 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Multi-criteria decision analysis (MCDA) was proposed to surmount arbitrary clinical decisions in the field of biological therapies for psoriatic patients. At the same time, MCDA may further highlight the potential of bimekizumab for the treatment of moderate-to-severe psoriasis, compared to placebo, adalimumab, ustekinumab, secukinumab, and even ixekizumab and risankizumab.
Research design and methods
The EVIDEM framework was adapted to reflect relevant criteria for the assessment. Estimated values were obtained by means of an additive linear model combining weights and scores assigned by a multidisciplinary committee of 12 experts. Consistency and replicability were evaluated through an alternative weighting method and a re-test.
Results
Bimekizumab was assessed by the committee as an intervention with a positive value contribution for the treatment of moderate-to-severe psoriasis in comparison to any of the alternatives. The drug provides a substantial therapeutical benefits and improves the health results reported by the patients, as it combines a higher level of clearance, rapidity, and persistence with a similar safety and tolerability profile.
Conclusions
Under a methodology with increasing use in the health field, bimekizumab was evaluated as a drug with a high added value for the treatment of moderate-to-severe psoriasis when compared to six different alternatives.
1. Introduction
The popularity of biological treatments for moderate-to-severe plaque psoriasis has increased over the last decades, resultant of their associated clinical benefits and safety profile. However, given the bounded resources available in healthcare budgets, allocation decisions play a critical role in determining the most appropriate alternatives to be employed. Cost-effectiveness analyses are progressively developing into the most prominent tools used in funding decisions, in general and also in dermatology [Citation1].
Notwithstanding, this methodology has been criticized for many reasons, such as the inadequacy on capturing the social value and an implicit judgment of other aspects outside the range of efficacy, safety, and cost, generating heterogeneity in coverage decisions across settings for the same treatment or indication. Although those could be explained by different budget constraints and priorities, an increased comprehension of the rationale used in the decision-making process could enhance the validity and acceptability of such determinations [Citation2].
The multi-criteria decision analysis (MCDA) framework, which is being used to an increased extent in healthcare decision making, yields ways of solving those hurdles, as it consists of a structured, multi-dimensional, transparent, and systematic approach, incorporating a vast set of criteria and their individual value contribution to the decision or allocation problem. As a result, it can be particularly useful as a complement to the standard economic evaluations in the assessment of drugs [Citation3].
Psoriasis is a chronic, systemic, inflammatory condition, characterized by a variable clinical course, usually chronic, with periods of relapse and remission of unpredictable duration [Citation4]. It affects approximately 41 million people globally, out of which, 1.1 million in Spain [Citation5]. Plaque psoriasis is the most common form of psoriasis, responsible for 90% of the cases [Citation6]. It manifests not only through the skin as visible plaques, pain, and itching, but also more widely, resulting in increased mortality, productivity losses, emotional and quality of life deterioration and risk of comorbidities, such as metabolic syndrome, obesity, and psoriatic arthritis compared to the general population, adversities, which are amplified according to psoriasis severity [Citation4]. Moderate-to-severe psoriasis accounts for about one-third of patients with plaque psoriasis in Spain [Citation7].
The use of biologic treatments for the management of moderate-to-severe psoriasis is swiftly growing, and may account for roughly 20% of systemic treatments [Citation8]. There is an extensive breadth of biologics therapies approved by the European Medicines Agency (EMA), entailing tumor necrosis factor (TNF) inhibitors (infliximab, etanercept, adalimumab, and certolizumab pegol), and different interleukin (IL) inhibitors, targeting IL12/23 (ustekinumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor (brodalumab), and IL-23p19 (guselkumab, tildrakizumab, and risankizumab) [Citation9].
Regardless of this extensive number of alternatives, people suffering from moderate-to-severe psoriasis still face multiple unmet needs. From an efficacy perspective, there is no cure for the disease, 40%–60% of patients do not obtain complete or almost complete skin clearance (Psoriasis Area and Severity Index [PASI]100 or PASI90 in clinical trials, PASI <2 in clinical practice), whilst for the half who achieve absolute clearance, it takes from 3 to 8 months after the start of treatment. Moreover, 50% of the patients discontinue their treatment with biologicals before the fifth year due to lack of efficacy [Citation10–14]. In addition, the need for biologic switch is increasing significantly, given failure related mainly to efficacy and safety issues, which generates additional costs and potential health risks, since persistent inflammation may trigger or worsen several cardiac, respiratory, and metabolic comorbidities [Citation15,Citation16]. Switching or combination of therapies, rather than persisting on a treatment that has primarily or secondarily failed, may improve outcomes [Citation17,Citation18]. In this sense, development of predictive models capable of estimating the probability of non-responses based on clinical features or endotypes may be of paramount clinical and pharmacoeconomic importance [Citation19,Citation20].
