ABSTRACT
Objectives
We assessed real-world healthcare resource utilization (HRU) and costs among US patients with relapsed or refractory mantle cell lymphoma (R/R MCL) by line of therapy (LoT).
Methods
We selected patients from MarketScan® (1/1/2016–12/31/2020): ≥1 claims of MCL-indicated first line (1L) therapies, ≥1 diagnoses of MCL pre-index date (1L initiation date), ≥6-month continuous enrollment pre-index date, second line (2L) therapy initiation, ≥18 years old at 2L, and no clinical trial enrollment. Outcomes included time to next treatment (TTNT), all-cause HRU, and costs.
Results
The cohort (N = 142) was 77.5% male, aged 62 years (median). Sixty-six percent and 23% advanced to 3L and 4L+, respectively. Mean (median) TTNT was 9.7 (5.9), 9.3 (5.0), and 6.3 (4.2) months for 2L, 3L, and 4L+, respectively. Mean (median) per patient per month (PPPM) costs were $29,999 ($21,313), $29,352 ($20,033), and $30,633 ($23,662) for 2L, 3L, and 4L+, respectively. Among those who received Bruton tyrosine kinase inhibitors, mean (median) PPPM costs were $24,702 ($17,203), $31,801 ($20,363), and $36,710 ($25,899) for 2L, 3L, and 4L+, respectively.
Conclusions
During the period ending in 2020, patients relapsed frequently, incurring high HRU and costs across LoTs. More effective treatments with long-lasting remissions in R/R MCL may reduce healthcare burden.
Plain Language Summary
Mantle cell lymphoma is a rare blood cancer of white blood cells. This type of cancer can be hard to treat, even with new treatments. In about 15% to 20% of people, the cancer will not get better or will come back within 2 years of starting treatment. When this happens, there are few good options for treatments that work. Using medical claims data, we looked at healthcare use and costs among US patients with mantle cell lymphoma that came back after treatment or did not respond to treatment. We found 142 patients who met the study criteria. Of these, 77.5% were men with a median age of 62 years. Sixty-six percent got a third of the treatment and 23% got a fourth treatment or more. The time until the next treatment was about 9–10 months for patients who got a second and third treatments.. It was about 6 months for people who got a fourth or more treatment. The average monthly cost of treatment was about $30,000 for those receiving a second or fourth or more treatment. It was slightly less for those who got a third treatment. For those who got Bruton’s tyrosine kinase inhibitors, the monthly costs went up with each treatment they needed. Overall, we found that during the study period, patients with mantle cell lymphoma worsened quickly, received multiple treatments, and had high costs of care. Better treatments that work longer are needed.
1. Introduction
Mantle cell lymphoma (MCL) is an aggressive, rare subtype of mature B-cell non-Hodgkin’s lymphoma (NHL) which accounts for approximately 3–6% of all newly diagnosed cases of NHL in the United States [Citation1]. MCL has an annual incidence of approximately 1 case per 100,000 people [Citation2] and is more common in males. The incidence increases with age, and the median age at diagnosis is generally between 65 and 70 years old [Citation3–5].
Roughly 75% of patients with MCL in the frontline setting present with aggressive disease, which requires treatment at the time of diagnosis. Despite recent therapeutic advances, MCL remains challenging to treat, and outcomes are generally poor [Citation6,Citation7]. Approximately 15% to 20% will relapse or become refractory within 2 years of initial treatment and most require multiple lines of therapy [Citation8]. Patients with relapsed/refractory (R/R) MCL have limited therapeutic options, which are generally characterized by short duration of remission and median overall survival, ranging from 5.5 to 12.5 months in the post-Bruton Tyrosine Kinase inhibitor (BTKi) setting [Citation7–10]. A recent meta-analysis showed that the objective response rate (ORR) among patients with R/R MCL with at least one prior line of therapy (LoT) was 52%; this drops to 43% with at least 2 LoTs and 28% post-BTKis [Citation11].
Recommended first-line (1L) treatment for younger (i.e. ≤65 years of age) patients without comorbidities involves aggressive therapy, including intensive combination chemotherapy incorporating rituximab and cytarabine, followed by consolidation with autologous stem cell therapy (auto-SCT) and maintenance rituximab [Citation12–15]. Recommended treatment for older or unfit patients (i.e. those >65 with comorbid conditions) includes less aggressive chemo-immunotherapy, with or without rituximab maintenance [Citation12,Citation16]. Despite initial response rates ranging from 65% to 100% ORR (CR and PR), disease relapse is near universal [Citation17–22]. The recommended regimens for second line (2L) therapy include covalent Bruton tyrosine kinase inhibitors (BTKis), including acalabrutinib or zanubrutinib, or lenalidomide with rituximab [Citation12]. Brexucabtagene autoleucel (TECARTUS) has also been approved by the Food and Drug Administration (FDA) for patients with R/R MCL [Citation23]. Most recently, a non-covalent BTKi, pirtobrutinib, was approved by the FDA and is recommended for 3L or subsequent therapy [Citation12,Citation24].
