ABSTRACT
Background
Several surgical treatments are available for managing lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH). Water vapor thermal therapy (WVTT) is a new minimally invasive therapy. This study estimates the budget impact of introducing WVTT for LUTS/BPH into the Spanish health care system.
Methods
A model simulated the evolution of men over 45 years of age with moderate-severe LUTS/BPH after surgical treatment, over a 4-year time horizon, from the Spanish public health care service´s perspective. The technologies in scope included those most used in Spain: WVTT, transurethral resection (TURP), photoselective laser vapourization (PVP) and holmium laser enucleation (HoLEP). Transition probabilities, adverse events and costs were identified from the scientific literature and validated by a panel of experts. Sensitivity analyses were performed by varying the most uncertain parameters.
Results
Per intervention, WVTT resulted in savings of €3,317, €1,933 and €2,661 compared to TURP, PVP and HoLEP. Over a 4-year time horizon, when performed in 10% of the cohort of 109,603 Spanish males with LUTS/BPH, WVTT saved €28,770,125 against the scenario without WVTT availability.
Conclusions
WVTT could reduce the cost of managing LUTS/BPH, increase the quality of health care and reduce the length of procedure and hospital stay.
1. Introduction
Benign prostatic hyperplasia (BPH) is a common condition in aging males caused by an overgrowth of prostatic tissue [Citation1]. For males in their 30s, the prevalence of BPH is estimated to be 10%, that rises to 50% in males over 50 and 90% in males over 80 [Citation2–4].
The main symptoms associated with BPH are lower urinary tract symptoms (LUTS), such as urinary voiding and storage, frequency, urgency and nocturia [Citation5]. Problems associated with urinary storage tend to have some of the greatest impacts on the health-related quality of life (HRQoL) of patients with BPH [Citation6,Citation7]. In addition to affecting the patient, BPH also impacts their partners’ quality of life, such as through sleep disturbances and impacts on social life, psychological status or sexual activity [Citation8]. Because of this impact, and promoted by several studies performed in last decades [Citation9–11], the management of BPH is now centered around LUTS.
The diagnosis of BPH might be done with different methods. There are several diagnostic tests available, but it is commonly diagnosed by a physical examination and the measurement of the maximum urinary flow to determine the obstruction grade. In some cases, the diagnosis includes the use of questionnaires fulfilled by the patient, as the International Prostatic Score System (IPSS). The determination of the prostatic size, the maximum flow and the evolution of the patient, reported in the IPSS, determine the future therapeutic approach [Citation1].
Currently, there are many different therapeutical strategies available to manage BPH. Most of the clinical guidelines recommend a ‘Watch-and-Wait’ strategy if the patient has mild LUTS, corresponding to a IPSS score under 7 [Citation12,Citation13]. When the patient´s IPSS increases, it is recommended to start a pharmaceutical treatment. However, this treatment is not effective in many patients, so the management of BPH in these cases must be performed with surgical treatments as transurethral resection of the prostate (TURP) or open prostatectomy.
TURP is the current gold-standard approach and involves the elimination of prostatic tissue fragments by conducting electricity via a diode into the prostate through the urethra. Traditionally, this technique has been performed by monopolar diode (mTURP). Bipolar diode (bTURP) is now becoming increasingly common due to its advantages, such as the possibility of using saline solution in the procedure, which allows the practitioner to perform longer interventions, and causes less damage in the peripheral tissue [Citation14].
Nevertheless, new minimally invasive surgical treatments (MISTs) alternatives to TURP have been developed in the last years. These MISTs include photoselective laser vapourization of the prostate (PVP) (Greenlight®, Boston Scientific) [Citation15], holmium laser enucleation (HoLEP) [Citation16] and water vapor thermal therapy (WVTT) (Rezum®, Boston Scientific) [Citation17].
PVP uses high-power potassium-titanyl-phosphate which emits visible green light with a wavelength of 532 nanometers (nm). This light is directed to the hemoglobin, present in high concentrations in the vascularized peripheral prostatic tissue, assessing the ablation and coagulation of the obstructive prostatic tissue in a hemostatic fashion [Citation15].
HoLEP is the most recent holmium laser prostatectomy technique, developed from Combined Endoscopic Laser Prostatectomy (CELAP), Holmium Laser Ablation of the Prostate (HoLAP) and Holmium Laser Resection of the Prostate (HoLRP). In HoLEP, the surgical times are shorter than other holmium laser prostatectomies because the holmium laser is used to dissect and transfer the prostatic lobes into the bladder, where the tissue is reduced into removable fragments with a morcellator [Citation16].
One of the newest interventions for BPH is WVTT, which is a minimally invasive procedure that uses water vapor to deliver targeted, controlled doses of stored thermal energy directly to the region of the prostate gland, relieving symptoms and obstructions, and reducing prostate tissue associated with BPH. The device creates and convectively delivers water vapor into the prostate tissue denaturing prostatic cells and causing their death. The cells are then absorbed by the body, reducing prostate volume [Citation17].