From a broader angle, other necessities can be highlighted, namely: treatment adequacy, patient’s satisfaction, ability to reduce emotional distress, route and frequency of administration of drugs, heterogeneity in access and the imperative to (re)define treatment goals beyond skin manifestations [Citation8,Citation21].
Bimekizumab (Bimzelx®, UCB Pharma S.A., Brussels, Belgium), an humanized IgG1/κ monoclonal antibody, is the first drug designed to selectively and directly inhibit both IL-17A and IL-17F cytokines, and has been authorized by EMA for the treatment of moderate-to-severe plaque psoriasis in August 2021 [Citation22].
The main objective of this study was to apply a multi-criteria decision analysis (MCDA) to assess the value of bimekizumab (BKZ) for the treatment of moderate-to-severe psoriasis, using placebo (PBO), adalimumab (ADA), ustekinumab (UST), secukinumab (SEC), ixekizumab (IXE) and risankizumab (RIS) as comparators.
2. Methods
2.1. Expert panel design and conduct of the study
The study was carried out through a multidisciplinary expert committee (MEC) of 12 individuals, with a balanced geographical representation from six autonomous regions. The participants were nationally recognized by their broad experience in the management of moderate-to-severe psoriasis and decision-making in Spain, who had participated as authors and coauthors in national and international publications. The number of experts included was in line with previous MCDA exercises undertaken in Spain [Citation23,Citation24]. The constitution of the MEC with these characteristics was done in order to factor in a wide-ranging volume of perspectives in the assessment of the value contribution of bimekizumab versus placebo and five biological drugs. Moreover, another selection criterion was the absence of any conflict of interest.
The MEC was composed by three dermatologists from three reference university hospitals in Spain (two from Catalonia [Germans Trias i Pujol University Hospital and Hospital de la Santa Creu i Sant Pau] and one from Madrid [La Paz University Hospital]; one nurse (from a referral hospital in Madrid, Universitary Hospital Gregorio Marañón); one psychologist specialized in psoriasis (Hospital de la Santa Creu i Sant Pau); two patients (representatives of the National Patient Association for Psoriasis, ‘Acción Psoriasis’); two healthcare managers (one hospital manager from a referral hospital in Valencia, west region of Spain [Hospital Doctor Peset], one regional healthcare manager in the area of Galicia, north region of Spain); one hospital pharmacist (from a reference hospital in the Balearic Islands [Universitary Hospital Son Espases]); one health economist (Department of Pharmacology and Clinical Therapeutics. Biomedical Research Institute of Málaga [IBIMA]); and one representative of the political sphere (Andalusian Public Health School, former General Secretary of Health and healthcare vocal at the Parliament).
The rationale used for the selection of the comparators combined the inclusion of at least one drug for each mechanism of action and recommendations based upon the practical experience of the members of the MEC.
Two online meetings were held with the MEC. In the first one (June 2021), experts received training on the MCDA methodology, based on a pre-read document sent prior to the meeting. Additionally, they agreed on adapting the EVIDEM (Evidence and Value: Impact on DEcision Making) framework (10th edition), a widely used and flexible methodology [Citation25], to the context of psoriasis (). Finally, the MEC assigned weights to each of the fifteen criteria, by distributing 100 points amongst them, that revealed their individual relative importance – the greater the points assigned, the higher the importance of the criterion.
Table 1. MCDA framework for the evaluation of drugs in moderate-to-severe psoriasis.
Prior to the second meeting, the experts scored (online) each criterion and intervention individually, based on the evidence matrices provided and their individual experience and perception. Some of the criteria are defined by EVIDEM as absolute (with scores ranging from 0 to 5, as no comparison between interventions are made) and some as relative (scores from −5 to 5, as they compare different interventions: a score of 5 means that bimekizumab is much better than the comparator in the criterion analyzed, whilst a score of −5, that bimekizumab is much worse than the comparator and 0, that there are no differences between bimekizumab and the alternative drug evaluated).
In the second meeting (September 2021), scores and value estimates were presented and debated amongst members of the MEC, with the objective to gather qualitative information around the rationale applied in their assessment. One week after that the MEC members were asked to assign (online) weights and scores again, as well as use an alternative weighting method, based on a nonhierarchical 5-point direct rating scale (1 = lowest relative importance, 5 = highest relative importance). The re-test weights and scores as well as the alternative weighting method generated new value estimates, which were used to check consistency and validity of the MCDA.
2.2. Literature review and evidence matrix
A comprehensive review of the literature was conducted to collect the available evidence regarding the fifteen criteria and each drug included in this MCDA. The information was assembled in seven evidence matrices and one summary document (supplementary file 1), which were reviewed and validated by the clinicians from the MEC. The search was performed using major biomedical databases, such as PubMed and Medline, clinical trial registries, clinical practice guidelines, official European and Spanish healthcare evaluation bodies webpages, namely European Medicines Agency (EMA), Spanish Medicines and Healthcare Products Agency (AEMPS), and Spanish regional and hospital evaluations, as well as gray literature. No date or language restrictions were applied.
