ABSTRACT
Objective
No consensus exists on the ideal methodology to evaluate the economic impact and value of new, potentially curative gene therapies. We aimed to identify and describe published methodologic recommendations for the economic evaluation of gene therapies and assess whether these recommendations have been applied in published evaluations.
Methods
This study was conducted in three stages: a systematic literature review of methodologic recommendations for economic evaluation of gene therapies; an assessment of the appropriateness of recommendations; and a review to assess the degree to which the recommendations were applied in published evaluations.
Results
A total of 2,888 references were screened, 83 articles were reviewed to assess eligibility, and 20 papers were included. Fifty recommendations were identified, and 21 reached consensus thresholds. Most evaluations were based on naive treatment comparisons and did not apply consensus recommendations. Innovative payment mechanisms for gene therapies were rarely considered. The only widely applied recommendations related to modeling choices and methods.
Conclusions
Methodological recommendations for economic evaluations of gene therapies are generally not being followed. Assessing the applicability and impact of the recommendations from this study may facilitate the implementation of consensus recommendations in future evaluations.
1. Introduction
Gene therapies function via several mechanisms, such as replacing a disease-causing gene with a healthy copy, inactivating a disease-causing gene, or introducing a new or modified gene to treat a disease [Citation1]. Gene therapies are potentially life-changing for a diverse range of diseases, such as neuromuscular diseases, inherited blindness, metabolic disorders, and hematologic malignancies [Citation2]. Because of technical limitations, medical ethics, and regulatory hurdles, very few approved gene therapies are available for treatment. However, more gene therapies are expected to be approved as technology advances and clinical trials progress [Citation3,Citation4]. An estimated >1 million patients will be treated with gene therapies by the year 2034, leading to an estimated global cost of >$300 billion [Citation5]. The innovative treatment paradigm and clinical benefits associated with the expected launch of additional gene therapies in the coming years may be met with reimbursement and funding challenges because of the need for health care payment structures to balance greater upfront costs with undetermined long-term clinical safety and effectiveness [Citation6–9].
The use of randomized controlled clinical trials is often unfeasible for gene therapies [Citation5]. Therefore, most clinical studies supporting the market authorization of gene therapies are small, open-label, and single-arm trials [Citation1,Citation5,Citation10]. In health technology assessments (HTAs), limited clinical evidence and greater upfront treatment costs for gene therapies have challenged reimbursement, and specific decision-making considerations have been necessary [Citation10–12]. Substantial challenges remain in how HTAs will appraise the relative degrees of effectiveness, safety, and value-for-money for gene therapies vs. non-gene therapies, based on less comprehensive evidence [Citation10,Citation11].
Several national health institutions and HTA bodies in Europe and North America, including the National Institute for Health and Care Excellence (NICE), the National Institute for Health Research, and the Institute for Clinical and Economic Review in the United States, have assessed the methodologic questions related to the economic evaluation of advanced therapeutic medical products (ATMPs) – including gene therapies – and have provided varying potential recommendations. These included additional scenario analyses to explore long-term benefits, threshold analyses to identify treatment effectiveness, cure proportion modeling in case a percentage of patients is likely to be cured, and reporting of net health benefits in addition to incremental cost-effectiveness (CE) ratios (ICERs) with a measurement of uncertainty [Citation13–17]. Experts from academia and industry have also addressed methodologic questions related to the evaluation of ATMPs and specifically gene therapies. These experts suggest that a completely new reference case is not needed for gene therapies, but some aspects of economic evaluation should be considered further, because of the unique aspects of gene therapies [Citation18–21].
1.1. Aim
The CE of gene therapies is important to assess because of greater upfront costs, but this assessment is challenging because of the limited relative effectiveness data and the uncertainty around long-term outcomes [Citation10,Citation11]. No consensus currently exists on the ideal methodology to evaluate the economic impact and benefits of new, potentially curative gene therapies, along with reimbursement and funding challenges for existing health care payment structures. Novel approaches for economic evaluations of gene therapies are therefore needed. We aimed to identify and describe the most widely accepted published methodologic recommendations for the economic evaluation of gene therapies and assess whether these recommendations were applied in published evaluations.
2. Methods
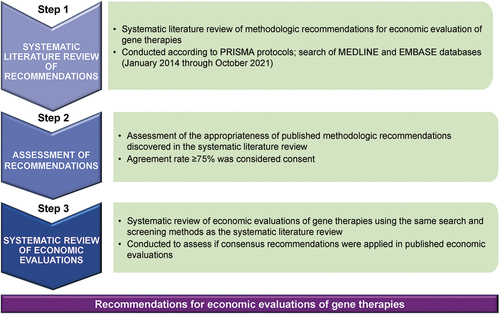
We conducted this study in three stages (): a systematic literature review of methodologic recommendations for the economic evaluation of gene therapies; an assessment of the appropriateness of the recommendations; and a review to assess if the consensus recommendations were applied in published evaluations.
Figure 1. Study design PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We conducted a systematic literature review of methodologic recommendations for economic evaluation of gene therapies, an assessment of the appropriateness of the recommendations, and a review to assess if consensus recommendations were applied in published economic evaluations.
2.1. Systematic literature review of recommendations
For stage 1, we completed a systematic literature review of recommendations for economic evaluations of gene therapies according to a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol. The review was based on a literature search performed in MEDLINE and EMBASE databases, covering the period from January 2014 through October 2021. Search key words were related to economic evaluations of gene therapies ().
Table 1. Search strategy: key words.
Publications were selected if the primary objective was to review, list, discuss, or provide recommendations or solutions for challenges related to economic evaluations of ATMPs (gene therapies and regenerative medicines). Evaluations had to consider both costs and health outcomes to be included, and one of the evaluated treatment strategies had to be a gene therapy or chimeric antigen receptor T-cell (CAR-T) therapy. The following were excluded: animal studies, articles addressing genetic tests, genotyping and whole-genome sequencing interventions, clinical trials, cost-minimization analyses, cost-of-illness or disease burden studies, conference abstracts, publications not offering economic evaluation methodologic recommendations, and articles not available in English. No restriction was placed on geographic scope.