While differences in clinical effectiveness is not well stablished between the surgical alternatives described previously [Citation18], performing one intervention or another has major implications for healthcare systems, due to the differences in costs between them. Thus, the economic evaluation of these health interventions has a key role in guaranteeing the sustainability of the system, yielding evidence that supports the choice of a therapy. In this context, Budget Impact Analysis (BIA) is an useful method to produce economic evidence, as it estimates the incremental cost of including a new treatment option in the healthcare system by comparing one scenario reflecting current clinical practice, against an hypothetical scenario considering the availability of the new treatment [Citation19,Citation20].
The aim of this study was to assess the economic impact of introducing WVTT for the surgical management of LUTS secondary to BPH into the Spanish public healthcare system.
2. Materials and methods
The present budget impact analysis (BIA) was conducted using an economic model developed in Microsoft Excel®, following the good practice principles published by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Task Force [Citation19,Citation20].
The analysis was performed from a Spanish health care system perspective with a 4-year time horizon. As explained previously, the total costs of two different scenarios were included in the analysis. The first scenario represented current clinical practice, without availability of WVTT, and where mTURP, bTURP, PVP and HoLEP were included. The second scenario simulated the introduction and availability of WVTT to the clinical practice of BPH surgical management.
The model was designed with a cohort state transition structure that mapped a hypothetical cohort of patients through defined health states according to a constant set of probabilities for each surgical alternative applied along a time horizon of 3-month duration model cycles (). The aim of this model was to chart the patients treated with the surgical alternatives, and to identify the clinical events which may incur additional costs. Given this purpose, and following ISPOR’s guidelines for conducting BIAs [Citation19,Citation20], the annual discount rate was not applied. Half cycle correction was not applied because of the simplicity of the model design and the relatively short Markov cycle duration considering that the absence of this adjustment would result in small calculation biases [Citation21].
Figure 1. Structure of the model designed for the budget impact analysis.
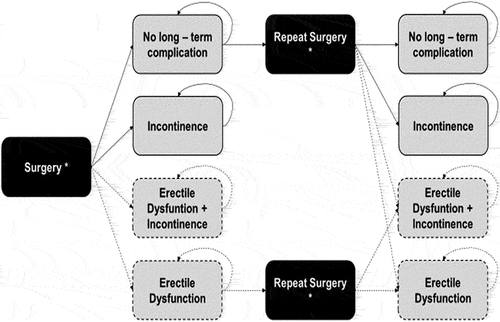
The simulation began with patients having a first surgical intervention. Transition to a non-long-term complication state or long-term complication state (urinary incontinence) was allowed.
A second surgical intervention was allowed for every intervention assessed, apart from those patients in an incontinence state post-initial surgery, because in concordance with the assumption applied in previous BPH models [Citation22,Citation23], permanent incontinence was considered a contraindication for further treatments.
The second intervention might be performed with the same technique used in the first surgery or with TURP. From this repeat surgery state, patients could evolve to the same states described earlier (non-long-term complications or urinary incontinence). The initial surgery and repeat surgery states were tunnel health states, meaning that these were states in which a patient could only remain for one model cycle.
All the data used for model customization were identified in the scientific literature and were validated by an advisory board composed of 3 Spanish urologists experienced in the surgical management of BPH.
A structured questionnaire developed for validation of available evidence and collection of information about clinical practice was individually filled by the expert panel members. Subsequently, an expert meeting was held to share answers and validate and agree upon all the values used in the model for the analysis.
The structure of the model matches the structure of the model submitted to the National Institute for Health and Care Excellence (NICE), accepted by it for the evaluation of the WVTT technology in the United Kingdom, which led to its authorization [Citation24]. In addition, the model was also adapted to other settings, such as Italy [Citation25].
2.1. Population
The target population comprised patients eligible for a surgical treatment to manage LUTS secondary to BPH. The number of patients with BPH and LUTS requiring surgical intervention was calculated by applying the prevalence of BPH with LUTS (25.0%) [Citation26] to the Spanish population of 11,328,492 males over 45 years of age reported in 2022 [Citation27]. Furthermore, the proportion of males with BPH and LUTS eligible for surgical intervention (38.7 surgeries for 1,000 patients/year) [Citation28] was applied to the prevalent patients to estimate the target population.
2.2. Surgical alternatives
The model considered the following technologies, which are currently used in the Spanish public health care system: TURP (with mono- or bipolar diode), PVP, HoLEP and WVTT.
The proportion of use of each of the techniques (60.00%, 30.00% and 10.00% for TURP, PVP and HoLEP respectively) reflected current national clinical practice, as validated by the expert panel. In the scenario including WVTT, it was assumed to be used in 10.00% of the patients, which experts considered a plausible rate of penetration for WVTT according to the Spanish setting. The hypothetical scenario was modified by reducing the use of TURP and PVP, but not the proportion of patients in whom HoLEP was indicated.
Given the lack of robust evidence on the distribution of mTURP and bTURP, the proportion of use applied was divided equally between them as validated by the expert panel.
In most studies, clinical effectiveness of the surgical treatments for BPH is measured by reduction of LUTS. However, evidence about differences in effectiveness between the alternatives is not robust [Citation18]. In order to remain conservative in the analysis and following the NICE criteria, which considered this approach a limitation but even though accepted the model [Citation24], the clinical effectiveness (measured by the reduction of LUTS) was assumed to be the same for each technology.