2.3. Data analysis
An overall estimated value (ranging from −1 to 1) was obtained for each comparison, through an additive linear model of all individual criteria value contributions, which were calculated as the product of normalized weights and scores:
where V is the total estimated value, Vx the value contribution of the criterion x, Wx the weighting of the criterion x, ∑Wn the sum of all weights, and Sx the normalized score for each criterion (Sx = score/5). A value estimation greater than 0 means that bimekizumab has a positive value contribution in relation to its comparator, whilst a value, which is lower than 0 represents a negative value contribution from the evaluated drug against the alternative. A more detailed explanation of how each parameter is evaluated is described in a previously published methodological guideline [Citation26].
The degree of agreement between the responses made at the two timepoints (test and re-test) was evaluated through the intra-rater correlation coefficients (ICC 3,1) using STATA® version 14 (STATA Corp., LP, College Station, TX, USA).
3. Results
3.1. Weights: relative importance of each criterion
The experts distributed 100 points between the 15 different criteria of the MCDA framework, disclosing their appreciation on the relative importance of each individual attribute when appraising any drug for the treatment of moderate-to-severe psoriasis. The analysis of the results by domain suggests that the parameters, which are usually assessed by economic evaluations (outcomes and costs) were given a relative importance of 55.9% over the total, whilst the other (need, type of benefit and knowledge about the intervention) represented a slightly lower, but still highly significant, share of the total (44.3%) ().
Figure 1. Relative importance of each individual criterion in the assessment of drugs for the treatment of moderate-to-severe psoriasis (mean, min, max and median weights and standard deviations). The 100-points distribution method was applied, by which the experts assigned a weight to each criterion, provided its aggregation resulted in 100. CPG: clinical practice guidelines.

Individually, the three most relevant criteria were level of clearance (11.3 ± 6.6), persistence of clearance and safety/tolerability (9.8 ± 2.2 each), which were considered to be between two and three times more important than the three least significant ones: impact on other direct costs (3.3 ± 1.8), impact on indirect costs (3.5 ± 2.5) and size of the affected population (3.7 ± 1.9). Variability in responses was low (SD: 1.7–3.4), except for the criterion level of clearance (SD: 6.6) which can be explained by one extreme case in the managers’ subgroup (30/100, being responsible for 54% of this deviation).
For healthcare professionals (dermatologists, nurse, and psychologist, n = 5) and managers (healthcare managers, hospital pharmacist, health economist, and politician, n = 5), the three most and least important criteria coincided with the global results. In contrast, for the patients (n = 2), unmet needs and consistency of the effect were two out of the three features considered as with upmost significance, while level of clearance and safety/tolerability occupied the seventh and eighth positions in order of importance for this subgroup (supplementary file 2).
3.2. Scores based on evidence and insights from the MEC
The fifteen criteria included in this MCDA and rated by the MEC are summarized in and Supplementary file 2. The overall average score (n = 12) for the ‘disease severity’ criterion was 3.8 ± 0.5 out of 5.0 (median: 4.0), reflecting the fact that moderate-to-severe psoriasis is considered a severe disease by the experts as, despite not being a life-threatening disease, it has a high impact on patients’ quality of life, work environment and emotional (psychological and psychiatric) spheres.
Table 2. Scores assigned per criterion, and main comments from the multidisciplinary experts committee or observation of subgroup results.
The ‘size of affected population’ is the only criterion in the EVIDEM framework that has a pre-specified scoring scale [Citation25], and the overall average score for this criterion (3.0 ± 0.4 out of 5.0 [median: 3.0]) showed a consensus in relation to the prevalence of moderate-to-severe psoriasis in Spain (0.6% of the population).
Moreover, in the opinion of the MEC, moderate-to-severe psoriasis is a disease with considerable ‘unmet needs,’ given the overall average score of 3.5 ± 0.9 out of 5.0 (median: 4.0) attributed to this criterion. Experts considered that the unmet needs are not only clinical, but also related to other aspects, such as psychological support and access to therapies.
‘Level of clearance’ received overall mean scores ranging from 4.8 ± 0.4 (median: 5.0) for BKZ vs. PBO to 2.1 ± 1.0 (median: 2.0) for BKZ vs. IXE. A score of 5.0 means that bimekizumab achieves a much higher level of clearance than the comparator. The experts agreed with the resulting ratings, adding that, for all efficacy criteria, they would have expected an absolute consensus of a 5.0 score in the comparison between BKZ and PBO.
Additionally, the scores for ‘rapidity of clearance’ were consistent with the evidence analyzed [Citation27–32] (ranging from 4.8 ± 0.6 [median: 5.0] for BKZ vs. PBO to 2.1 ± 0.8 [median: 2.0] for BKZ vs. IXE. A score of 5.0 means that bimekizumab is much quicker than the comparator in achieving the expected results), reflecting that the IL-17 (BKZ IL-17A/F, SEC IL-17A, and IXE IL-17A) are the drugs, which provide the fastest onset of action.