Although treatment options for patients with MCL have recently evolved, literature describing the real-world treatment patterns, healthcare resource utilization (HRU), and economic burden of MCL remains limited. In 2019, a retrospective cohort study found an association between MCL-related adverse events (AEs), hospitalizations, and costs utilizing a large administrative database from November 2012 to January 2018 [Citation25]. Goyal et al. assessed the treatment patterns, AEs, HRU, and economic burden of patients with MCL who had commercial or Medicare Supplemental health plans in the US from July 2011 to June 2016 [Citation26]. Total all-cause and MCL-related mean (standard deviation [SD]) monthly costs were considerable, at $10,964 ($17,530) and $8,613 ($16,166), respectively. Compared to mean (SD) monthly all-cause costs for those with no AEs, costs were nearly 3 times higher for those with six or more AEs (no AEs: $5131 ($10,352) vs 6+ AEs: $13,560 ($18,466)) [Citation26]. While these findings provide valuable insights into the HRU and economic burden associated with MCL, both studies focused on the overall MCL population, did not report the time to next treatment, the costs by specific LoT, and did not include more recently approved therapies, due to the analysis timeframes. The lack of available research focused on the healthcare utilization and costs of care by line of therapy and the inclusion of more recently approved therapies, particularly newly approved BTKi therapies, for patients with R/R MCL represents a crucial evidence gap. Furthermore, research is needed to better understand the duration of time to next treatment (TTNT) to gain further insight into the durability of remission by LOT in R/R MCL. Finally, an updated assessment of treatment patterns and costs associated with more recently approved therapies is vital to understanding the current therapeutic landscape for R/R MCL.
The objective of this study is to address these critical evidence gaps by characterizing the treatment patterns, including TTNT, health resource utilization, and costs of care among US-based patients with R/R MCL specifically by line of therapy. We also aimed to describe the treatment patterns, HRU, and costs specifically for those patients with R/R MCL who received BTKis. The most recent US commercial claims data available at the time of the study was utilized to include more recently approved therapies.
2. Methods
2.1. Data source
This retrospective observational cohort study used administrative claims data from the MarketScan Commercial and Medicare Supplemental Claims and Encounters Database, which contains medical and pharmacy data from approximately 137.6 million individuals enrolled in a variety of fee-for-service and managed care health plans. This database provides detailed longitudinal payer costs, pharmacy and medical services resource utilization, and outcome data provided in inpatient and outpatient settings. All data utilized in this study were de-identified and institutional review board approval was not required.
2.2. Patient selection and study period
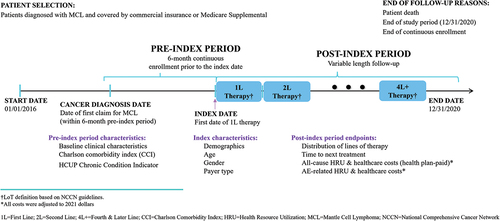
The study design is summarized in . Patients with a diagnosis of MCL during the sample selection time period of 1 January 2016 through 31 December 2020, representing the most recent 5 years of data available at the time of the analysis, were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes. A list of MCL therapies based on the National Comprehensive Cancer Network (NCCN) clinical practice guidelines was applied to identify patients who received treatment [Citation12]. The index date was the first date of 1L MCL therapy. Six months of continuous health plan enrollment prior to the index date served as the pre-index period during which baseline clinical characteristics, including comorbidity variables, were identified. Patients were followed until death, health plan disenrollment, or end of the study period (31 December 2020), whichever was earliest.
2.3. Inclusion criteria
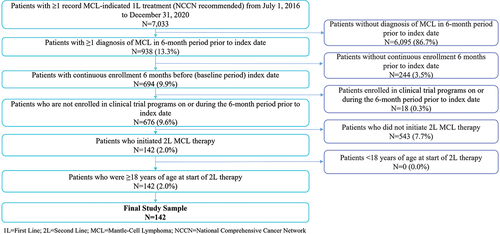
presents the patient sample selection flow for this study. Patients were required to meet the following inclusion criteria for the study: one or more claims for an MCL-indicated, 1L NCCN recommended treatment, continuous health plan enrollment for at least 6 months before the index date, one or more primary or secondary diagnoses of MCL (ICD-10-CM codes C83.1 and C83.1×), initiation of 2L MCL therapy and at least 18 years of age at the initiation of 2L MCL treatment.