The review of recommendations was completed by a search for relevant white papers published by HTA agencies (NICE, Scottish Medicines Consortium, Haute Autorité de Santé, Canadian Agency for Drugs and Technologies in Health, US Institute for Clinical and Economic Review, and Institute for Quality and Efficiency in Health Care) and a manual cross-referencing search through published literature reviews on the topic.
Abstracts and full texts were independently screened by two reviewers. After a first selection of references based on title and abstract, full texts were screened again by two different analysts. Detailed information, including product class, intervention of investigation, target diseases, population, region, sponsor, use of surrogate endpoints, and time frame, was extracted from selected articles and summarized. Disagreements in the screening, extraction, and summary process were resolved by discussions between analysts and a senior researcher. We listed recommendations in a comprehensive manner, without judging relevance, and then classified the recommendations by theme.
2.2. Assessment of recommendations
For stage 2, we evaluated the appropriateness of published methodologic recommendations discovered in the systematic literature review and selected which recommendations that we, in our collective judgment, believed to be worthwhile and valuable. Our assessment was based on our relevant health economic experience from academia or HTA-related organization membership(s) in Europe and the United States along with our experience related to economic evaluations of gene therapies.
In reviewing the recommendations, we (the eight authors of this paper) indicated if each proposed recommendation was relevant for the economic evaluation of gene therapy (‘Agree,’ ‘Neutral,’ or ‘Disagree’) and whether publications should explicitly report how corresponding issues were addressed. An agreement rate of ≥75% (n = 6/8) was determined to identify a consensus recommendation. We grouped the final list of consensus recommendations into five categories: input data (recommendations addressing limitations of clinical data, such as small patient numbers and single-arm trials); modeling choices (methods, considering in particular the uncertainty around long-term effects of gene therapies); health-related quality of life (HRQOL; measurement and evaluation of outcomes, including the challenges related to the pediatric population); estimation of costs; and evaluation framework.
2.3. Systematic review of economic evaluations of gene and cell therapies
For stage 3, we conducted a separate review of economic evaluations of ATMPs in order to assess the degree of concordance by analysts to our consensus methodologic recommendations. The systematic review of economic evaluations was conducted using the same key words and methods used in the prior review.
Detailed information was extracted from selected articles, including product class (e.g. gene therapy or CAR-T); intervention of investigation (generic name); target disease (classified based on the International Classification of Diseases, 11th edition); population (e.g. children or adults); region of study; study sponsor (e.g. private or public); utilization of surrogate endpoints; clinical trial design; use of observational data; indirect treatment comparison (ITC) methods; time frame; discount rates; perspective; utility elicitation methods; types of scenario analyses; types of sensitivity analyses; details of innovative payment mechanisms; and use of real-world evidence post-launch.
Data extraction was conducted by one analyst and checked for completeness and accuracy by a second analyst. A senior analyst was consulted in any case of discrepancy. The quality of reporting was assessed using the Drummond checklist for assessing economic evaluations for gene therapies [Citation22]. This checklist was developed to clarify the extent to which various factors, including clinical effectiveness, elements of value (value to caregivers and insurance carriers, and improvement in life expectancy), and other influences (such as discounts/alternative payment methods), are identified and considered in an economic evaluation [Citation22].
3. Results
3.1. Systematic literature review of recommendations
An initial search retrieved 4,302 records. Of these, 2,888 records remained for title and abstract screening after duplicates were removed (). Based on the inclusion/exclusion criteria, 2,805 articles were excluded by the reviewers, and after the first selection of references based on title and abstract, 85 full-text articles were excluded. We reviewed the full texts of the remaining 83 articles to assess eligibility, and 20 papers (18 methodologic publications and two white papers from U.S. and Canadian HTA agencies) were included in the descriptive synthesis () [Citation14,Citation18,Citation19,Citation21,Citation23–38].
Figure 2. PRISMA Diagram for the Search on Methodologic Recommendations HTA, health technology assessment; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
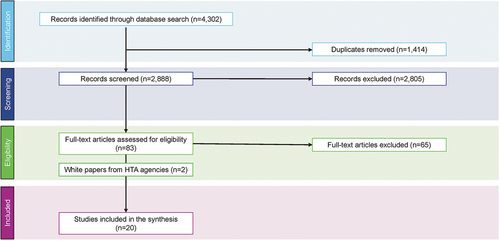
Table 2. Publications identified in the systematic literature review.
3.2. Assessment of recommendations
We identified 50 recommendations from the systematic literature review and grouped these recommendations into five categories for review and assessment (). After the review, 21 consensus recommendations were identified per the agreement rate of ≥75% (n = 6/8).
Table 3. Assessment of identified recommendations for the economic evaluations of gene therapies.
3.2.1. Input data
Seventeen of the identified input data recommendations were associated with limitations to clinical data for gene therapies, including surrogate endpoint validations, use of nonrandomized trial data, methods for estimating relative efficacy and safety, and utilization of expert opinion to obtain information and make a probabilistic representation in the absence of data.
There is scarcity of long-term observed data for gene therapies, particularly at the time of initial regulatory approval or reimbursement consideration [Citation14,Citation21,Citation24]. There was agreement (n = 7/8) with using surrogate endpoint data to assess clinical efficacy; however, only half (n = 4/8) reported that evidence of a correlation between treatment effects on surrogate endpoints and final endpoints should be presented.
Several publications documented that the rationale for conducting noncomparative studies (single-arm trials) should be clearly elucidated [Citation14,Citation21–24,Citation32] and others recommended the use of other nonrandomized data to provide complementary information to a single-arm trial to allow for an estimation of relative effectiveness [Citation19,Citation24,Citation25,Citation31]. We unanimously agreed (n = 8/8) that the rationale behind conducting noncomparative studies should be clearly provided and with the use of observational data to serve as a control arm.
Some publications recommended an assessment of the feasibility of conducting indirect comparisons using network meta-analysis or other statistical approaches when direct comparison is not possible [Citation14,Citation24]. We supported the use of network meta-analysis when feasible in these cases (n = 6/8). In addition, we agreed that matching-adjusted indirect comparisons (MAIC) (n = 6/8) and propensity score matching (PSM) (n = 7/8) may be considered when multivariate and network meta-analysis are not feasible.
3.2.2. Modeling choices
We identified 12 recommendations around modeling choices and methods, covering time horizon and extrapolation, scenario analyses, parametric sensitivity analysis, value of information, and discount rate.