In the analysis, transition to the repeat surgery state represented the main difference between interventions, principally in terms of resources consumption and costs. Despite the 4-year time horizon selected for the analysis, five-year follow-up retreatment rates identified in the scientific literature were used (9.3% for TURP [Citation29], 6.9% for PVP [Citation30,Citation31] – estimated by applying the relative risk of 1.18 observed in PVP GOLIATH study [Citation31], to TURP incidence of reoperation estimated in 5.8% [Citation30] –, 3.0% for HoLEP [Citation32] and 4.4% for WVTT [Citation33]), since the experts considered those as the most representative of Spanish clinical practice.
In case of reintervention, patients could be treated with the same surgical alternative as in first intervention or with TURP. TURP was considered the surgical technique for reintervention in 50.0% of patients with previous PVP, 60.0% of patients from WVTT and 100.0% of patients with previous TURP (mTURP or bTURP). Patients initially treated with HoLEP requiring additional intervention were assumed to be reoperated with HoLEP, according to the three urologists´ criteria.
The safety profile of the alternatives was captured by means of adverse events, which were classified as short- or long-term adverse events. The short-term adverse events were further split into mild/moderate or severe complications. Mild/moderate complications considered include non-acute urinary retention (AUR), mild urinary tract infection (UTI) and bleeding. Severe complications included AUR, severe UTI, hemorrhage or transfusion requirement, bladder neck constriction and transurethral resection syndrome (TURS). Long-term adverse events incorporated into the analysis were urinary incontinence and erectile dysfunction.
The frequencies of the complications for each therapy were identified from multiple sources including the clinical trials of the techniques, network meta-analyses (NMA) or national studies ().
Table 1. Clinical parameters of the surgical treatments of lower urinary tract symptoms in benign prostatic hyperplasia.
2.3. Resource utilization and costs
The estimation of the total costs of the different surgical alternatives included the medical device cost, surgical procedure cost, and cost of managing adverse events.
The medical device-related costs were estimated by assuming that the capital equipment required to perform each of the interventions was leased. It was also assumed that every hospital owned a resectoscope, so only the costs of disposable resources and health care professionals were considered for each intervention (). The disaggregated consumption of disposables and utilization of health care professional time for performing every technique were provided by the expert panel (Supplementary Table 1).
Table 2. Costs included in the economic evaluation.
The surgical procedures were characterized by four parameters: the length of the intervention, defined by the minutes required to perform the intervention in the operating room, the length of hospital stay following surgery and the number of pre- and postdiagnostic visits and tests required.
The length of intervention and hospital stay for each technique were sourced from the literature and validated by the expert panel as representative of current clinical practice in Spain ().
Preoperative and follow-up visits and tests were considered equivalent for all alternatives. One preoperative and one follow-up visit to the urologist per patient were considered. Preoperative tests included one ultrasound echography and urinary flowmetry in every patient, and one urodynamic test and flexible cystoscopy in 5% of patients. The postoperative tests included one ultrasound echography and urinary flowmetry in every patient, and 1 hemogram, the measurement of prostatic specific antigen (PSA), urea, creatinine, and urinary sediment in 20% of patients.
Finally, the cost of managing adverse events was identified in the scientific literature for AUR [Citation42], acute UTI [Citation43] and urinary incontinence. The latter was estimated assuming 5% severe incontinence (€11,633.58) [Citation43], 40% moderate incontinence (€2,519.44) [Citation44] and 55% mild incontinence (€1,278.75) [Citation45] ().
The disaggregated consumption of health resources provided by the expert panel (Supplementary Table 1), was used to estimate the management cost for non-AUR, nonacute UTI, nonacute bleeding, bleeding, TURS and bladder neck constriction ().
All costs are expressed in euros with values of the year 2023. Unit costs for the health resources were obtained from a national database of tariffs and other published costs cited in the scientific literature, applying to the Spanish population and procedures (Supplementary Table 2). Where required, costs were updated to 2023 values, by applying the consumer price index (CPI) provided by the Statistics National Institute [Citation27].
2.4. Alternative scenario
An alternative scenario was assessed considering that the equipment is acquired by hospital centers instead of being leased. The amortization rate of the equipment was applied (3.5%) for 10 years, assuming an average of 150 interventions per year with each of the techniques.
The acquisition of equipment is usually associated with agreements in device acquisition costs different from those applied in the leasing scenario. Alternative resources and unit costs considered in this scenario are detailed in Supplementary Table 1 and Supplementary Table 2, respectively.
2.5. Sensitivity analyses
Due to uncertainty concerning some of the inputs and to test the model’s robustness, several deterministic and probabilistic sensitivity analyses were conducted.
One-way deterministic sensitivity analyses were performed by varying the following parameters: the proportion of use of mTURP vs. bTURP, mTURP mild UTI frequency [Citation46], PVP duration, the surgery theater cost per minute [Citation47], the proportion of patients retreated with TURP, and the proportion of patients treated with each surgical alternative in the scenario with WVTT.