2.4. Exclusion criteria
Patients were excluded if they participated in clinical trials on or during the 6-month period prior to the index date.
2.5. Patient characteristics
Demographic characteristics reported in this study included age, gender, and health plan type. The Charlson Comorbidity Index (CCI) and Healthcare Cost and Utilization Project (H-CUP) Chronic Condition Indicator scores were calculated during the pre-index period [Citation27].
2.6. Lines of therapy
The rules governing the start and end of each MCL LoT were defined as part of the analysis. MCL-specific LoTs were first identified based on the most recent NCCN Guidelines for the treatment of B-cell lymphoma. The LoT rules were further refined by expert clinical review of selected data to ensure they were clinically meaningful. Details regarding the specific LoT rules are included in Appendix C, Table C1.
2.7. Outcome measures
Study outcomes included the number of LoTs received, TTNT, and all-cause HRU including hospitalization rates, inpatient length of stay, emergency room (ER) visits, office visits, other outpatient services, and outpatient prescription medications. Additionally, all-cause total payer costs (health plan-paid) and costs for each of these healthcare categories were calculated. HRU and costs associated with treatment-related AEs were also calculated. All costs were adjusted to 2021 US dollars using the medical care component of the US Consumer Price Index. The AEs included in this analysis were identified based on the published literature that reported treatment-related AEs. AE-related HRU was assessed using medical claims containing an applicable diagnosis and treatment code (at primary position or elsewhere) for the corresponding AE. An AE-related HRU event was defined as the AE occurring during the time the treatment was administered (see Appendix D, Table D1 for list of AEs by treatment and corresponding diagnosis codes). Similarly for AE-related costs, if the AE diagnosis was included in the primary or secondary position in the claim, the costs for this event was included in the AE-related healthcare costs. All outcomes in this analysis were stratified by LoT (second line, third line: 3L, and fourth line or later: 4L+). The fourth line was combined with later lines of therapy for reporting purposes due to the small sample sizes in these LoTs.
2.8. Data analysis
All study outcomes were analyzed descriptively. Univariate analyses were conducted to describe treatment patterns and cost drivers. Categorical variables were calculated as counts and percentages, and continuous variables were calculated as the number of observations, means, standard deviations (SDs), medians, and ranges. All-cause and AE-related HRU and costs were assessed overall as well as on a per patient per month (PPPM) basis to standardize the outcomes as well as total costs by LoT. For cost variables, patients with zero costs in any of the healthcare categories were excluded from the cost analysis for that category. Two subgroup analyses of the R/R MCL cohort (1) who received BTKis within each LoT and (2) did not receive BTKis within each LoT were conducted to understand the real-world cost and HRU associated with the newest class of therapies.
All analyses were conducted using SAS statistical software version 9.4 (SAS Institute Inc., Cary, North Carolina).
3. Results
3.1. Patient selection and demographics
depicts the sample selection process to identify the R/R MCL cohort for this study. A total of 142 adult patients with R/R MCL met the selection criteria. describes baseline characteristics of these patients. The median age was 62 years and 77.5% were male. Approximately two-thirds of the cohort were from commercial plans (66.2%), while one-third (33.8%) had Medicare Supplemental insurance. Patients with MCL had mean (SD) CCI and H-CUP Chronic Condition Indicator scores of 3.7 (2.3) and 15.0 (7.5), respectively. Patients had a mean (SD) length of follow-up of 17.7 (13.1) months during the study period.
Table 1. Baseline characteristics of patients with R/R MCL.
3.2. Treatment patterns
Among patients who received 2L therapy during the observation period (n = 142), 94 (66.2%) received 3L, and 33 (23.2%) received 4L+. Within each LoT, 60 out of 142 (42.3%), 55 out of 94 (58.5%), and 20 out of 33 (60.6%) patients received BTKis during 2L, 3L, and 4L+, respectively. Of the 60 patients who received BTKis during 2L, 39 (65.0%) received ibrutinib, 20 (33.3%) received acalabrutinib, and 1 (1.6%) received zanubrutinib. Of the 55 patients who received BTKis during 3L, 35 (63.6%), 18 (32.7%), and 2 (3.6%) received ibrutinib, acalabrutinib, and zanubrutinib, respectively. Of the 20 patients who received BTKis for 4L+, 10 (50.0%), 9 (45.0%), and 1 (5.0%) received ibrutinib, acalabrutinib, and zanubrutinib, respectively. The 1L treatments received are shown in Table 6 of Appendix A, Table A1. For the R/R MCL cohort, the mean/median (SD) TTNT for 2L was approximately 9.7/5.9 (9.7) months, 9.3/5.0 (10.0) months for 3L, and 6.3/4.2 (7.5) months for 4L+. Among those who received BTKs within each LoT, the mean/median (SD) TTNT was 4.4/1.0 (8.0) months for 2L, 5.1/1.8 (8.3) months for 3L, and 3.8/2.3 (3.9) months for 4L+ (see ).