‘Persistence of clearance’ (which means the durability of the clearance achieved) was considered by the MEC as a criterion, which would need more long-term results and data to allow for a full assessment of its effect after the first year. Nevertheless, they were able to provide ratings based on the available evidence related to the persistence of clearance at 1 year, resulting in aggregated scores ranging from 4.8 ± 0.6 (median: 5.0) for BKZ vs. PBO to 2.2 ± 1.6 (median: 3.0) for BKZ vs. RIS. A score of 5.0 means that the effects produced by bimekizumab are much more persistent over time than the ones produced by the comparator.
The criterion ‘safety and tolerability’ received scores between 1.7 ± 1.6 (median: 2.0) for BKZ vs. ADA, and −0.3 ± 2.6 (median: −1.0) for BKZ vs. PBO. A score of +5.0 means that bimekizumab is much safer than the comparator, and −5.0, less safe. The experts commented that bimekizumab, overall, has a similar safety/tolerability profile compared to most of the comparators and that, some of the low scores assigned to this drug could be explained by the adverse event related to candidiasis, although they highlighted that this is perfectly manageable, not leading to treatment discontinuation.
The scoring of the criterion ‘patient reported outcomes’ was based on both quality-of-life scales and convenience of treatment, with results ranging from 4.6 ± 0.7 (median: 5.0) for BKZ vs. PBO to 0.8 ± 1.3 (median: 1.0) for BKZ vs. RIS. A score of 5.0 means that bimekizumab provides much better patient reported outcomes than the comparator. According to the MEC, there is a correlation between level of clearance and patient reported outcomes, fact which posed a challenge for an independent assessment of this criterion.
‘Type of therapeutic benefit,’ received a mean score of 3.6 ± 0.7 (median 4.0), reflecting the belief that bimekizumab produces a high therapeutic benefit, given it provides a high level of clearance and a rapid onset of action, which are sustained over time. In addition, the therapeutical benefits were associated with an improvement in patients’ quality of life and the relief of the main symptoms, such as pain, itching, and scaling.
Furthermore, ‘consistency of the effect’ was scored between 4.5 ± 0.8 (median: 5.0) for BKZ vs. PBO, and 0.9 ± 1.6 (median: 0.5) for BKZ vs. IXE. A score of 5.0 means that bimekizumab is much more consistent than the comparator. Some of the experts based their score on whether or not the drug was approved for the indication of psoriatic arthritis, or could potentially receive this indication in the future, based on published or ongoing clinical trials [Citation33,Citation34], while others also considered aspects that stand out in each drug (i.e. secukinumab for the treatment of nail psoriasis).
The economic consequences were appraised in three criteria, the first being ‘cost of the intervention.’ In the absence of a price for bimekizumab in Spain (not defined at the time this study was being carried on), we assumed the same annual acquisition cost per patient as that of an IL-17A inhibitor already marketed, such as secukinumab (€19,400 for the first year and €14,900 for the maintenance period, based on ‘notified prices’ [laboratory sales prices: PVL]).
Overall mean scores for the criterion ‘cost of the intervention’ ranged from 0.8 ± 0.8 (median: 1.0) for BKZ vs. RIS to −3.8 ± 1.9 (median: −5.0) for BKZ vs. PBO. A score of +5.0 means that the acquisition of bimekizumab generates substantial savings to the system versus the comparator, and −5.0, substantial additional costs. There was a consensus on the large difference that exists between the notified price reported and the price paid in practice, especially for the biologics for which there are biosimilars. Thereupon, for the scoring of bimekizumab vs. adalimumab, the MEC took into account the real cost of adalimumab, which was informed to be nearly €3500 per year.
The median score for all comparisons (except BKZ vs. PBO) related to the ‘impact on other direct costs’ and the ‘impact on indirect costs’ were 1.0 (SD ranged from 0.6 to 1.2), and no negative scores were given, reflecting that bimekizumab appears to be slightly superior in terms of the impact that its implementation would have on all other costs for the system, such as hospitalizations, medical visits and productivity losses. The experts commented that the results are consistent, based on the narrow evidence available for these criteria.
Overall, the ‘quality of evidence’ provided by the clinical trials analyzed was considered as very relevant and valid (medians between 4.0 and 4.5; SD between 1.0 and 1.2) for the head-to-head comparisons (BKZ vs. PBO, ADA, UST, and SEC), whilst the indirect comparisons (BKZ vs. IXE and RIS) were considered as relatively less relevant (medians: 2.0 ± 1.4), as comparisons had less scientific rigor.
The last criterion appraised was ‘expert consensus/clinical practice guidelines’. The MEC considered that bimekizumab will be positioned similarly to other first-line biological drugs for the treatment of moderate-to-severe psoriasis in the future updates of clinical practice guidelines (medians between 1.0 and 2.0; SD between 1.1 and 2.0). Some of the elements that could differentiate bimekizumab from the others, according to the experts, are the form of administration (every 2 months); the potential approval for the treatment of psoriatic arthritis and the maintenance of efficacy levels.