Five publications recommended reporting analyses over different time frames and/or to consider different curative time frames or variance in treatment waning or to use a threshold analysis to determine the duration of beneficial effect that would be needed to achieve standard CE thresholds [Citation19,Citation23,Citation25,Citation32,Citation38]. Extrapolation approaches, such as cure proportion modeling, were suggested as the standard reference case for gene therapies whenever relevant, whereas survival analysis was suggested to address uncertainty based on other modeling approaches [Citation19,Citation25]. We agreed (n = 7/8) that scenario analyses with different time frames or efficacy waning parameters should be performed, but we did not reach agreement consensus regarding reporting a threshold analysis on the minimum duration of effect required to reach CE thresholds or the use of cure proportion modeling.
Several publications highlighted the importance of conducting and reporting both deterministic and probabilistic sensitivity analyses in the case of gene therapies [Citation14,Citation19,Citation21,Citation23,Citation31,Citation32,Citation38]. We agreed with the importance of these analyses (n = 6/8). We also agreed (n = 6/8) with the Institute for Clinical and Economic Review’s recommendation to conduct optimistic and conservative scenarios on treatment benefits (e.g. duration of benefit, magnitude/quality of benefit, proportion that achieve a specific benefit, different types of survival models, and relative treatment benefit under alternative assumptions), with the selection of assumptions and inputs used in the optimistic and conservative scenarios being described and justified [Citation38].
Three publications supported lesser discount rates for health outcomes than for costs [Citation19,Citation24,Citation32], five recommended retaining standard reference case discount rates for health outcomes and costs (usually equal) in base-case analysis and to conduct sensitivity analyses with different discount rates for benefits and costs [Citation14,Citation21,Citation23,Citation25,Citation38], and one stated that variable discount rates over time would be more appropriate than a uniform and constant discount rate [Citation9]. We agreed (n = 7/8) with applying the reference case discount rates in the base-case analysis for gene therapies and conducting sensitivity analyses with different rates.
3.2.3. Health-related quality of life
Thirteen recommendations identified concerned the measurement and valuation of outcomes. These included recommendations related to HRQOL for children and caregivers.
In the absence of validated instruments to assess and value HRQOL for children, alternative approaches, such as vignette studies, are recommended [Citation19]. We supported this recommendation (n = 6/8).
Two publications highlighted the importance of considering the impact of gene therapies on the HRQOL of caregivers and families, irrespective of whether costs are assessed from a health care payer or societal perspective [Citation25,Citation27]. We agreed (n = 7/8) that HRQOL of caregivers and patient families should be considered in economic evaluations of gene therapies.
3.2.4. Estimation of costs
Publications addressed several aspects of the estimation of costs: perspective, additional costs not usually considered in economic evaluations, innovative payment mechanisms for gene therapies, and cost offsets.
Many publications recommended conducting economic eval-uations from both health care payer and societal perspectives [Citation19,Citation21,Citation23–25,Citation27,Citation30,Citation33]. We unanimously agreed (n = 8/8) with this recommendation. Some costs not usually considered in economic evaluations could be substantial for gene therapies, particularly those related to infrastructure changes and travel to distant, specialized facilities where gene therapies may be delivered [Citation32]. Although half (n = 4/8) agreed with the suggestion to account for other costs, consensus was not reached.
Several publications recommended exploring the impact of innovative payment mechanisms in CE analyses [Citation14,Citation21,Citation23–25,Citation35–37]. We all agreed (n = 8/8) with the recommendation to consider innovative payment mechanisms to facilitate access to gene therapies while recognizing the sustainability of health care budgets.
3.2.5. Evaluation framework
Publications assessed general recommendations related to the framework of evaluation and decision-making, including elements of value to consider beyond QALYs, analytical frameworks in which those elements may be considered, whether a greater CE threshold is relevant for gene therapies, and collection of real-world evidence after launch.
Elements of importance not normally captured in QALYs for gene therapies according to reviewed publications were severity of disease, scientific spillovers [Citation28], insurance value, value of hope, value of cure, fear of contagion, and reduction in inequity [Citation27,Citation28,Citation32]. We unanimously agreed (n = 8/8) that severity of disease should be considered, and we also agreed (n = 7/8) that value of caregivers should be considered. None of the other elements reached consensus. Four publications discussed whether the cost per QALY model could be adapted to account for the elements of value cited above or if the cost-utility analysis (CUA) framework needed to be changed more fundamentally (i.e. using SAVEs instead of QALYs or multiple criteria decision analysis [MCDA] or cost-benefit analysis [CBA] instead of CUA) [Citation20,Citation24,Citation28,Citation32]. There was very little support for such approaches.
Four publications proposed the use of a greater CE ratio for gene therapies [Citation18,Citation21,Citation24,Citation35], with one proposing the establishment of explicit budget impact thresholds to highlight access challenges and to trigger negotiation with manufacturers [Citation25]. Only one out of eight supported a greater threshold for gene therapies, while the others (n = 7/8) were neutral.
A few publications posited that post-launch real-world evidence collection is critical to confirm the treatment benefits and fill the evidence gaps from the initial regulatory submission [Citation19,Citation21,Citation24,Citation27]. We all agreed (n = 8/8) that, after the launch of a gene therapy, real-world evidence is important to confirm the benefits of treatment and to provide further evidence on other elements of value.
3.3. Systematic review of economic evaluations of gene and cell therapies
One hundred and sixty references were selected for full-text review, and 126 articles were excluded by the reviewers based on the exclusionary criteria. Three articles were added based on a manual cross-referencing search through published literature reviews on the topic. A total of 37 publications were included after we screened the titles and abstracts () [Citation36,Citation39–74]. These 37 economic evaluations investigated 10 different marketed gene therapies. Other publications covered adeno-associated virus (AAV)-mediated gene therapies (in three studies) and hypothetical cell or gene therapies (in two studies). CAR-T cell therapies were assessed in 16 studies, and gene therapies other than CAR-T cell therapies were assessed in 21 economic evaluations. These interventions were assessed in 12 different pathologies. Fifteen studies included adult patients only, 11 studies included pediatric patients only, and 11 studies included both children and adults. Most studies were conducted in the United States (n = 22) and the United Kingdom (n = 8). Half of the economic evaluations (n = 19) were funded by private companies and 15 were funded by public and private organizations.