Table 2. LoT and time to next treatment for R/R MCL Cohort and among those who received BTKis by LoT.*
3.3. All-cause and AE-related HRU and costs among R/R MCL Cohort
presents the all-cause and AE-related hospitalization rates, inpatient LOS, and healthcare costs (overall and PPPM) for the R/R MCL cohort during the study period. For all-cause inpatient visits, the proportion of those hospitalized increased by LoT, with 48.6% hospitalized in 2L, 56.4% in 3L, and 63.6% in 4L+. Mean (SD) all-cause LOS also increased by LoT, from 7.6 (10.2) days in 2L, to 11.2 (11.2) in 3L, to 11.8 (10.9) in 4L+. Rates of AE-related inpatient visits increased similarly, with 46.5%, 55.3%, and 63.6% experiencing an AE-related hospitalization during 2L, 3L, and 4L+ LoTs, respectively. Correspondingly, the mean AE-related LOS followed the same pattern of increasing days by LoT, at 7.6 (10.3), 10.4 (9.1), and 12.5 (11.4) days, for 2L, 3L, and 4L+, respectively.
Table 3. All-cause and AE-related HRU and costs (overall and PPPM) for patients with R/R MCL by LoT.*
The mean (SD) all-cause overall total costs were $198,798 ($191,682) for 2L, $163,775 ($151,414) for 3L, and $187,409 ($189,542) for 4L+. The mean (SD) monthly all-cause total costs increased in later LoTs, from $29,999 ($28,423) for 2L, $29,352 ($26,958) for 3L, to $30,633 ($27,954) for 4L+. Inpatient hospitalization costs were consistently the largest driver of total costs across lines of therapy; however, both outpatient service and prescription medication costs also contributed to the total costs.
Examination of the AE-related costs revealed high rates of healthcare service utilization and associated costs, suggesting that managing AEs play a significant role in contributing to the economic burden among this population. The mean (SD) overall total costs for the management of AEs increased with each LoT from $110,601 ($134,504) for 2L, $113,698 ($127,677) for 3L, to $130,500 ($136,018) for 4L+. The mean (SD) monthly total costs for the management of AEs were $17,756 ($22,244), $21,072 ($23,321), and $22,267 ($24,431) for 2L, 3L, and 4L+, respectively. Similar to all-cause costs, AE-related hospitalization costs were the primary driver of total costs across LoTs.
3.4. All-cause and AE-related HRU and costs among patients receiving BTKi within each LoT
presents the all-cause and AE-related HRU and costs for the subgroup of patients who received BTKis within each LoT during the study period. The rates of all-cause hospitalizations increased with each LoT, from 38.3% for 2L, to 47.3% for 3L, and 65.0% for 4L+. The mean (SD) LOS days were consistently high, at 8.2 (11.3), 7.7 (5.6), and 9.5 (6.8) for 2L, 3L, and 4L+, respectively.
Table 4. All-cause and AE-related HRU and costs (overall and PPPM) for patients with R/R MCL who received BTKis within each LoT.*
The mean (SD) all-cause overall total costs in the BTKi subgroup were $190,550 ($229,129) for 2L, $158,181 ($162,026) for 3L, and $222,265 ($205,094) for 4L+. The mean (SD) all-cause monthly total costs were $24,702 ($28,839) for 2L, $31,801 ($27,131) for 3L and $36,710 ($28,994) for 4L+.
AE-related hospitalizations among those who received BTKi treatments were nearly identical to all-cause hospitalizations, indicating that the vast majority of inpatient visits for this subgroup were for treatment-related AEs. The AE-related, mean (SD) overall total costs ranged from $123,377 ($166,209) for 2L, $119,720 ($136,131) for 3L, to $160,493 ($147,712) for 4L+. AE-related, mean (SD) monthly total costs revealed increasing costs with each LoT ($16,261 [$23,408], $23,889 [$23,315], and $30,049 [$27,502] for 2L, 3L, and 4L+, respectively). Similar to the entire R/R MCL cohort, hospitalizations remained the key driver of total costs for this subgroup.