Additionally, another deterministic sensitivity analysis was performed including the probability of the occurrence of erectile dysfunction, alone or with incontinence. The occurrence frequency of erectile dysfunction with each intervention, obtained from the literature, was 0.0% for WVTT [Citation48,Citation49], 10.0% and 1.8% for mTURP and bTURP respectively [Citation50], 1.1% for PVP [Citation50] and 2.3% [Citation51] for HoLEP. The cost of managing erectile dysfunction (€1,965.07) was derived from the scientific literature [Citation52,Citation53].
The probabilistic sensitivity analysis included 1,000 Monte Carlo simulations modifying simultaneously all the parameters according to beta, log-normal and gamma probabilistic distributions (Supplementary Table 3).
3. Results
3.1. Base case results
The cohort of patients with LUTS secondary to BPH requiring surgical treatment in Spain was estimated to be 109,603 males annually.
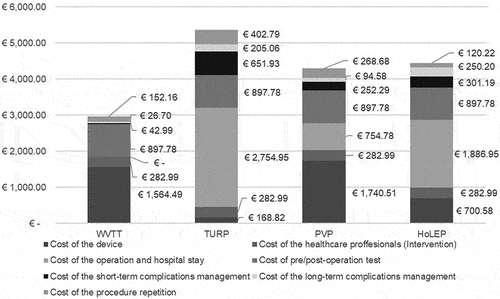
The average cost per patient treated, considering the resource consumption and cost of pre-operation tests, devices and surgical procedure, in addition to the resource consumption and cost of post-operation test, short and long-term complications management and reinterventions for 4 years, was estimated to be €6,616 for TURP, €5,232 for PVP, €5,960 for HoLEP and €3,299 for WVTT.
WVTT had the highest consumable cost, but lower costs associated with the surgical procedure, hospital stay and management of adverse events (), resulting in cost savings per operated patient of €3,317, €1,933 and €2,661 compared to TURP, PVP and HoLEP, respectively (disaggregated cost savings are presented in Supplementary Table 4).
Figure 2. Cost breakdown per first intervention with the techniques in the base case.
For the whole target cohort in the scenario without WVTT, the total cost was €672,419,688. In the scenario considering the use of WVTT in 10% of eligible patients, the total cost resulted €643,649,562, so the budget impact associated with the introduction of WVTT for the surgical management of patients with LUTS associated with BPH, would result in €28,770,125 for the 4-year period. Savings were greatest in the 1st year, at €26,115,733, reached €893,883, in the 2nd year, reached €884,740 in the 3rd year and reached €875,769 in the 4th year ().
Table 3. Base case and alternative scenario results.
The management of 10% eligible patients with WVTT was also associated with savings of 25,483 operation hours and 7,362 bed days for the whole 4-year period (Supplementary Table 5).
3.2. Alternative scenario results
In the alternative scenario, which considered the acquisition of the equipment, the average cost per patient estimated for each therapeutic alternative was €3,169 for WVTT, €6,620 for TURP, €5,106 for PVP and €6,101 for HoLEP.
The budget impact estimated in this scenario resulted in total savings of €29,522,641 over the 4-year period for the whole target population.
Savings of €26,866,479 were observed in the 1st year, €894,458 in the 2nd year, €885,330 in the 3rd year and €876,374 in the 4th year ().
The capacity analysis was the same as in the base case (Supplementary Table 5).
3.3. Sensitivity analyses results
The results of all sensitivity analyses demonstrated savings associated with the introduction of the WVTT for the surgical management of LUTS in BPH ().
Table 4. One-way sensitivity analyses results.
The parameter generating the greatest variation compared to the base case was the increase in the proportion of the use of WVTT, which resulted in total savings of €57,540,250 and €86,310,376 when it was used in 20% or 30% of patients, respectively.
The clinical parameter that had the most significant impact on savings was the duration of operation when performing PVP. When this duration was increased from 49.6 to 100 minutes, savings derived from the availability of WVTT were estimated to raise up to €34,134,699 for the 4-year period. The parameter that produced the most notable reduction in savings was the cost of the surgery theater, reducing savings to €25,550,816 when the cost applied was €12.04 [Citation54], instead of the cost considered in the base case €18.96 [Citation47].The analysis performed including erectile dysfunction resulted in savings of €31,639,242 for the 4-year time horizon (Supplementary Table 6).
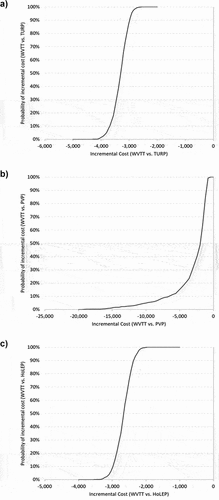
The probabilistic sensitivity analysis results () showed average savings per patient of €3,319.10 when comparing WVTT against TURP (SD: 273.16; CI 95%: €3,302.17 – €3,336.03; Median: €3,296.59 [P25: €3,499.75 – P75: €3,129.55]), €3,098.83 when comparing WVTT against PVP (SD: 2,941.35; CI 95%: €3,281.13 – €2,916.52; Median: €1,942.61 [P25: €3,474.79 – P75: €1,385.72]), and €2,665.57 when comparing WVTT against HoLEP (SD: 238.70; CI 95%: €2,650.77 – €2,680.36; Median: €2,654.39 [P25: €2,821.24 – P75: €2,504.51]). As shown in , there is a 100% probability of having savings over €2,571.77 when comparing WVTT vs. TURP, over €651.25 when comparing WVTT vs. PVP and over €1,960.69 when comparing WVTT vs. HoLEP.