Table 4. Characteristics of the studies in the publications included for the review of economic evaluations.
Criteria of the Drummond checklist [Citation22] were applied in reviewed economic evaluations. However, there were methodologic weaknesses related to the identification of relevant costs and lack of or limited sensitivity analysis.
3.3.1. Review of input data
Four consensus recommendations regarding limitations of clinical data were retained: justifying the validity of surrogate endpoints, at least with a biologic argument; justifying the utilization of single-arm trial designs when applicable; using observational data for patients not receiving gene therapy as a control arm in the absence of comparative clinical trials; and methods for ITCs. Most published economic evaluations reviewed did not adhere to the consensus recommendations, with only one study providing information on validation of surrogate endpoints [Citation56].
Of the publications reporting the use of single-arm clinical trials, only five justified their use [Citation47,Citation49,Citation63,Citation65,Citation74]. Justifications covered practical and ethical reasons, low incidence/rarity of the disease, and lack of confounding variables without details provided. Approximately 70% of studies (n = 26/37) used other nonrandomized data, such as natural history studies or registries. Some studies provided precise comparison information between populations, and others provided complementary information on the rationale and robustness of the data without providing additional details.
Where comparators were not included in pivotal clinical trials, most of the studies (n = 26/32) employed naive comparisons. Two studies used MAIC [Citation47,Citation65] and one used a scenario analysis [Citation48]. Two studies used published network meta-analyses [Citation67,Citation72].
3.3.2. Review of modeling choices
Consensus recommendations related to modeling choices and methods included conducting analyses over different time frames or with different durations of treatment effect; using the reference case discount rates and conducting sensitivity analyses with different rates for costs and outcomes; conducting deterministic and probabilistic sensitivity analyses on model parameters; and reporting optimistic and pessimistic scenarios related to treatment benefits. Our systematic review of economic evaluations found that the degree of adherence to these recommendations was generally positive, except for reporting optimistic and pessimistic scenarios related to treatment benefits.
Most studies (86% [n = 32/37]) used a lifetime time horizon in the base-case scenario. Different time frames and/or assumptions on treatment effects over time were reported in 18 economic evaluations as sensitivity analyses. The time frame or duration of effect often had a greater impact on the results.
A majority of the economic evaluations (78% [n = 29/37]) used standard discount rates, identical for both health outcomes and costs, and ran sensitivity analyses to vary discount rates. The choice of the discount rate was identified as significantly affecting the results [Citation48,Citation71].
Twenty-six of 37 publications included both deterministic and probabilistic sensitivity analyses. For the 11 remaining publications, four provided a deterministic sensitivity analysis only [Citation41,Citation55,Citation57,Citation63], two provided a probabilistic sensitivity analysis only [Citation53,Citation54], and the others did not conduct sensitivity analyses [Citation36,Citation56,Citation61,Citation66,Citation72].
Only five of the 37 publications (14%) reported pessimistic/optimistic scenarios on treatment benefits [Citation50,Citation52,Citation67,Citation69,Citation70]. These two extreme scenarios were reported in addition to the standard sensitivity analyses. ICER values varied widely between optimistic and pessimistic scenarios.
3.3.3. Review of health-related quality of life
Consensus recommendations related to the measurement and valuation of outcomes included the use of alternative approaches, such as vignette studies to obtain utility values when no valid generic instruments (such as EuroQol-5D [EQ-5D], a standard measure of clinical and economic HRQOL via surveys) exist for the targeted population and the need to account for caregivers’ and families’ HRQOL when impacted by the patient’s disease. Our systematic review of economic evaluations determined that the degree of adherence to these consensus recommendations was poor.
Of the 14 evaluations including children aged 5 years or younger, five evaluations used utility values elicited from vignette studies [Citation58,Citation59,Citation62,Citation73,Citation74]. Indications covered retinal dystrophy, SMA, and TDT. Other evaluations including very young children considered utility values based on EQ-5D (youth version).
Six studies considered HRQOL for caregivers and families, including only four of 14 studies of pediatric populations [Citation46,Citation50,Citation58,Citation62,Citation69,Citation73]. The impact of HRQOL inclusion on the results of economic evaluations for caregivers depends on the gene therapy and assessed indications, and differences also can be seen within the same indication.
3.3.4. Review of estimation of costs
Two recommendations for estimating costs reached consensus: conducting analyses from both health care payer and societal perspectives and exploring the impact of innovative payment mechanisms on incremental costs. Twenty-four studies considered a payer perspective [Citation36,Citation39,Citation40,Citation42,Citation44,Citation45,Citation47–51,Citation54,Citation55,Citation58,Citation61,Citation64–70,Citation72,Citation74], and three studies considered the societal perspective [Citation57,Citation71,Citation73]. Four studies conducted both health care payer and societal analyses [Citation46,Citation52,Citation60,Citation62].
Discussions and analyses considering innovative/alternative payment mechanisms were reported in approximately 20% of the economic evaluations (n = 7/37) [Citation36,Citation50,Citation52,Citation54,Citation55,Citation69,Citation74]. Several forms of performance-based payments were considered, such as assuming payment for treatment acquisition for responders at 1 month, payment triggered by a remission duration reaching a given threshold, and payment only for initial complete response. Thus, payment mechanisms varied according to the nature and duration of treatment effects. The impact of innovative payment mechanisms on ICERs varied substantially between studies.
Many published evaluations were conducted pre-launch, and no real-world evidence was available. Therefore, only three economic evaluations considered real-world evidence (two included sensitivity analyses), using real-world data on adverse events and health care resource utilization [Citation48,Citation49,Citation51]. The use of estimates of treatment effects based on real-world evidence often led to a reduction of the ICER. We reviewed several economic evaluations that discussed the importance of collecting real-world evidence.