3.5. All-cause and AE-related HRU and costs among patients who did not receive BTKi within each LoT
The all-cause and AE-related HRU and costs for the subgroup of patients who did not receive BTKis within each LoT during the study period are presented in . The all-cause hospitalization rates were highest for 3L, with 56.1% for 1L, 69.2% for 2L, and 61.5% or 4L+. The mean (SD) hospital LOS days increased with each LoT, from 7.2 (9.7) for 1L, to 14.5 (14.0) for 2L and 15.4 (15.2) for 4L+.
Table 5. All-cause and AE-related HRU and costs (overall and PPPM) for patients with R/R MCL who did not receive BTKis within each LoT.*
The mean (SD) all-cause overall total costs for those not receiving BTKis within each LoT were highest for 2L and decreased with each LoT, with $204,833 ($103,848) for 2L, $171,664 ($136,725) for 3L, and $133,785 ($155,120) for 4L+.
AE-related hospitalization rates among those not receiving BTKis within each LoT were similar to the rates for all-cause hospitalizations, with 54.9% at 1L, 66.7% at 2L, and 61.5% at 4L+. However, the total AE-related costs of care for patients were consistently lower within each LoT than the all-cause costs at $99,904 ($105,171), $105,205 ($115,907), and $80,511 ($100,450) for 2L, 3L, and 4L+, respectively.
3.6. Sensitivity analysis
A sensitivity analysis was performed to assess the impact of excluding patients with other forms of NHL on the strength of the findings. An additional exclusion criterion of patients diagnosed with other forms of NHL on or during the 6-month period pre-index date was applied, with all other eligibility criteria remaining consistent. The results of the sensitivity analysis found that both the HRU and cost outcomes were consistent with the main results, suggesting reliability of the study findings. The results of this sensitivity analysis are included in Appendix E, Table E1 and E2.
4. Discussion
4.1. 4.1 Summary and key findings
This study demonstrated that in the time period examined (2016–2020) after first-line therapy, patients with MCL generally relapsed and received multiple additional lines of therapy. Resource utilization and overall costs of treating R/R MCL were considerably higher, particularly in comparison to other cancer tumor types (see Appendix B, Table B1 for costs of other cancer types). Mean monthly costs for R/R MCL were consistently high within and across lines of therapy, and those costs increased with additional LoTs. Notably, there was a considerable range in costs within the healthcare services utilized by this cohort, reflecting the high variability in the rate of disease progression, the care received, and the outcomes experienced by these patients. While the mean monthly costs in this analysis were higher than previously reported [Citation29–32], this was not surprising given that our study focused specifically on patients who were relapsed or refractory, while prior studies included all patients with MCL. Additionally, other factors including new therapies that have become available more recently and their associated AEs could account for the higher costs found in our study. Overall, the results of this study supported prior analyses that showed the HRU and economic burden associated with MCL were substantial across the lines of therapy. Hospitalizations were the primary cost driver; rates of hospitalization and mean LOS increased with each LoT, which was also consistent with observations in other published analyses [Citation28,Citation29,Citation33]. In addition, in our study other outpatient services and prescription medications also accounted for a considerable portion of costs. This study also revealed a shorter duration of TTNT with each successive LoT, suggesting a low durability of current therapies in later LoTs. In parallel, AE-related hospitalizations and associated LOS also grew with each LoT, indicating that patients receiving multiple LoTs also experienced increasing AEs that resulted in hospital readmissions. This suggests that subsequent LoTs are resulting in a greater frequency of AEs, with more prolonged hospital admissions.
Similar trends were observed among those who received BTKis within each LoT, with patients receiving multiple LoTs. Patients who received BTKis also had increasing rates of hospitalizations and mean monthly costs with each LoT. Furthermore, the mean TTNT among those receiving BTKis within each LoT were 4.4, 5.1, and 3.8 months for 2L, 3L, and 4L+, respectively.
When assessing HRU patterns for those who received BTKis versus those who did not receive BTKis within each LoT, the hospitalization rates for 2L and 3L were somewhat higher for those who did not receive BTKis. Mean total costs followed a similar pattern whereby those who did not receive BTKis had slightly higher costs for 2L and 3L compared to those receiving BTKis. By 4L+; however, both those who received BTKis and those who did not had similar rates of hospitalizations and mean total costs of care. It should be noted that the sample sizes for those receiving 4L+ in both groups were very small, so results should be interpreted with caution. Interestingly, while all-cause mean costs were slightly higher for non-BTKi-treated patients, mean AE-related costs were consistently higher for those receiving BTKis across each LoT. The descriptive differences of cost and HRUs between the BTKi- and non-BTKi-treated groups along each LoT should be interpreted with caution due to lack of risk adjustment and the small sample sizes especially in 4L+. These findings suggest that despite the increased adoption of BTKis, patients with R/R MCL treated with this newly introduced class of therapies in clinical practice still experience poor outcomes at each LoT, particularly AE-related, that require substantial healthcare services and incur considerable costs of care.