3.3. Value estimates: combining weights and scores
The integration of weights and scores of each panelist resulted in overall value estimates scaling between −1 and 1. Bimekizumab provided a high added value against all comparators analyzed. Specifically, the value contribution of bimekizumab for the treatment of moderate-to-severe psoriasis was: BKZ vs. PBO (0.65 ± 0.10; median: 0.64), BKZ vs. ADA (0.53 ± 0.09; median: 0.53), BKZ vs. UST (0.51 ± 0.10; median: 0.50), BKZ vs. SEC (0.46 ± 0.09; median: 0.45), BKZ vs. IXE (0.41 ± 0.12; median: 0.41) and BKZ vs. RIS (0.42 ± 0.13; median: 0.47) ().
Figure 2. Value contribution of bimekizumab compared to placebo and five biological drugs according to the MCDA framework for the assessment of drugs in moderate-to-severe psoriasis. Mean value contribution per domain and overall value estimates are shown. Error bars show standard deviations across the twelve participants. BKZ: Bimekizumab. PBO: placebo. ADA: Adalimumab. UST: Ustekinumab. SEC: Secukinumab. IXE: Ixekizumab. RIS: Risankizumab.
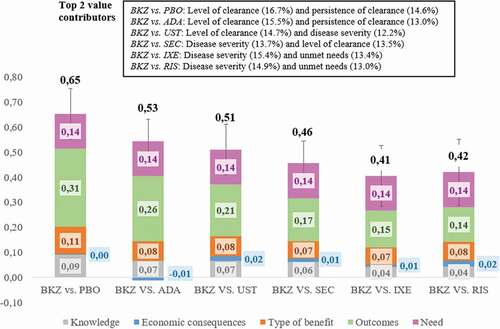
The five criteria with the greatest contribution to the final estimated values were the level of clearance (12%-17%), persistence of clearance (10%–15%), disease severity (10%–15%), unmet needs (8%–13%) and type of therapeutic benefit (8%–13%). Negative contributions were only revealed in two comparisons related to the safety criterion and in four comparisons regarding drug acquisition costs, suggesting that BKZ is less safe than PBO and RIS and more costly than PBO, ADA, UST, and IXE.
In the analysis by domains, comparative outcomes of the intervention contributed between 30% and 56% of the final estimated values, followed by the need for the intervention (21%–34%), the type of benefit (11%–22%), the knowledge about the intervention (10%–17%) and the economic consequences of the intervention (−4% to +9%) (Supplementary file 2).
3.4. Replicability and consistency
The consistency of the weights between the test and the retest was high, with an average intraclass correlation coefficient (ICC) of 0.8422. Similarly, the retest scores were very resemblant to those of the test, in all comparisons performed, with mean intraclass correlation coefficients ranging from 0.9178 to 0.9775. The consistency of the final estimated values between the test and the retest was also high, with total average ICCs ranging between 0.6160 and 0.8898. The final mean values obtained in the retest were lower than those obtained in the test, with variations between −2.8% and −10.6%. The final value estimates obtained by the application of an alternative weighting method (direct 1–5 rating scale) were almost identical as the ones obtained by the 100-point distribution method (∆-2.0% to +1.3%) (Supplementary file 3).
4. Discussion
The decision-making processes related to the appraisal of new drugs in moderate-to-severe psoriasis are complex, as they involve the need to balance multiple needs from a wide range of stakeholders [Citation1]. The mainstream methodological approach currently used to support evaluations are cost-effectiveness models [Citation2].
However, the MCDA methodology can be particularly useful as a complement to this approach, as it consists of a structured (validated stepwise methodology), multi-dimensional (participation of experts from a broad range of professional fields), transparent (criteria, weights, and scores are explicit) and systematic approach (replicable), incorporating various criteria and their individual value contribution to the decision or allocation problem [Citation3]. Its popularity has become evident in the healthcare field in the recent years, both nationally and internationally [Citation3,Citation35–38], in diverse areas, such as oncology [Citation39–42], rare diseases [Citation43–46] and dermatology [Citation23,Citation24].
This study has adopted a holistic and transparent methodological approach in the assessment of the value contribution of bimekizumab in comparison to placebo and five biological drugs for the treatment of moderate-to-severe psoriasis in Spain, through a multidisciplinary panel of experts involved in the clinical, management, and political decision-making aspects of the pathology. The EVIDEM framework was adapted into a set of 15 criteria relevant to the drug appraisal context of psoriasis. Notably, this is the first MCDA to include three efficacy criteria in a disaggregated manner (level of clearance, rapidity, and persistence of clearance), as well as to integrate another relevant criterion to this setting, which is consistency of the effect in the treatment of other indications and specific psoriasis locations.