4. Discussion
New gene therapies will be reaching the market in upcoming years, and the cumulative budget impact of these therapies is expected to be substantial [Citation5]. Health economic evaluations will play an important role in pricing and reimbursement decisions related to gene therapies. However, economic eval-uations of gene therapies raise many methodologic challenges, which have been discussed in the literature [Citation1–12,Citation14]. We found many recent publications that provided methodologic recommendations for economic evaluations of gene therapies or, more broadly, ATMPs [Citation14,Citation18,Citation19,Citation21,Citation23–38]. In the current study, we summarized these recommendations, critically appraised the recommendations, and then assessed their applicability and impact in published economic evaluations of gene therapies. We found that, although analysts conducting economic evaluations of gene therapies have access to many publications that provide methodologic recommendations and guidelines, most of these recommendations were generally not followed.
The recommendations originated from academia, HTA agencies, and industry. Some consensus for these recommendations was observed between these publications, including conducting analyses from both health care payer and societal perspectives; considering the impact of innovative payment mechanisms on CE; collecting real-world evidence and updating evaluations after launch; conducting analyses over different time frames; and reviewing the evidence supporting the validation of surrogate endpoints. Several other recommendations appeared in one or two publications only. However, areas of disagreement were not observed between publications, except for divergent views on discount rates.
Some recommendations aimed to resolve important issues associated with economic evaluations of gene therapies, but would require paradigmatic changes in evaluation methodology, and are therefore unlikely to be implemented (e.g. the use of CBA or MCDA instead of CUA or the use of SAVEs instead of QALYs).
Recommendations that would provide more information to decision-makers and improve transparency without changing results of the reference case (e.g. presenting evidence on validation of surrogate endpoints; justifying the use of single-arm studies; providing scenario analyses; discussing elements of value that are not captured in QALYs, such as scientific spillovers, insurance value, or reduction in inequities) achieved consensus, and some of these recommendations were frequently implemented in reviewed economic evaluations.
The importance of providing some justification of the validity of surrogate endpoints reached consensus, but not on the exact criteria of validation. With one exception, reviewed studies did not provide any evidence of validation of surrogate endpoints. This is not surprising because a published review [Citation75] reported that the pivotal trial evidence supporting marketing approvals for products going through expedited approval pathways were often based on non-validated surrogate endpoints.
Published economic evaluations often involved comparisons between a gene therapy and standard of care, using a single-arm trial to inform health outcomes with the gene therapy and an observational study to inform outcomes of standard of care. There was relative consensus that investigators using such comparisons should be able to justify the objective and reproducible nature of the endpoints, assess the consequences of heterogeneity in patient population and study outcomes, and control for confounding factors. However, justifications for using such comparisons in reviewed economic evaluations were missing in a majority of publications or limited to comments about the comparability between populations.
Indirect treatment comparisons are an important area of possible improvement for future economic evaluations of gene therapies [Citation76]. In the absence of head-to-head studies, it is generally recommended to perform ITCs using network meta-analyses when feasible, which requires randomized controlled trials. When only single-arm studies are available, ITCs may be performed using MAIC or PSM, if individual patient data are available for the comparator [Citation76]. There was consensus about these recommendations.
Another recurring challenge in economic evaluations of gene therapies is the measure and valuation of HRQOL. A recommendation reaching consensus was to conduct vignette studies to obtain health state utility values for this population. Several publications used vignette studies, but most of those studies were also flawed, and in experimental vignette studies, flaws in study design or conduct may limit data integrity or introduce biased results [Citation77]. The full potential of vignette studies has not yet been realized [Citation77]. Only one evaluation actually used the approach from the consensus recommendation, having vignettes valued by a sample of the general public using a direct utility method [Citation56].
Only four of 14 economic evaluations for pediatric populations considered HRQOL for caregivers, even though severe pediatric conditions may be expected to have a substantial impact on caregiver HRQOL [Citation78]. According to studies that incorporated disutility values for caregivers, the impact on the ICER appeared to be small to moderate.
Several recommendation papers argued that costs should be valued from two perspectives – health care payer and societal [Citation19,Citation21,Citation23–25]. We fully agreed with this, but only 11% of studies reported analyses from both perspectives, possibly because economic evaluations may be related to a specific country’s HTA guidelines [Citation17]. Another explanation may be that authors of economic evaluations simply considered that the fraction of costs not paid by the health care payer was modest. Thus, the differences in ICERs between societal and health care payer perspectives, in articles reporting both, never exceeded 25%. While we all agreed with the recommendation to explore the influence of alternative payment mechanisms in terms of CE, <20% of reviewed economic evaluations reported such analyses.
Consensus recommendations related to modeling methods and choices were more frequently followed than other recommendations, specifically deterministic and probabilistic sensitivity analyses and sensitivity analyses around discount rates. The time frame and discount rates often had a large impact on results, which confirms the importance of conducting these sensitivity or scenario analyses. The greater variability in results according to discount rates raises the question of which discount rates are the most appropriate. We generally recommended following standard methodologic guidelines for the base-case analysis, which will ensure comparability between studies. However, debate exists on whether the discount rates recommended by some HTA agencies are truly appropriate [Citation79].
There were several limitations to our study. Reviewed studies were in the English language only. In addition, economic evaluations were identified through MEDLINE and EMBASE, and we did not search for reports published on the websites of HTA agencies. A substantial number of articles were not included because of the exclusion criteria implemented during the systematic literature review (e.g. articles addressing genetic tests, genotyping and whole-genome sequencing interventions, clinical trials, cost-minimization analyses, and cost-of-illness analyses). Finally, we reviewed recommendations without providing additional specific insight into our assessment of the recommendations. Having specific insight for recommendations that did not reach the consensus threshold may have provided additional understanding.
5. Expert opinion
Despite the potential clinical benefits associated with some gene therapies, obstacles to efficient market access, including reimbursement and funding challenges, prevail. Although it is important to assess the CE of gene therapies because there are greater upfront costs associated with these treatments, it is challenging to perform this assessment because of the limited relative effect-iveness data available and the uncertainty around the long-term effectiveness and safety of these treatments. No consensus exists on the ideal methodology to evaluate the economic impact and benefits of new, potentially curative gene therapies or the associated reimbursement and funding challenges for existing health care payments structures. This is concerning because a growing number of gene therapies are expected to be approved in the coming years. Therefore, novel approaches for economic evaluation of gene therapies are urgently needed to address these issues.