Assessment of all-cause versus AE-related, mean monthly costs for those who received a BTKi within each LoT revealed that nearly all of the inpatient visits and associated costs were for AE-related causes. The mean monthly total costs for AE-related causes consisted of 66% (2L), 75% (3L), and 82% (4L+) of the all-cause costs, suggesting that the majority of the costs may be attributable to the management of AEs.
The results of this study suggest that effective, well-tolerated therapeutic options that may result in longer remission times for patients with R/R MCL remain a critical unmet need. More recently, innovative immunotherapies such as chimeric antigen receptor T-cell therapy (CAR T), reversible non-covalent BTK inhibitors, CD20 × CD3 bispecific antibodies, phosphatidylinositol 3-kinase inhibitors, and antibody-drug conjugates [Citation33] have emerged that show promise in addressing this gap in care. In July 2020, the FDA approved brexucabtagene autoleucel under the Accelerated Approval pathway, as the first and only CAR T immunotherapy for adult patients with R/R MCL. Results for adult patients with R/R MCL in ZUMA-2 (a multicenter, open-label phase 2 study) demonstrated a strong treatment response with brexucabtagene autoleucel, where 91% of the patients with R/R MCL after BTKi therapies achieved objective response (OR) and 68% achieved complete remission (CR) after a median follow-up of 35.6 months. The median duration of response (DOR), progression-free survival (PFS), and overall survival (OS) was 28.2 months, 25.8 months, and 46.6 months, respectively, suggesting a durable remission period [Citation23,Citation32].
Pirtobrutinib, a recently approved, highly selective, reversible non-covalent BTK inhibitor, has demonstrated efficacy and safety for R/R MCL. In a phase I/II open-label BRUIN study among 120 patients with R/R MCL who previously received BTKis, the ORR was 50%, with 13% CR, 38% with partial response (PR), and a median DOR of 8.3 months [Citation24,Citation34].
Additional new therapeutic approaches are currently in early to mid-phase development for R/R MCL, including glofitamab and epcoritamab, which are bispecific antibodies targeting CD20/CD3 proteins, and parsaclisib, a next-generation, highly selective phosphatidylinositol 3-kinase inhibitor. Finally, antibody-drug conjugates, which selectively target receptor tyrosine kinase-like orphan receptor-1 (ROR-1), represent another potential new treatment for MCL.
Given the short duration of remission and the multiple LoTs that patients cycle through on chemotherapies and BTKis, innovative therapies such as CAR T, reversible non-covalent BTK inhibitors, CD20 × CD3 bispecific antibodies, phosphatidylinositol 3-kinase inhibitors, and antibody-drug conjugates may hold promise for enhanced durability of remission for MCL [Citation33].
This study expands upon prior analyses in examining the time to next LoT and the evaluation of the HRU and cost burden within each LoT. This study also provides a more up-to-date assessment of utilization and costs as it included newer second-generation BTKi therapies, such as acalabrutinib and zanubrutinib, which may not have been approved when prior analyses were conducted. This research should be updated in the future when there is a sufficient sample of patients who received CAR T and other innovative therapies. Future research could also examine the full cost of care, including in 1L, for patients with R/R MCL.
4.2. Limitations
This observational study had several limitations that should be acknowledged. The relatively small sample size may have introduced variability or bias, and the generalizability of our cohort to patients with R/R MCL with other types of insurance may be limited. Additionally, the data were analyzed descriptively, without any adjustments for potential confounders, such as demographics or risk factors. There were also limitations that stem from the nature of claims data, which are collected for financial reporting purposes and are not designed to accurately capture certain clinical and prognostic factors. Particularly, the study estimated AE-related costs and HRU outcomes based on claims with diagnosis codes of AEs related to corresponding MCL treatment regimens. However, causality between HRU and costs in a specified AE (e.g. upper respiratory tract infection) and the MCL treatment regimens (e.g. acalabrutinib) cannot be established based on claims data. Moreover, while this dataset does include Medicare Supplemental claims data, it does not capture all costs associated with other Medicare types such as Medicare Fee for Service (FFS) or Medicare Advantage (MA), which limits generalizability to this subpopulation.