Figure 3. Probabilistic Sensitivity Analysis Results: Probability of Incremental Cost (WVTT vs. Other surgical therapies).
4. Discussion
Innovative medical devices might achieve better clinical results than traditional care; however, the decision to fund innovations from the public purse must also consider the economic impact of their introduction.
Risk of BPH increases significantly with age. Given the aging of the Spanish population, an increase in the burden of LUTS secondary to BPH, and consequently the cost of the surgical management of cases, the overall cost burden is high and expected to rise in the next years.
The results of this analysis suggest that the introduction of WVTT would generate savings for the Spanish NHS of almost €29 million over a 4-year period.
Higher savings were observed during the 1st year, mainly derived from the reduction in operating room time and length of stay associated with WVTT. The ambulatory management of patients treated with WVTT would imply savings of approximately 7,000 operation hours and 25,500 bed days for the whole 4-year period.
In Spain, operation hours and bed days are the main drivers of wait times for patients. Moreover, given the increase in waiting lists exacerbated by the COVID crisis, solutions that help reduce the burden on capacity in the health care system might be more relevant than ever [Citation55]. Thus, the shorter procedure time and length of stay for WVTT relative to other surgical procedures should accelerate patient access to surgical treatments, allowing the health care systems to improve their health care quality and efficiency.
Additionally, the lower retreatment rate and rate of adverse events of WVTT compared to other interventions, as well as benefitting the patient, are expected to generate cost savings.
WVTT constitutes a new alternative that, although it implies a higher outlay for the acquisition of the devices in comparison with the other therapies, it also entails a significant reduction in the cost of the surgical procedure, reinterventions and adverse events. Beyond reducing the cost of management of a patient with LUTS secondary to BPH, WVTT contributes to the improvement of patient’s well-being because of its low rates of adverse events and reintervention, as well as for its shorter duration of intervention and outpatient management.
The sensitivity analysis demonstrated the robustness of the results, even when parameters controversial when validated by experts were modified. These analyses confirmed that the main cost drivers in the model were duration of the procedure and length of stay. However, modifying these parameters did not lead to important changes in the results.
The results of the probabilistic sensitivity analysis estimated, as an average of the 1,000 Monte Carlo simulations, incremental costs of WVTT against the alternatives similar to those of the base case, demonstrating either the robustness of the model. Furthermore, every simulation resulted in savings when comparing WVTT against TURP, PVP or HoLEP, having a probability of 100% of cost savings.
In addition, the sensitivity analysis revealed that taking into account the management of erectile dysfunction complications implies a significant cost for the Spanish health care system. In this analysis, savings increase by almost €32 million against the savings observed in the base case. However, as not all patients are sexually active, erectile dysfunction was excluded in the base case to remain conservative.
To the knowledge of the authors, this study is the first BIA of WVTT in Spain. Previous publications attempted to estimate the efficiency of this treatment in other settings. One cost-effectiveness analysis (CEA) in the United States of America (USA) found WVTT cost-effective vs. drug therapy, conductive radiofrequency thermal therapy (Prostiva®, Urologix®), prostatic urethral lift (PUL) (Urolift®, Teleflex Medical®), PVP and TURP [Citation56]. Another CEA showed WVTT cost-effective against PUL [Citation57]. In absence of head to head comparisons of the MISTs available for the management of LUTS secondary to BPH, scores of IPSS from individual studies were used to perform the CEA mentioned before [Citation56,Citation57]. However, indirect comparisons or NMA would be preferable to compare all the interventions.
A published NMA did not reported statistically significant differences in IPSS scores between therapies [Citation18], that serve as a robust basis for performing a CEA. Therefore, modeling a partial economic evaluation, such as a BIA, was considered preferable than a complete economic evaluation [Citation58].
Other authors estimated the budget impact derived from the introduction of WVTT in healthcare systems. In USA, a BIA derived from one of the CEA reported cost savings of WVTT vs. PUL [Citation57]. Furthermore, savings with WVTT against TURP were also observed in a BIA performed in Italy [Citation25]. Our study, which considered multiple comparators instead of a single alternative, is in line with these results.
Future direct or indirect comparisons or NMAs between the whole MISTs available for the management of BPH providing accurate evidence about the presence or absence of real differences in effectiveness, could be useful and would allow more complex economic evaluations.
The present analysis is not exempted from constraints, which should be evaluated when the results are being interpreted. First, as this study is a model-based analysis, it is subject to uncertainty. An expert panel was consulted to minimize this issue and to obtain accurate insight into clinical practice. To address the potential bias, several sensitivity analyses were performed on the most uncertain parameters.