We aimed to identify and describe the most widely accepted published methodologic recommendations for the economic evaluation of gene therapies and to assess whether these recommendations were applied in published evaluations. Economic evaluations of gene therapies in the current medical literature highlight several issues that have been accepted as limitations of economic evaluations in other therapeutic areas. The studies we reviewed in this systematic literature review largely presented ways to evaluate gene therapies as appropriately as possible within the standard CE analysis framework.
We reviewed the recommendations in the current medical literature associated with the economic evaluation of gene therapies and identified several consensus recommendations. Because of a lack of long-term observed clinical benefits for gene therapies, surrogate endpoint data should be used to assess clinical efficacy. The rationale for conducting noncomparative studies should be clearly elucidated, and the inclusion of other nonrandomized data should be considered to provide complementary information to a single- arm trial. For access to gene therapies, consideration of innovative payment mechanisms was supported, which would facilitate patient access while maintaining sustainable health care budgets. The HRQOL of caregivers and patients’ families must be considered in economic evaluations of gene therapies. After the launch of a gene therapy, gathering real-world evidence is important to confirm the benefits of treatment and to provide further evidence following initial evaluations.
Fully addressing the limitations of economic evaluation in the context of gene therapies may require methodologic changes beyond those that health economists currently appear willing to accept. However, with the clinical progress made over recent years, gene therapies are now considered a potentially paradigm-shifting treatment, making it possible to treat incurable diseases with unmet needs. The results of the current study highlight the considerable HTA challenges that remain. It is important to understand what additional data are needed to convince decision-makers to pay for the potential long-term treatment benefits associated with gene therapies. These guidelines were summarized to critically appraise and assess the applicability and impact of the recommendations in published evaluations, which may facilitate the implementation of more important recommendations in future evaluations.
6. Conclusions
Health economists conducting evaluations of gene therapies have access to a relatively large number of publications that provide methodologic recommendations. Economic evaluations of gene therapies highlight several issues that have been accepted as limitations of economic evaluations in other therapeutic areas. Studies from the reviewed literature generally presented ways to evaluate gene therapies as appropriately as possible within the standard CE analysis framework. Fully addressing the limitations of economic evaluation in the context of gene therapies may require methodologic changes beyond those that health economists may readily accept. Most of these recommendations are currently generally not followed by published economic evaluations of gene therapies. Assessing the applicability and impact of the recommendations from this study may facilitate the implementation of important recommendations in future evaluations.
Article highlights
Currently, no consensus exists on the ideal methodology to evaluate the economic impact and benefits of new, potentially curative gene therapies, so novel approaches for economic evaluations of gene therapies are needed.
Fifty methodologic recommendations for the economic evaluation of gene therapies were identified in the published literature, summarized, and critically appraised, with 21 recommendations reaching consensus thresholds and then assessed for applicability and impact in the published economic evaluations.
Our study found that most evaluations were based on naive treatment comparisons and did not apply consensus recommendations, innovative payment mechanisms for gene therapies were rarely considered, and the only widely applied recommendations related to modeling choices and methods.
Although analysts conducting economic evaluations of gene therapies have access to many publications that provide methodologic recommendations and guidelines, these published recommendations are generally not being followed.
Assessing the applicability and impact of the recommendations from this study may facilitate the implementation of consensus recommendations in future economic evaluations of gene therapies to assist with reimbursement and funding challenges for existing healthcare payment structures.
Declaration of interest
M Toumi and S Aballea are former employees of Creativ-Ceutical and consultants for Novartis Gene Therapies, Inc. (NGT). O Dabbous is an employee of NGT and owns stock/other equities. S Sullivan is a consultant for AbbVie, Bayer, Incyte, Nanoscope, Neurocrine, NGT, Novo Nordisk, and Spark Therapeutics. P Neumann served on advisory boards/as a consultant for AbbVie, Amgen, Bayer, the Congressional Budget Office, Janssen, Merck, NGT, Novartis, Novo Nordisk, Vertex, and Curta Inc., and received research grants from the Alzheimer’s Association, Amgen, the Bill and Melinda Gates Foundation, Lundbeck, the National Institutes of Health, and the National Pharmaceutical Council. J-M Graf von der Schulenburg and M Drummond are consultants for NGT. S Tunis has received honoraria from NGT and served on advisory boards for BioMarin and UCB Pharma. D Malone has served as a consultant for Novartis, NGT, Sarepta, Pharmacyclics, Currax, and Seres, and served on advisory boards for BioMarin and Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Manuscript concept and design: all. Drafting of manuscript: all. Critical reviews: all. All authors approved and agreed for the final version of the manuscript to be published.
Acknowledgments
Editorial support was provided by Wynne Dillon, MS, of Kay Square Scientific. This support was funded by Novartis Gene Therapies, Inc.
Additional information
Funding
References
- Alnasser SM. Review on mechanistic strategy of gene therapy in the treatment of disease. Gene. 2021;769:145246.
- Bulaklak K, Gersbach CA. The once and future gene therapy. Nat Commun. 2020;11(1):5820.
- Lapteva L, Purohit-Sheth T, Serabian M, et al. Clinical development of gene therapies: the first three decades and counting. Mol Ther Methods Clin Dev. 2020;19:387–397. DOI:10.1016/j.omtm.2020.10.004
- Zhou W, Wang X. Human gene therapy: a patent analysis. Gene. 2021;803:145889.
- Wong CH, Li D, Wang N, et al. Estimating the financial impact of gene therapy. 2020 Oct 3. [cited 2022 Jul 7]. Available from: https://www.medrxiv.org/content/10.1101/2020.10.27.20220871v1.full-text
- Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the EU market. Cell Stem Cell. 2016;19:293–297.
- Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833.
- Iancu EM, Kandalaft LE. Challenges and advantages of cell therapy manufacturing under good manufacturing practices within the hospital setting. Curr Opin Biotechnol. 2020;65:233–241.
- Nestler-Parr S, Korchagina D, Toumi M, et al. Challenges in research and health technology assessment of rare disease technologies: report of the ISPOR rare disease special interest group. Value Health. 2018;21:493–500.
- Qiu T, Hanna E, Dabbous M, et al. Health technology assessment of gene therapies in Europe and the USA: analysis and future considerations. Cell Gene Ther Insights. 2019;5:1043–1059.