This is also the first MCDA in moderate-to-severe psoriasis that has included six comparators, which were evaluated against bimekizumab by a MEC with a broader professional profile in relation to previous MCDA in this area. In other MCDA conducted in Spain in the area of moderate-to-severe psoriasis, the EVIDEM framework was used without any adaptations, with a smaller number of comparators, and expert committees that lacked the vision of the profiles included in this MCDA, such as nursing, psychology, and political professions [Citation23,Citation24]. In one of them, 45 experts (national and regional evaluators in Spain) weighted the 13 criteria of the EVIDEM framework, and five of them (two hospital pharmacists, one regional payer, one psoriasis expert, and one patient representative) scored ixekizumab versus four comparators [Citation23]. In another, which was performed through a committee of 10 experts (three dermatologists, four patients, two regional payers, and one health economist), the EVIDEM framework was also applied in a comparison between secukinumab and three other drugs [Citation24].
According to this MCDA, bimekizumab provides a positive value contribution in the treatment of moderate-to-severe psoriasis in Spain, in comparison to all drugs analyzed. The final estimated values ranged from 0.42 (vs. risankizumab) to 0.65 (vs. placebo). These results, despite the methodological differences explained above, are in line with those obtained in the other two MCDA applied to moderate-to-severe psoriasis in Spain [Citation23,Citation24]. The final values estimated by Badia et al. (2017) for ixekizumab ranged between 0.36 (vs. secukinumab) and 0.45 (vs. adalimumab) [Citation23], whilst Zozaya et al. (2018) estimated values for secukinumab between 0.39 (vs. ustekinumab) and 0.45 (vs. etanercept) [Citation24].
In any case, the importance of this type of study does not lie so much in the exact amounts of the value estimates, but in understanding the value drivers of the drug that is being evaluated. In this sense, the multidisciplinary debate generated was key to understanding the strengths and weaknesses of bimekizumab compared to other drugs in each of the attributes considered, from a qualitative perspective based on insights provided by the MEC.
This study is not exempt from certain limitations, inherent to any MCDA, which should be pointed out. The first limitation stems from the composition of the expert committee itself, as the limited number of experts may not be representative of the opinions of all the stakeholders involved. On the other hand, the small panel size facilitated discussions and sharing of insights, allowing for a more in-depth analysis of different value contributors. Secondly, the evidence matrix gathered information which were limited to the publicly available data at the time of the study, and some evidence was scarce (i.e. impact on other direct and indirect costs). Hence, results could be different if faced with new information, meaning that a follow-up of this study could be of added value in the future. Thirdly, misinterpretation of some evidence or scoring scale may have occurred, due to the cognitive complexity of the exercise. To minimize this potential limitation, scoring was preceded by a thorough explanation of the MCDA methodology, the assumptions made and the interpretation of the values. Fourthly, for two of the comparisons done in this MCDA (BKZ vs. IXE and BKZ vs. RIS), there were no head-to-head trials available, so that value judgment was mainly based on evidence from indirect comparisons, through studies, which were undertaken in different patient populations and conditions. This added difficulty was partially gathered by the MCDA methodology, as one criterion allows for a weighting according to the quality of evidence. Fifthly, all data related to pricing and, when applicable, to other criteria, were based on the Spanish reality, and results may differ if applied in other countries. Finally, this MCDA could have been enhanced by the addition of some aspects, such as the inclusion of all comparators available, the use of real practiced prices (versus notified prices), or the inclusion of the qualitative criteria.
5. Conclusions
Under this methodology of increasing use in the healthcare, bimekizumab has been evaluated by a multidisciplinary committee as a drug that adds value to the treatment of moderate-severe psoriasis, by providing a high degree of clearance, rapidly and persistently over time, with a very similar safety profile to other drugs.
Exercises of this type allow us to understand where the value of health interventions lies for the different agents, encourage communication between them and can serve as a reference in decision-making on evaluation, financing, and reimbursement. In the future, it would be desirable to continue advancing in the development of the MCDA methodology and to extend its use, so that health care decision making can be carried out in a framework of greater transparency, consistency, and efficiency.