Second, the reintervention rates and frequency of occurrence of complications were obtained from different sources because of a lack of direct comparisons between the therapies evaluated [Citation18]. As it was mentioned, further research conducted via direct or indirect comparisons and NMA would address this issue and support the economic modeling approach and its results.
Third, it was assumed that retreatment could only be performed with the same technique or with TURP when designing the model. This might not completely reflect clinical practice, where other techniques for treating patients are available, replacing TURP or the original intervention. However, given the low rates of reintervention identified, this parameter is not expected to have an important impact on the results.
Finally, the costs identified might be variable between regions and hospitals in Spain, which is reason why, wherever possible, the average cost was calculated with every cost identified.
Despite these limitations, this study suggests that WVTT, in addition to facilitating health care sustainability, might increase the health care quality for patients. As this technique reduces time spent in hospital, it might be particularly suited to patients who are working and want a quick procedure. Likewise, due to lower rates of adverse events, WVTT can meet the expectations of patients who would like to protect their sexual function.
5. Conclusion
The present analysis shows that the introduction of WVTT could reduce the cost of surgical treatment of BPH patients reporting LUTS and help reduce the hospital capacity burden. This is principally due to the shorter intervention duration and absence of hospital stay after operation, as well as the lower rates of adverse events associated with WVTT.
Given that WVTT is expected to reduce procedure time and length of stay and does not impact sexual function, the procedure may be particularly attractive to patients with a busy lifestyle who are sexually active. A reduction in the risk of side effects and retreatment rates compared to other therapies may also bring additional patient benefits.
These findings may help clinicians, payers and policy-makers, form their decisions regarding the introduction of WVTT to the Spanish public health care setting.
Declaration of interest
A De la Cuadra and I Oyagüez are employees of PORIB, a consultant company, specialized in economic evaluation of health interventions, which has received financial support for developing the local customization of the model, including literature search, model feeding, results interpretation and manuscript drafting. M Fernández-Arjona, J Rioja-Zuazu and M Dominguez-Esteban have received honoraries from Boston Scientific for consultant activities related to validation of the parameters and results. E Torres and E Woodward are employees of Boston Scientific. R Blissett is a director of MedTech Economics Ltd which received financial compensation for the development of the original economic model and subsequent country adaptations. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
A de la Cuadra: Conception and design of the study, analysis and interpretation of data, drafting of the paper and revising it critically for intellectual content. J Rioja-Zuazu: Drafting of the paper, revising it critically for intellectual content and final approval. M Domínguez-Esteban: Drafting of the paper, revising it critically for intellectual content and final approval. E Torres: Conception and design of the study, analysis and interpretation of data, drafting of the paper and final approval. R Blisset: Conception and design of the study, analysis and interpretation of data, drafting of the paper and final approval. E Woodward: Conception and design of the study, drafting of the paper, analysis and interpretation of data and final approval. I Oyagüez: Conception and design of the study, analysis and interpretation of data, drafting of the paper and revising it critically for intellectual content. M Fernández-Arjona: Drafting of the paper, revising it critically for intellectual content and final approval. All authors agree to be accountable for all aspects of the work. All authors read and approved the final manuscript to be published.
Supplemental Material
Download MS Word (65.4 KB)Acknowledgments
The authors would like to thank Ramón Burgos-Pol, former employee of Boston Scientific, for his support in the study.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2189591
Additional information
Funding
References
- Cozar JM, Solsona E, Brenes F, et al. Manejo asistencial del paciente con hiperplasia benigna de próstata en España. Actas Urológicas Españolas. 2011;35(10):580–588.
- Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132(3):474–479.
- Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3–S14.
- Bushman W. Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol Clin North Am. 2009;36:403–415.
- O’Leary MP. Lower urinary tract symptoms/benign prostatic hyperplasia: maintaining symptom control and reducing complications. Urology. 2003;62:15–23.
- Van Dijk MM, Wijkstra H, Debruyne FM, et al. The role of nocturia in the quality of life of men with lower urinary tract symptoms. BJU Int. 2010;105:1141–1146.
- Bruskewitz RC. Quality of life and sexual function in patients with benign prostatic hyperplasia. Rev Urol. 2003;5:72–80.
- Mitropoulos D, Anastasiou I, Giannopoulou C, et al. Symptomatic benign prostate hyperplasia: impact on partners’ quality of life. Eur Urol. 2002;41:240–244. discussion 244-245
- Abrams P. New words for old: lower urinary tract symptoms for “prostatism.” BMJ. 1994;308:929–930.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49.
- Carrero-López VM, Cózar-Olmo JM, Miñana-López B. Benign prostatic hyperplasia and lower urinary tract symptoms. A review of current evidence. Actas Urol Esp. 2016;40:288–294.
- Brenes Bermúdez FJ, Brotons Muntó F, Castiñeiras Fernández J, et al. [Consensus document on the management and follow-up of the male with lower urinary tract symptoms secondary to benign prostate hyperplasia]. Semergen. 2016;42:547–556.