- Bubela T, McCabe C, Archibald P, et al. Bringing regenerative medicines to the clinic: the future for regulation and reimbursement. Regener Med. 2015;10:897–911.
- Iglesias-Lopez C, Obach M, Vallano A, et al. Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States. Cytotherapy. 2021;23:261–274.
- US Food and Drug Administration, Gottlieb S. Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D, Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and GTs. 2019 Jan 15 . [cited 2022 Jul 7]. Available from: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics
- Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21:1–204.
- Marsden G, Towse A Exploring the assessment and appraisal of regenerative medicines and cell therapy products: is the NICE approach fit for purpose? OHE Consulting Report. 2017 Feb. [cited 2022 Jul 7]. Available from: https://www.ohe.org/publications/exploring-assessment-and-appraisal-regenerative-medicines-and-cell-therapy-products
- Cowles E, Marsden G, Cole A, et al. A review of NICE methods and processes across health technology assessment programmes: why the differences and what is the impact? Appl Health Econ Health Policy. 2017;15:469–477.
- National Institute for Health Care and Excellence (NICE). NICE health technology evaluations: the manual. 2022 Jan 31. [cited 2022 Jul 7]. Available from: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation
- Pochopień M, Qiu T, Aballea S, et al. Considering potential solutions for limitations and challenges in the health economic evaluation of GTs. Expert Rev Pharmacoecon Outcomes Res. 2021;21:1145–1158. .
- Aballéa S, Thokagevistk K, Velikanova R, et al. Health economic evaluation of gene replacement therapies: methodological issues and recommendations. J Mark Access Health Policy. 2020;8:1822666.
- Qiu T, Pochopień M, Hanna E, et al. Challenges in the market access of regenerative medicines, and implications for manufacturers and decision-makers: a systematic review. Regen Med. 2022;17:119–139.
- Drummond MF, Neumann PJ, Sullivan SD, et al. Analytic considerations in applying a general economic evaluation reference case to gene therapy. Value Health. 2019;22:661–668.
- Drummond M, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford University Press; 2015. cited 2022 Jul 7. Available from: https://www.nlm.nih.gov/nichsr/edu/healthecon/drummond_list.html
- Ten Ham RMT, Klungel OH, Leufkens HGM, et al. A review of methodological considerations for economic evaluations of gene therapies and their application in literature. Value Health. 2020;23:1268–1280.
- Coyle D, Durand-Zaleski I, Farrington J, et al. HTA methodology and value frameworks for evaluation and policy making for cell and GTs. Eur J Health Econ. 2020;21:1421–1437.
- Angelis A, Naci H, Hackshaw A. Recalibrating health technology assessment methods for cell and gene therapies. Pharmacoeconomics. 2020;38:1297–1308.
- Annemans L, Makady A. TRUST4RD: tool for reducing uncertainties in the evidence generation for specialised treatments for rare diseases. Orphanet J Rare Dis. 2020;15:127.
- Gonçalves E. Advanced therapy medicinal products: value judgement and ethical evaluation in health technology assessment. Eur J Health Econ. 2020;21:311–320.
- Garrison LP, Jackson T, Paul D, et al. Value-based pricing for emerging GTs: the economic case for a higher cost-effectiveness threshold. J Manag Care Spec Pharm. 2019;25:793–799.
- Petrou P. Is it a Chimera? A systematic review of the economic evaluations of CAR-T cell therapy. Expert Rev Pharmacoecon Outcomes Res. 2019;19:529–536.
- Gavan SP, Lu CY, Payne K. Assessing the joint value of genomic-based diagnostic tests and gene therapies. J Pers Med. 2019;9:28.
- Raymakers AJN, Regier DA, Peacock SJ. Modelling uncertainty in survival and cost-effectiveness is vital in the era of GTs: the case of axicabtagene ciloleucel. Health Policy Technol. 2019;8:103–104.
- Jönsson B, Hampson G, Michaels J, et al. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur J Health Econ. 2019;20:427–438.
- Hampson G, Towse A, Pearson SD, et al. Gene therapy: evidence, value and affordability in the US health care system. J Comp Eff Res. 2018;7:15–28.
- Chapman RH, Kumar VM, Whittington MD, et al. Does cost-effectiveness analysis overvalue potential cures? Exploring alternative methods for applying a “shared savings” approach to cost offsets. Value Health. 2021;24:839–845.
- Gonçalves E. Value-based pricing for advanced therapy medicinal products: emerging affordability solutions. Eur J Health Econ. 2022;23:155–163.
- Jørgensen J, Servos S, Kefalas P. The potential price and access implications of the cost-utility and budget impact methodologies applied by NICE in England and ICER in the US for a novel gene therapy in Parkinson’s disease. J Mark Access Health Policy. 2018;6:1500419.
- Canada’s Drug and Health Technology Agency. Gene therapy: international regulatory and health technology assessment (HTA) activities and reimbursement status. 2018 Mar 27. [cited 2022 Jul 7]. Available from: https://www.cadth.ca/gene-therapy-international-regulatory-and-health-technology-assessment-activities-and-reimbursement
- Marsden G, Towse A, Pearson SD, et al. Gene therapy: understanding the science, assessing the evidence, and paying for value. OHE Research Paper. 2017 Mar. [cited 2022 Jul 7]. Available from: https://www.ohe.org/publications/gene-therapy-understanding-science-assessing-evidence-and-paying-value
- Bolous NS, Chen Y, Wang H, et al. The cost-effectiveness of gene therapy for severe hemophilia B: a microsimulation study from the United States perspective. Blood. 2021;138:1677–1690.
- Machin N, Ragni MV, Smith KJ. Gene therapy in hemophilia A: a cost-effectiveness analysis. Blood Adv. 2018;2(14):1792–1798.
- Halioua-Haubold CL, Jolly JK, Smith JA, et al. Potential lifetime quality of life benefits of choroideremia gene therapy: projections from a clinically informed decision model. Eye (Lond). 2019;33:1215–1223.
- Salcedo J, Bulovic J, Young CM. Cost-effectiveness of a hypothetical cell or gene therapy cure for sickle cell disease. Sci Rep. 2021;11:10838.
- Fleeman N, Bagust A, Boland A, et al. Talimogene laherparepvec for treating metastatic melanoma: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2017;35:1035–1046.
