analyses were conducted to estimate the impacts on population-level health outcomes of these innovative drugs for the six conditions.
The results of the counterfactual analyses suggested that health outcomes would have been worse for each condition had the innovations not been introduced.
Our results are consistent with outcomes from other longitudinal observational database studies in these conditions and with previously published estimates on the impact of pharmaceutical innovation.
Declaration of interest
W. Herring and J. Mauskopf are employees of RTI Health Solutions, a business unit of RTI International. Funding for the analysis was provided in a contract to RTI Health Solutions from the National Pharmaceutical Council. W. Herring and J. Mauskopf perform a variety of consulting services for many pharmaceutical companies as part of their employment of RTI Health Solutions. M. Ciarametaro, B. Sils, and R. Dubois are employees of the National Pharmaceutical Council, which is funded by pharmaceutical companies in the United States. The National Pharmaceutical Council is the sponsor for this study and also collaborated with the other authors in the design and implementation of the study and the preparation of the manuscript. D. Wamble was an employee of RTI Health Solutions who received a grant from the National Pharmaceutical Council (NPC) to perform this study in collaboration with NPC. D. Wamble also performed economic evaluation studies that are funded by many pharmaceutical companies as part of his responsibilities when he was an employee of RTI Health Solutions. D Wamble no longer works at RTI Health Solutions and now works at a Pharmaceutical Company but he has continued to contribute to the article and has reviewed the final study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors participated in the conceptualization and design of the study, the interpretation of the results, and writing and commenting on the final manuscript. All authors agree for the final version of the manuscript to be published.
Supplemental Material
Download Zip (234.3 KB)Acknowledgments
The authors acknowledge John Forbes for editing and formatting the document and Emily Gill for preparing the figures.
Data availability statement
RA functional status from: Krishnan E, Lingala B, Bruce B, et al. Disability in rheumatoid arthritis in the era of biological treatments. Ann Rheum Dis. 2012;71:213–218.
GBD data from: Global Health Data Exchange. Global burden of disease study 2017 (GBD 2017) data resources. BD results tool. 23 July 2019[cited 23 April 2021]. Available from: http://ghdx.healthdata.org/gbd-2017
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Centers for Medicare and Medicaid Services. National health expenditure data. Historical. [updated 2020 December 16 cited 2021 December 16]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical
- Medina L, Sabo S, Vespa J. Living longer: historical and projected life expectancy in the United States,1960 to 2060: population estimates and projections. Current Population Reports. 25–1145 [2020 Feb cited 2021 Apr 23]. Available from: https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1145.pdf
- Centers for Medicare and Medicaid Services. Design and development of the Diagnosis Related Group (DRG) [2019 Oct cited 2021 Apr 23] Available from: Design and development of the Diagnosis Related Group (DRG (cms.gov)
- MedicareResources,org. Medical Advantage (Part C) private health plans. [updated 2020 Dec 8 cited 2021 Apr 23]. Available from: https://www.medicareresources.org/medicare-benefits/medicare-advantage/#:~:text=Key%20takeaways,B%20benefits%2C%20except%20hospice%20services
- Bennett L. Low-value health care on downward trend in Washington state. Press Release. [updated 2019 Oct 29 cited 2021 Apr 23]. Available from: https://wahealthalliance.org/low-value-health-care-on-downward-trend-in-washington-state/
- Lee J. Covid-19, rare diseases frame drug pricing debate in congress. Bloomberg Law. [updated 2021 Apr 7 cited 2021 Apr 23]. Available from: https://news.bloomberglaw.com/health-law-and-business/covid-19-rare-diseases-frame-drug-pricing-debate-in-congress
- Hellmann J. Democrats plan crackdown on rising drug costs. The hill. [2021 Feb 21 cited 2021 Apr 23]. Available from: https://thehill.com/policy/healthcare/539724-democrats-plan-crackdown-on-rising-drug-costs
- Lower Drug Costs Now Act. HR 3, 116th congress. 2019–2021. cited 2021 Apr 26. Available from: https://www.congress.gov/bill/116th-congress/house-bill/3
- Congressional Budget Office. Effects of drug price negotiation stemming from title 1 of H.R. 3, the lower drug costs now act of 2019, on spending and revenues related to part D of medicare. Letter to the Honorable Frank Pallone Jr. [updated 2019 Oct 11 cited 2021 Mar 19]. Available from: https://www.cbo.gov/system/files/2019-10/hr3ltr.pdf?wpisrc=nl_health202&wpmm=1
- VitalTransformation. H.R. 3 - International reference pricing. Calculating the impact on the US biopharmaceutical innovation ecosystem. [2019 Nov 21 cited 2021 Apr 26]. Available from: http://vitaltransformation.com/wp-content/uploads/2020/10/PhRMA-Deck_v7.11.21.19.pdf
- Lichtenberg FR. The effect of new drugs on HIV mortality in the U.S., 1987–1998. Econ Hum Biol. 2003;1:259–266.