Authors contributions
N Zozaya and A Hidalgo participated in the conception and design of the work. N Zozaya, F Abdalla, S Alfonso, J Balea, J Carrascosa, O Delgado, F Dolz, A Garcia-Ruiz, P Herranz, A Manfredi, J Martinez, P Morales de los Rios Luna, L Puig and S Ros have contributed to the acquisition of data. N Zozaya and F Abdalla have analyzed and interpreted the data. F Abdalla has drafted the manuscript, and N Zozaya has substantively reviewed it. All authors have approved the manuscript and have agreed both to be personally accountable for the author’s contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
List of abbreviations
ADA Adalimumab
AEMPS Spanish Medicines and Healthcare Products Agency
BKZ Bimekizumab
EMA European Medicines Agency
EVIDEMEvidence and Value: Impact on DEcision Making
IL Interleukin inhibitors
IXE Ixekizumab
MCDA Multi-Criteria Decision Analysis
MEC Multidisciplinary Experts Committee
PASI Psoriasis Area and Severity Index
PBO Placebo
RIS Risankizumab
SEC Secukinumab
TNF Tumor Necrosis Factor inhibitors
UST Ustekinumab
Declaration of interest
N Zozaya, F Abdalla and A Hidalgo are employed by the consultancy firm Weber, which received funding for the development of this project. S Alfonso, J Balea, J Carrascosa, O Delgado, F Dolz, A Garcia-Ruiz, P Herranz, A Manfredi, J Martinez, P Morales de los Rios Luna, L Puig and S Ros received fees from the consultancy firm Weber for the participation in the project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (1.9 MB)Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Bray N, Wolf P. Allocation of biologics: health economics and clinical decision making in plaque psoriasis. Br J Dermatol. 2018;178(5):997–998.
- Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. 2018;19(1):123–152.
- Drake JI, de Hart JCT, Monleón C, et al. Utilization of multiple-criteria decision analysis (MCDA) to support healthcare decision-making. J Mark Access Health Policy. [cited 2017 Nov 21]. Available from 2017 Nov 21] 2017;5(1):1360545. Internet: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5645903/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315.
- Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. [cited 2022 Mar 17]. Available from 2022 Mar 17] 2021;8. Internet: https://www.frontiersin.org/article/10.3389/fmed.2021.743180
- Boehncke W-H, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994.
- Carrascosa J, Pujol R, Daudén E, et al. A prospective evaluation of the cost of psoriasis in Spain (EPIDERMA project: phase II). J Eur Acad Dermatol Venereol. 2006;20(7):840–845.
- Acción Psoriasis. Encuesta NEXT sobre necesidades actuales y expectativas de futuro en psoriasis en España. Informe de resultados 2019 [Internet]. 2020 [cited 2021 Apr 29]. Available from: https://www.accionpsoriasis.org/recursos/publicaciones/otras-publicaciones.html
- Reid C, Griffiths CEM. Psoriasis and treatment: past, present and future aspects. Acta Derm Venereol. 2020;100(3):adv00032.
- Kerkhof PCM, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population‐based multinational assessment of psoriasis and psoriatic arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002–2010.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Cochrane Database Syst Rev. [Internet]. 2021 [cited 2021 Apr 20]; Available from]. [];4.: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011535.pub4/full
- Armstrong AW, Soliman AM, Betts KA, et al. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther. 2021;11(3):885–905.
- Egeberg A, Andersen YMF, Halling-Overgaard A-S, et al. Systematic review on rapidity of onset of action for interleukin-17 and interleukin-23 inhibitors for psoriasis. J Eur Acad Dermatol Venereol JEADV. 2020;34(1):39–46.
- Lin P-T, Wang S-H, Chi -C-C. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8(1):16068.
- Tsai Y-C, Tsai T-F. Switching biologics in psoriasis - practical guidance and evidence to support. Expert Rev Clin Pharmacol. 2020;13(5):493–503.
- Usha M, Abrar Q, Raquel L. The economic burden of switching biologics in psoriasis: a real-world analysis in the US population. J Am Acad Dermatol. 2014;70:AB191.
- Conti A, Damiani G, Ruggeri R, et al. Switching infliximab in psoriatic patients during COVID −19 pandemics: a real-life retrospective study comparing intra-versus interclass switching strategies. Dermatol Ther. 2021;34(5):e15088.
- Damiani G, Odorici G, Pacifico A, et al. Secukinumab loss of efficacy is perfectly counteracted by the introduction of combination therapy (rescue therapy): data from a multicenter real-life study in a cohort of Italian psoriatic patients that avoided secukinumab switching. Pharmaceuticals. 2022;15(1):95.
- Damiani G, Conic RRZ, Pigatto PDM, et al. Predicting secukinumab fast-responder profile in psoriatic patients: advanced application of artificial-neural-networks (ANNs). J Drugs Dermatol JDD. 2020;19(12):1241–1246.
- Monks G, Rivera-Oyola R, Lebwohl M. The psoriasis decision tree. J Clin Aesthetic Dermatol. 2021;14:14–22.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol. 2014;70(5):871–881.e30.
- European Medicines Agency (EMA). Bimzelx. summary of product characteristics. 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf [cited 2021 Jun 23]
- Badia X, de la Cueva P, Moreda FR, et al. Application of multi-criteria decision analysis (MCDA) to determine the value of treatments for the moderate to severe plaque psoriasis in Spain. Value Health. 2017;20(9):A564.
- Zozaya N, Martínez-Galdeano L, Alcalá B, et al. Determining the value of two biologic drugs for chronic inflammatory skin diseases: results of a multi-criteria decision analysis. BioDrugs Clin Immunother Biopharm Gene Ther. 2018;32:281–291.