Claims data are also subject to potential miscoding, and data elements may be missing or inaccurate. The timeframe of this study (particularly the last 9 months of 2020) overlapped with the outbreak of COVID-19 and the subsequent reduction in healthcare services due to pandemic shutdowns in many parts of the US. This may have impacted the care that patients in this cohort received and may not reflect standard care in non-pandemic circumstances. The timeframe of this study also did not capture the newest MCL treatments, including CAR T therapy. As utilization of novel MCL therapies is expected to increase, further real-world research is needed to capture real-world treatment patterns and costs to understand the scope of economic burden of this disease.
5. Conclusions
This study found that in the time period (2016–2020), patients with R/R MCL relapsed quickly, received multiple LoTs, incurred considerable HRU, of which the majority were attributable to AEs, and costs were consistently high across lines of therapy. These findings highlight the accumulated total costs of subsequent lines of therapy and the critical and continuing unmet need for effective, well-tolerated therapeutic options with longer remission times for R/R MCL. The emergence of new therapies may hold promise in potentially addressing the continuing unmet need in R/R MCL.
Article highlights
Despite recent therapeutic advances, MCL remains a challenging condition to treat with generally poor outcomes.
Patients with R/R MCL have limited therapeutic options.
We assessed the real-world HRU and costs among US patients with R/R MCL by a line of therapy utilizing the Marketscan database from 1/1/2016 to 12/31/2020.
During the study period, patients with R/R MCL relapsed frequently, received multiple LoTs, incurred high HRU, and costs across LoTs.
These findings highlight the accumulated total costs of subsequent lines of therapy and the critical and continuing unmet need for effective, well-tolerated therapeutic options with longer remission times for R/R MCL.
The emergence of new therapies may hold promise in potentially addressing the continuing unmet need in R/R MCL.
Declaration of interest
D Ito, J Epstein, and C Kim (employee at the time of the study) are employees of Stratevi, a consulting firm that received research funding from Kite Pharma, a Gilead company, to conduct this study. C Feng, C Fu, J Wu, and J Snider are employees of Kite Pharma, who in the course of their employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Gilead Sciences. A DuVall is an unpaid independent joint researcher to this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer declares work at Concertai, conducting oncology research. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
D Ito, C Feng, C Fu, C Kim, J Wu, J Epstein, and J Snider were involved in the conception and design of this study, the analysis, interpretation of the results, and drafting/revising the paper for intellectual content. A DuVall was involved in the interpretation of the results and drafting/revising the paper for intellectual content. All authors gave the final approval for this manuscript to be published and agree to be accountable for all aspects of the work herein.
Previous presentation
Ito D, Feng C, Fu C, Kim C, Wu J, Epstein J, Snider JT, DuVall AS. Health resource utilization (HRU) and total costs of care for adult patients with relapsed or refractory mantle cell lymphoma in the United States: A retrospective claims analysis. Poster presented at Academy of Managed Care Pharmacy (AMCP) Nexus Conference, October 11–14, 2022; National Harbor, MD.
Acknowledgments
The authors gratefully acknowledge Timothy Niecko for his contributions to the data analysis for this study.
Data availability statement
The data that support the findings of this study are available from Merative. Restrictions apply to the availability of these data, which were used under license for this study. Data are available with permission from Merative.
Additional information
Funding
References
- Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. JCO. 2016;34(11):1256–1269. DOI:10.1200/JCO.2015.63.5904
- Fu S, Wang M, Lairson DR, et al. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: a comparative study between Texas and National SEER areas. Oncotarget. 2017;8(68):112516–112529.
- Fakhri B, Kahl B. Current and emerging treatment options for mantle cell lymphoma. Ther Adv Hematol. 2017;8(8):223–234.
- Yang KK, Lucas E, Lesher B, et al. A systematic review of the epidemiology and economic burden of mantle cell lymphoma (MCL). Blood. 2019;134(Supplement_1):5831.
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791.
- Arcaini L, Lamy T, Walewski J, et al. Prospective subgroup analyses of the randomized MCL-002 (SPRINT) study: lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma. Br J Haematol. 2018;180(2):224–235. DOI:10.1111/bjh.15025
- Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26(6):1175–1179. DOI:10.1093/annonc/mdv111
- Bond DA, Switchenko JM, Villa D, et al. Early relapse identifies MCL patients with inferior survival after intensive or less intensive frontline therapy. Blood Adv. 2015;5(23):5179–5189. DOI:10.1182/bloodadvances.2021004765
- Vose JM. Mantle cell lymphoma: 2017 update on diagnosis, risk‐stratification, and clinical management. Am J Hematol. 2017;92(8):806–813.