- Juliao AA, Plata M, Kazzazi A, et al. American urological association and European Association of urology guidelines in the management of benign prostatic hypertrophy: revisited. Curr Opin Urol. 2012;22:34–39.
- Rassweiler J, Schulze M, Stock C, et al. Bipolar transurethral resection of the prostate–technical modifications and early clinical experience. Minim Invasive Ther Allied Technol MITAT Off J Soc Minim Invasive Ther. 2007;16:11–21.
- Ajib K, Mansour M, Zanaty M, et al. Photoselective vaporization of the prostate with the 180-W XPS-Greenlight laser: five-year experience of safety, efficiency, and functional outcomes. Can Urol Assoc J J Assoc Urol Can. 2018;12:E318–24.
- Tan AHH, Gilling PJ. Holmium laser prostatectomy. BJU Int. 2003;92:527–530.
- Green Z, Westwood J, Somani BK. What’s new in rezum: a transurethral water vapour therapy for BPH. Curr Urol Rep. 2019;20:39.
- Lourenco T, Pickard R, Vale L, et al. Alternative approaches to endoscopic ablation for benign enlargement of the prostate: systematic review of randomised controlled trials. BMJ. 2008;337:a449.
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices—budget impact analysis. Value Health. 2007;10:336–347.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014;17:5–14.
- Naimark DMJ, Kabboul NN, Krahn MD. The half-cycle correction revisited: redemption of a kludge. Med Decis Mak Int J Soc Med Decis Mak. 2013;33:961–970.
- Lourenco T, Armstrong N, N’Dow J, et al. Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement. Health Technol Assess Winch Engl. 2008;12(iii,ix–x, 1–146):169–515.
- National Institute for Health and Care Excellence (NICE). In: Overview | uroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia | guidance | NICE [Internet]. London: NICE. 2015. Available from: https://www.nice.org.uk/guidance/mtg26
- National Institute for Health and Care Excellence (NICE). Overview | rezum for treating lower urinary tract symptoms secondary to benign prostatic hyperplasia | guidance | NICE [Internet]. London: NICE; 2020 [cited 2021 Feb 4]. Available from: https://www.nice.org.uk/guidance/mtg49.
- Blissett RS, Blissett DB, Oselin M, et al. Transurethral water vapor therapy for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: a cost-minimization and budget impact analysis from an Italian healthcare perspective. Minerva Urol Nephrol. 2022. in press.
- Garraway WM, Russell EB, Lee RJ, et al. Impact of previously unrecognized benign prostatic hyperplasia on the daily activities of middle-aged and elderly men. Br J Gen Pract J R Coll Gen Pract. 1993;43:318–321.
- Instituto Nacional de Estadística (INE). Población residente por fecha, sexo y edad [Internet]. Madrid: INE; 2021 [cited 2022 Mar 25]. Available from: https://www.ine.es/jaxiT3/Datos.htm?t=31304#!tabs-tabla.
- Verhamme KMC, Dieleman JP, Bleumink GS, et al. Treatment strategies, patterns of drug use and treatment discontinuation in men with LUTS suggestive of benign prostatic hyperplasia: the Triumph project. Eur Urol. 2003;44:539–545.
- Roos NP, Wennberg JE, Malenka DJ, et al. Mortality and reoperation after open and transurethral resection of the prostate for benign prostatic hyperplasia. N Engl J Med. 1989;320:1120–1124.
- Madersbacher S, Lackner J, Brössner C, et al. Reoperation, myocardial infarction and mortality after transurethral and open prostatectomy: a nation-wide, long-term analysis of 23,123 cases. Eur Urol. 2005;47:499–504.
- Thomas JA, Tubaro A, Barber N, et al. A multicenter randomized noninferiority trial comparing greenlight-XPS laser vaporization of the prostate and transurethral resection of the prostate for the treatment of benign prostatic obstruction: two-yr outcomes of the GOLIATH study. Eur Urol. 2016;69:94–102.
- Enikeev D, Taratkin M, Morozov A, et al. Long-term outcomes of holmium laser enucleation of the prostate: a 5-year single-center experience. J Endourol. 2020;34:1055–1063.
- McVary K, Roehrborn CG. Lba01-06 five year results of the prospective, randomized controlled trial of water vapor thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2020; 203:e1021.
- Bachmann A, Tubaro A, Barber N, et al. 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial–the GOLIATH study. Eur Urol. 2014;65:931–942.
- Peyronnet B, Robert G, Comat V, et al. Learning curves and perioperative outcomes after endoscopic enucleation of the prostate: a comparison between GreenLight 532-nm and holmium lasers. World J Urol. 2017;35:973–983.
- Johnston MJ, Hindley R. Rezum water vapour therapy for benign prostatic hyperplasia. Trends in Urology and Men´s Disease. 2019:24–29.
- Ray A, Morgan H, Wilkes A, et al. The Urolift System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: a NICE Medical Technology Guidance. Appl Health Econ Health Policy. 2016;14:515–526.
- Li T, Wilcox CS, Lipkowitz MS, et al. Rationale and strategies for preserving residual kidney function in dialysis patients. Am J Nephrol. 2019;50:411–421.