- Lichtenberg FR. The impact of new drugs on U.S. longevity and medical expenditure, 1990–2003. Amer Econ Rev. 2007b;97:438–443.
- Lichtenberg FR. The effect of new cancer drug approvals on the life expectancy of American cancer patients, 1978–2004. Econ Innovation New Technol. 2009;18:407–428.
- Lichtenberg FR. Pharmaceutical innovation and longevity growth in 30 developing and high-income countries, 2000– 2009. NBER Working Paper 18235. 2012. Accessed 14 August 2021; Available from: http://www.nber.org/papers/w18235
- Lichtenberg FR. The effect of pharmaceutical innovation on longevity: patient level evidence from the 1996–2002 medical expenditure panel survey and linked mortality public-use files. Forum Health Econ Policy. 2013a;16:1–33.
- Lichtenberg FR. The impact of new (orphan) drug approvals on premature mortality from rare diseases in the U.S. and France, 1999–2007. Euro J Health Econ. 2013b;14:41–56.
- Lichtenberg FR. How many life-years have new drugs saved? A 3-way fixed-effects analysis of 66 diseases in 27 countries, 2000–2013. Working Paper 25483. 2019. Accessed 14 August 2021. Available from: http://www.nber.org/papers/w25483
- Buxbaum JD, Chernaw ME, Fendrick AM, et al. Contributions of public health, pharmaceuticals, and other medical care to US life expectancy changes, 1990–2015. Health Aff (Millwood). 2020 Dep;39:1546–1556.
- Cutler DM, Ghosh K, Messer KL, et al. Explaining the slowdown in medical spending growth among the elderly, 1999–2012. Health Aff (Millwood). 2019 Feb;38:222–229.
- Global Health Data Exchange. Global burden of disease study 2017 (GBD 2017) data resources. BD results tool. [updated 2019 Jul 23 cited 2021 Apr 23]. Available from. http://ghdx.healthdata.org/gbd-2017
- Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the global burden of disease 2013 study. Lancet Glob Health. 2015 Nov 1;3:e712–23.
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13:S38–S44.
- Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017 Feb 1;46:348–355.
- Flint AC, Conell C, Klingman JG, et al. Impact of increased early statin administration on ischemic stroke outcomes: a multicenter electronic medical record intervention. J Am Heart Assoc. 2016;5:e003413.
- Perrett KP, Jachno K, Nolan TM, et al. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatr. 2019;173:280–282.
- Islam N, Sharp SJ, Chowell G, et al. Physical distancing interventions and incidence of coronavirus disease 2019: natural experiment in 149 countries. BMJ. 2020 Jul 15;370:m2743.
- Williams AJ, Henley W, and Frank J. Impact of abolishing prescription fees in Scotland on hospital admissions and prescribed medicines: an interrupted time series evaluation. BMJ Open. 2018 Dec 18; 8:e021318.
- GBD. Disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2017;392:1789–1858. [2018 Nov 10].
- Krishnan E, Lingala B, Bruce B, et al. Disability in rheumatoid arthritis in the era of biological treatments. Ann Rheum Dis. 2012;71:213–218.
- Lichtenberg FR. The impact of pharmaceutical innovation on longevity and medical expenditure in France, 2000–2009. Econ Hum Biol. 2014;13:107–127.
- Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012 Apr 17;125:1848–1857.
- Howlader N, Noone AM, Krapcho M, et al. eds. SEER cancer statistics review. 1975–2016. Bethesda MD: National Cancer Institute; based on November 2018 SEER data submission, posted to the SEER web site. 2019 April. [citedApr 2021] https://seer.cancer.gov/csr/1975_2016/
- Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. 2018 Nov;2: ky062.
- Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr. 2016 Sep 1;73:39–46.
- Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care. 2010 Jan;33:73–77.
- Burrows NR, Hora I, Geiss LS, et al. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes - United States and Puerto Rico, 2000–2014. MMWR Morb Mortal Wkly Rep. 2017 Nov 3;66:1165–1170.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983–2007. Ann Rheum Dis. 2010 May;69:868–871.
- Pennington B, Davis S. Mapping from the health assessment questionnaire to the EQ-5D: the impact of different algorithms on cost-effectiveness results. Value Health. 2014;17:762–771.
- Lichtenberg FR. Effects of new drugs on overall health spending: Frank Lichtenberg responds. Health Affairs. 2007a;26:887–890.
- Shahrbaf FG, Assadi F. Drug-induced renal disorders. J Renal Injury Prev. 2015 Sep 1;4:57–60.
- Teschke R, Danan G. Worldwide use of rucam for causality assessment in 81,856 idiosyncratic Dili and 14,029 hili cases published 1993-mid 2020: a comprehensive analysis. Medicines (Basel). 2020 sep 29;7:62.