- Almutairi AR, Alkhatib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22–28.
- Cher BP, Gan KY, Aziz MIA, et al. Cost utility analysis of tisagenlecleucel vs salvage chemotherapy in the treatment of relapsed/refractory diffuse large B-cell lymphoma from Singapore’s healthcare system perspective. J Med Econ. 2020;23:1321–1329.
- Thielen FW, van Dongen-Leunis A, Arons AMM, et al. Cost-effectiveness of anti-CD19 chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020;105:203–215.
- Moradi-Lakeh M, Yaghoubi M, Seitz P, et al. Cost-effectiveness of tisagenlecleucel in paediatric acute lymphoblastic leukaemia (pALL) and adult diffuse large B-cell lymphoma (DLBCL) in Switzerland. Adv Ther. 2021;38:3427–3443.
- Wakase S, Teshima T, Zhang J, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia in Japan. Transplant Cell Ther. 2021;27:241.e1.
- Qi CZ, Bollu V, Yang H, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma in the United States. Clin Ther. 2021;43:1300–19.e8.
- Lin JK, Lerman BJ, Barnes JI, et al. Cost effectiveness of chimeric antigen receptor T-Cell therapy in relapsed or refractory pediatric B-Cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36:3192–3202.
- Furzer J, Gupta S, Nathan PC, et al. Cost-effectiveness of tisagenlecleucel vs standard care in high-risk relapsed pediatric acute lymphoblastic leukemia in Canada. JAMA Oncol. 2020;6:393–401.
- Sarkar RR, Gloude NJ, Schiff D, et al. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111:719–726.
- Walton M, Sharif S, Simmonds M, et al. Tisagenlecleucel for the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37:1209–1217.
- Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172:1161–1168.
- Ribera Santasusana JM, de Andrés Saldaña A, García-Muñoz N, et al. Cost-effectiveness analysis of tisagenlecleucel in the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in children and young adults in Spain. Clinicoecon Outcomes Res. 2020;12:253–264.
- Farmer C, Bullement A, Packman D, et al. Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations: an evidence review group perspective of a NICE highly specialised technology appraisal. Pharmacoeconomics. 2020;38:1309–1318.
- Uhrmann MF, Lorenz B, Gissel C. Cost effectiveness of voretigene neparvovec for RPE65-mediated inherited retinal degeneration in Germany. Transl Vis Sci Technol. 2020;9:17.
- Viriato D, Bennett N, Sidhu R, et al. An economic evaluation of voretigene neparvovec for the treatment of biallelic RPE65-mediated inherited retinal dystrophies in the UK. Adv Ther. 2020;37:1233–1247.
- Johnson S, Buessing M, O’Connell T, et al. Cost-effectiveness of voretigene neparvovec-rzyl vs standard care for RPE65-mediated inherited retinal disease. JAMA Ophthalmol. 2019;137:1115–1123.
- Zimmermann M, Lubinga SJ, Banken R, et al. Cost utility of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease. Value Health. 2019;22:161–167.
- Cook K, Forbes SP, Adamski K, et al. Assessing the potential cost-effectiveness of a gene therapy for the treatment of hemophilia A. J Med Econ. 2020;23:501–512.
- Zuluaga-Sanchez S, Teynor M, Knight C, et al. Cost effectiveness of nusinersen in the treatment of patients with infantile-onset and later-onset spinal muscular atrophy in Sweden. Pharmacoeconomics. 2019;37:845–865.
- South E, Cox E, Meader N, et al. Strimvelis® for treating severe combined immunodeficiency caused by adenosine deaminase deficiency: an evidence review group perspective of a NICE highly specialised technology evaluation. Pharmacoecon Open. 2019;3:151–161.
- Simons CL, Malone D, Wang M, et al. Cost-effectiveness for KTE-X19 CAR T therapy for adult patients with relapsed/refractory mantle cell lymphoma in the United States. J Med Econ. 2021;24:421–431.
- Liu R, Oluwole OO, Diakite I, et al. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2021;24:458–468.
- Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2:e190035.
- Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21:1238–1245.
- Lin JK, Muffly LS, Spinner MA, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019;37:2105–2119.
- Dean R, Jensen I, Cyr P, et al. An updated cost-utility model for onasemnogene abeparvovec (Zolgensma®) in spinal muscular atrophy type 1 patients and comparison with evaluation by the Institute for Clinical and Effectiveness Review (ICER). J Mark Access Health Policy. 2021;9:1889841.
- Malone DC, Dean R, Arjunji R, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7:1601484.
- Broekhoff TF, Sweegers CCG, Krijkamp EM, et al. Early cost-effectiveness of onasemnogene abeparvovec-xioi (Zolgensma) and nusinersen (Spinraza) treatment for spinal muscular atrophy I in the Netherlands with relapse scenarios. Value Health. 2021;24:759–769.
- Connock M, Andronis L, Auguste P, et al. Will the US$5 million onasemnogene abeparvosec treatment for spinal muscular atrophy represent ‘value for money’ for the NHS? A rapid inquiry into suggestions that it may be cost-effective. Expert Opin Biol Ther. 2020;20:823–827.
- Shih ST, Farrar MA, Wiley V, et al. Newborn screening for spinal muscular atrophy with disease-modifying therapies: a cost-effectiveness analysis. J Neurol Neurosurg Psychiatry. 2021;92:1296–1304.
- Kansal AR, Reifsnider OS, Brand SB, et al. Economic evaluation of betibeglogene autotemcel (Beti-cel) gene addition therapy in transfusion-dependent β-thalassemia. J Mark Access Health Policy. 2021;9:1922028.
- Schuster Bruce C, Brhlikova P, Heath J, et al. The use of validated and nonvalidated surrogate endpoints in two European Medicines Agency expedited approval pathways: a cross-sectional study of products authorised 2011-2018. PLoS Med. 2019;16:e1002873.
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38:200–211.
- Sheringham J, Kuhn I, Burt J. The use of experimental vignette studies to identify drivers of variations in the delivery of health care: a scoping review. BMC Med Res Methodol. 2021;21:81.
- Javalkar K, Rak E, Phillips A, et al. Predictors of caregiver burden among mothers of children with chronic conditions. Children (Basel). 2017;4:39.
- Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36:745–758.