- Mitjana Biosca S, Povo Martín I, Pallás Costa YS, et al. Rezum “Water Vapor Thermal Therapy” primeras experiencias en pacientes con crecimiento prostático benigno [Internet]. I Congreso Nacional Virtual de Urología: Asociación Española de Urología (AEU); 2020. [cited 9 Feb 2021]. Available from: https://aeu.es/reuniones/aeu2020/libroResumenes/verabstract.aspx?Sesion=0&Num=C-225.
- Abascal Junquera JM, Cecchini Rosell L, Salvador Lacambra C, et al. [Bipolar versus monopolar transurethral resection of the prostate: peroperative analysis of the results]. Actas Urol Esp. 2006;30:661–666.
- Placer J, Salvador C, Narváez MA, et al. Holmium laser enucleation of the prostate (HoLEP): evaluation of the first thousand patients. Eur Urol Suppl. 2019;18:e1907.
- Antoñanzas F, Brenes F, Molero JM, et al. [Cost-effectiveness of the combination therapy of dutasteride and tamsulosin in the treatment of benign prostatic hyperlasia in Spain]. Actas Urol Esp. 2011;35:65–71.
- Ministerio de Sanidad. Consumo y Bienestar Social (MSCBS). Consulta Interactiva del SNS [Internet]. Madrid: MSCBS; 2021 [cited 2021 Feb 9]. Available from: https://pestadistico.inteligenciadegestion.mscbs.es/publicoSNS/C/rae-cmbd/rae-cmbd/grupos-relacionados-por-el-diagnostico-grd/grd-estadisticos-por-comunidad-autonoma-grupo-de-hospitales-servicios.
- Arlandis-Guzmán S, Brenes-Bermúdez F, Jiménez-Cidre MA, et al. [Cost effectiveness of Mirabegron and antimuscarinic drugs in patients with hiperactive bladder.]. Arch Esp Urol. 2018;71:809–824.
- Montesino-Semper MF, Jimenez-Calvo JM, Cabases JM, et al. Cost-effectiveness analysis of the surgical treatment of female urinary incontinence using slings and meshes. Eur J Obstet Gynecol Reprod Biol. 2013;171:180–186.
- Insausti I, Sáez de Ocáriz A, Galbete A, et al. Randomized comparison of prostatic artery embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. J Vasc Interv Radiol JVIR. 2020;31:882–890.
- Callejo Velasco D, López-Polín D´Olhaberriague A, Guerra Rodríguez M, et al. Evaluación económica de la vaporización fotoselectiva de la próstata para el tratamiento de la hiperplasia benigna de próstata. Madrid: Ministerio de Ciencia e Innovación; 2011.
- Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral. L.I.F.T. Study. Can J Urol 2017;24:8802–8813.
- McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016;195:1529–1538.
- Miner M, Rosenberg MT, Perelman MA. Treatment of lower urinary tract symptoms in benign prostatic hyperplasia and its impact on sexual function. Clin Ther. 2006;28:13–25.
- Romero-Otero J, García-Gómez B, García-González L, et al. Critical analysis of a multicentric experience with holmium laser enucleation of the prostate for benign prostatic hyperplasia: outcomes and complications of 10 years of routine clinical practice. BJU Int. 2020;126:177–182.
- Diario Oficial de la Generalitat de Catalunya (DOGC). ORDEN SLT-248-2016, de 29 de agosto, por la que se determinan para el año 2016 los precios unitarios para la contraprestación de la atención hospitalaria y especializada. [Internet]. Barcelona: DOGC; 2016 [cited 2021 Sep 10]. Available from: https://vlex.es/vid/orden-slt-248-2016-649669485.
- Diario Oficial de la Generalitat de Catalunya (DOGC). In: RESOLUCIÓ SLT/353/2013, de 13 de febrer, sobre la revisió de preus públics corresponents als serveis sanitaris que presta l’Institut Català de la Salut. [Internet]. Barcelona: DOGC. 2013. Available from: https://portaldogc.gencat.cat/utilsEADOP/PDF/6326/1287494.pdf
- Benejam-Gual JM, Sanz-Granda A, García-Miralles Grávalos R, et al. Análisis coste efectividad a 2 años del tratamiento quirúrgico de la hiperplasia benigna de próstata mediante vaporización fotoselectiva de la próstata con GreenLight–PhotoVaporization 120W versus resección transuretral de la próstata. Actas Urol Esp. 2014;38:238–243.
- Escobar LDP, García-Centeno MC. Impacto de la Covid-19 sobre las listas de espera quirúrgicas. Rev Esp Salud Pública. 2021;95:e1–12.
- Ulchaker JC, Martinson MS. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Clin Outcomes Res CEOR. 2018;10:29–43.
- Chughtai B, Rojanasarot S, Neeser K, et al. Cost-effectiveness and budget impact of emerging minimally invasive surgical treatments for benign prostatic hyperplasia. J Health Econ Outcomes Res. 2021;8:42–50.
- Drummond M, O’Brien B, Stoddarts G, et al. Methods for economic evaluation of health care programmes. 2nd. Madrid: Ediciones Díaz Santos; 2001.