- Hess G, Dreyling M, Oberic L, et al. Real-world experience among patients with relapsed/refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor failure in Europe: the SCHOLAR-2 retrospective chart review study. Br J Haematol. 2022;00:1–11.
- Dreyling M, Shah B, Wu JJ, et al. Efficacy outcomes following treatment with Bruton tyrosine kinase inhibitors (BTKi) for relapsed/refractory mantle cell lymphoma (R/R MCL): a literature-based meta-analysis. Poster presented at ISPOR; May 2022; Washington, DC.
- National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: b-cell lymphomas. Version 5.2021. Sep 22, 2021.
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–2952. DOI:10.1182/blood-2013-11-531327
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520–531. DOI:10.1056/NEJMoa1200920
- Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German low grade lymphoma study group (GLSG). JCO. 2005;23:1984–1992.
- Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132(16):1647–1656.
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. DOI:10.1016/S0140-6736(15)00667-4
- Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378(13):1211–1223. DOI:10.1056/NEJMoa1715519
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–3695. DOI:10.1200/JCO.2013.49.2835
- Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–4874. DOI:10.1200/JCO.2006.07.9665
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–667. DOI:10.1016/S0140-6736(17)33108-2
- Monga N, Tam C, Garside J. Clinical efficacy and safety of first-line treatments in patients with mantle cell lymphoma: a systematic literature review. Crit Rev In Oncol/Hematol. 2021;158:103212.
- TECARTUS [package insert]. Santa Monica CA: Kite, A Gilead Company; 2021.
- JAYPIRCA [package insert]. (IN)polis IN: Lilly USA, LLC; 2023.
- Kabadi SM, Near A, Wada K, et al. Treatment patterns, adverse events, healthcare resource use and costs among commercially insured patients with mantle cell lymphoma in the United States. Cancer Med. 2019;8(17):7174–7185.
- Goyal RK, Nagar SP, Kabadi SM, et al. Adverse events, resource use and economic burden associated with mantle cell lymphoma: a real-world assessment of privately insured patients in the US. Leukemia & Lymphoma. Leukemia & Lymphoma. 2018;60(4):955–963. DOI:10.1080/10428194.2018.1509320
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med care. 2005;43(11):1130–1139. DOI:10.1097/01.mlr.0000182534.19832.83
- Agency for Healthcare Research and Quality. HCUP Chronic Condition Indicator. Healthcare Cost and Utilization Project (HCUP). [Accessed 2022 Oct 6]. Available at. https://www.hcup-us.ahrq.gov/toolssoftware/chronic_icd10/chronic_icd10.jsp
- Wade RL, Hu HX, Chen JY, et al. Increased treatment cost associated with mantle cell lymphoma disease progression. Haematologica. 2015;100:149.
- Owen C, Berinstein NL, Chistofiedes A, et al. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26(2):e233–240.
- O’Brien SM, Brown JR, Byrd JC, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Front Oncol. 2021;11. doi:10.3389/fonc.2021.720704.
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. DOI:10.1056/NEJMoa1914347
- Kumar A, Eyre TA, Lewis KL, et al. New Directions for Mantle Cell Lymphoma in 2022. Am Soc Clin Oncol Educ Book. 2022;42(42):614–628. DOI:10.1200/EDBK_349509
- National Cancer Institute. Financial burden of cancer care. Updated April 2022. Accessed May 2, 2022. https://www.progressreport.cancer.gov/after/economic_burden.
APPENDIX A
Table A1. First-line therapies for patients with R/R MCL.
RDHA = rituximab, dexamethasone, cytarabine + platinum (carboplatin, cisplatin, or oxaliplatin); RCHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; RDHAP = rituximab, dexamethasone, cytarabine, cisplatin; Nordic regimen = dose-intensified induction immunochemotherapy with rituximab + cyclophosphamide, vincristine, doxorubicin, prednisone [maxi-CHOP]; HyperCVAD = cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine; VR+CAP = bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone; RBAC500 = rituximab, bendamustine, cytarabine.
APPENDIX B
Table B1. Average (per patient) annualized 2007–2013 cancer-attributable costs in 2020 US dollars for cancer-related medical services by selected cancer site and phase of care.
APPENDIX C
Table C1. Line of therapy definitions.
APPENDIX D
Table D1. Table of adverse events for MCL-related treatments.
APPENDIX
Table E1. Baseline characteristics of patients with R/R MCLŦ.
Table E2. All-cause HRU and costs (overall and PPPM) for patients with R/R MCL by LoTŦ.
2L = second line; 3 L = third line; 4L+ = fourth line and higher; ER = emergency room; HRU = healthcare resource utilization; LoT = line of therapy; PPPM = per patient per month; SD = standard deviation