- Goetghebeur MM, Cellier MS. Can reflective multicriteria be the new paradigm for healthcare decision-making? The EVIDEM journey. Cost Eff Resour Alloc CE. [Internet]. 2018 cited 2020 Sep 23;16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6225552/
- Zozaya N, Oliva Moreno J, HIdalgo-Vega A. Multi-criteria decision analysis in healthcare. Its isefulness and limitations for decision making . [Internet]. Weber Foundation; 2018. Available from: https://weber.org.es/wp-content/uploads/2021/03/libro_admc_17_x_24_ingles_digital.pdf [cited 2020 Sep 24]
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet Lond Engl. 2021;397(10273):475–486.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–141.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet Lond Engl. 2021;397(10273):487–498.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–152.
- Griffiths CEM, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet Lond Engl. 2015;386(9993):541–551.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet Lond Engl. 2020;395(10222):427–440.
- ClinicalTrials.gov (NIH U.S. National Library of Medicine). A phase 3, multicenter, randomized, double-blind, placebo-controlled, active reference (Adalimumab) study evaluating the efficacy and safety of bimekizumab in the treatment of subjects with active psoriatic arthritis (BE OPTIMAL) [internet]. clinicaltrials.gov; 2021 [cited 2021 Dec 13]. Report No.: NCT03895203. Available from: https://clinicaltrials.gov/ct2/show/NCT03895203
- Bayón Yusta J, Gutiérrez Iglesias A, Galnares-Cordero L, et al. Proyecto metodológico. Síntesis de información relevante de apoyo a los MCDA (análisis de decisión multicriterio) para la toma de decisiones [Internet]. Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Tecnologías Sanitarias del País Vasco; 2019 [cited 2019 Apr 26]. Available from 2019 Apr 26: http://www.ogasun.ejgv.euskadi.eus/r51-catpub/es/k75aWebPublicacionesWar/k75aObtenerPublicacionDigitalServlet?R01HNoPortal=true&N_LIBR=052312&N_EDIC=0001&C_IDIOM=es&FORMATO=.pdf
- Marsh K, Lanitis T, Neasham D, et al. Assessing the value of healthcare interventions using multi-criteria decision analysis: a review of the literature. PharmacoEconomics. 2014;32(4):345–365.
- Gilabert-Perramon A, Lens C, Betolaza JI, et al. Multi-criteria decision analysis (MCDA): common tools for different needs supporting healthcare decision making in Spain. Value Health. 2016;19(7):A489–90.
- Guarga L, Badia X, Obach M, et al. Implementing reflective multicriteria decision analysis (MCDA) to assess orphan drugs value in the Catalan health service (CatSalut). Orphanet J Rare Dis. 2019;14(1):157.
- Hsu JC, Lin J-Y, Lin P-C, et al. Comprehensive value assessment of drugs using a multi-criteria decision analysis: an example of targeted therapies for metastatic colorectal cancer treatment. PloS One. 2019;14(12):e0225938.
- Wagner M, Samaha D, Khoury H, et al. Development of a framework based on reflective MCDA to support patient-clinician shared decision-making: the case of the management of gastroenteropancreatic neuroendocrine tumors (GEP-NET) in the United States. Adv Ther. 2018;35(1):81–99.
- Camps C, Badia X, García-Campelo R, et al. Development of a multicriteria decision analysis framework for evaluating and positioning oncologic treatments in clinical practice. JCO Oncol Pract. 2020;16(3):e298–e305.
- Garau M, Hampson G, Devlin N, et al. Applying a multicriteria decision analysis (MCDA) approach to elicit stakeholders’ preferences in Italy: the case of obinutuzumab for rituximab-refractory indolent non-Hodgkin lymphoma (iNHL). PharmacoEconomics Open. 2017;2(2):153–163.
- Kolasa K, Zwolinski KM, Zah V, et al. Revealed preferences towards the appraisal of orphan drugs in Poland - multi criteria decision analysis. Orphanet J Rare Dis. [Internet]. 2018 [cited 2020 Jul 23];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5922020/
- Zozaya N, Galindo J, Alcalá B, et al. El análisis de decisión multi-criterio como herramienta para la toma de decisiones en medicamentos huérfanos: una revisión de la literatura. IX Congreso Internacional de Medicamentos Huérfanos y Enfermedades Raras, Sevilla; 2019. [cited 2021 Oct 21]. Available from: www.farmaceuticosdesevilla.es
- Schey C, Krabbe PFM, Postma MJ, et al. Multi-criteria decision analysis (MCDA): testing a proposed MCDA framework for orphan drugs. Orphanet J Rare Dis. 2017;12(1):10.
- Wagner M, Khoury H, Bennetts L, et al. Appraising the holistic value of Lenvatinib for radio-iodine refractory differentiated thyroid cancer: a multi-country study applying pragmatic MCDA. BMC Cancer. Internet]. 2017;17(1). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5393009/ [cited 2020 Sep 14]
