ABSTRACT
Objectives
This real-world study evaluated the impact of dapagliflozin on short-term medical costs in patients with stage 3 chronic kidney disease (CKD).
Methods
This retrospective, observational cohort study used medical and pharmacy claims data from IQVIA PharMetrics Plus. Patients aged ≥18 years with a filled dapagliflozin prescription after stage 3 CKD diagnosis between September 2020 and December 2021 were 1:1 propensity score matched with patients with stage 3 CKD who did not receive dapagliflozin. The primary endpoint was cardiorenal medical costs to payers over 6 months; all-cause medical and pharmacy costs were also analyzed. Within the overall population, there was a new-user subgroup of patients with no sodium-glucose co-transporter-2 use during baseline.
Results
The new-user subgroup included 503 matched patients per cohort. Mean per-patient cardiorenal medical costs were reduced by 49.0% in the dapagliflozin versus non-dapagliflozin cohort ($3172.15 vs $6219.50; P < 0.001). Mean all-cause medical costs were reduced ($8043.58 vs $12,194.87; P < 0.001) and mean all-cause pharmacy costs were increased ($9056.98 vs $7453.23; P = 0.22). Results were similar for the overall population.
Conclusion
This study showed dapagliflozin was associated with reduced cardiorenal medical costs over 6 months compared with no dapagliflozin treatment in patients with stage 3 CKD, demonstrating real-world medical cost savings.
Plain Language Summary
Chronic kidney disease (CKD) is a condition in which the kidneys become progressively less effective at filtering blood. Patients with CKD also have an increased risk of cardiovascular disease, high blood pressure, and stroke. Dapagliflozin is a drug that can be prescribed for adults with CKD to reduce the risk of CKD worsening, hospitalization for heart failure, and death from cardiovascular disease. Because the cost of medications could affect whether they are prescribed to patients who could benefit from them, our goal was to study the impact of dapagliflozin treatment on short-term costs for patients in the United States with CKD. We used health insurance claims data to compare medical costs (sum of costs for treatment during hospital admissions and outpatient and emergency department visits) and pharmacy costs over 6 months between patients with stage 3 CKD treated with dapagliflozin with those for a matching group of patients who were not treated with dapagliflozin. The dapagliflozin group had a lower average medical cost for cardiorenal causes (related to CKD, including hospitalization for heart failure) paid by health insurance than the non-dapagliflozin group; the average cardiorenal medical cost patients paid themselves (out-of-pocket) was also lower for the dapagliflozin group. The average medical cost for all causes paid by insurance was also lower for the dapagliflozin group; this reduction was larger than the increase in the average all-cause pharmacy cost in the dapagliflozin group. Our study showed that treatment with dapagliflozin can lead to medical cost savings for patients with CKD.
1. Introduction
Chronic kidney disease (CKD) is associated with significant disease burden and is a growing public health concern. The United States (US) Centers for Disease Control and Prevention estimates that more than 37 million US adults (approximately 15%) have CKD [Citation1]. This is expected to increase as the number of individuals with risk factors for CKD, such as type 2 diabetes (T2D), hypertension, obesity, and older age, increases in the general population [Citation2–4].
Healthcare resource utilization and expenditure is higher for individuals with CKD than that for those without CKD, regardless of age (≥66 years or 18–65 years) or insurance type [Citation3]. Furthermore, progression of CKD is associated with increased healthcare resource utilization and costs [Citation3]. For all insurance types, annual per-patient healthcare costs in 2020 were higher for patients with stage 4–5 CKD (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) than those for patients with stage 3 CKD (eGFR 30–59 mL/min/1.73 m2), with the greatest increase among patients aged 18–65 years with commercial insurance [Citation3]. Hospitalization for heart failure (HF) is an important driver of medical cost for patients with CKD and is more likely with increased CKD severity [Citation5]. In a prospective cohort study, the rate of adjudicated primary hospitalization for HF was 3.1 per 100 person-years among patients with stage 3a CKD, compared with 7.4 and 10.3 per 100 person-years for patients with stage 3b and stage 4–5 CKD, respectively [Citation5]. Therefore, slowing the progression of CKD in patients with stage 3 CKD could reduce healthcare resource utilization and costs.
Sodium-glucose co-transporter-2 inhibitors (SGLT-2is), originally developed and approved as treatments for T2D, demonstrated additional clinical benefits with respect to cardiovascular and renal outcomes in clinical trials [Citation6–13]. Subsequent studies in patients with HF and CKD, including those without T2D [Citation11,Citation14–17], have led to the approval of some SGLT-2is for these indications [Citation18–20].
The SGLT-2i dapagliflozin showed potential benefit with respect to renal outcomes in DECLARE-TIMI 58, a cardiovascular outcomes trial in patients with T2D and established cardiovascular disease or multiple cardiovascular risk factors [Citation6,Citation7]. In this study, significantly fewer dapagliflozin-treated patients experienced a sustained decrease in eGFR (P < 0.0001), defined as a 40% decrease from baseline to <60 mL/min/1.73 m2, or progression to end-stage kidney disease (ESKD; P = 0.013) during a median follow-up of 4.2 years [Citation6].
The DAPA-CKD study clearly demonstrated that dapagliflozin improved renal outcomes in patients with CKD (eGFR 25–75 mL/min/1.73 m2) with or without T2D [Citation15]. There was a significant reduction in the composite primary outcome of sustained eGFR decline of ≥50%, ESKD, or death from renal or cardiovascular causes in patients treated with dapagliflozin 10 mg compared with that with placebo (P < 0.001), irrespective of diabetes status [Citation15]. In addition, dapagliflozin-treated patients had significant reductions in the secondary outcomes of the composite of sustained eGFR decline of ≥50%, ESKD, or death from renal causes (P < 0.001), the composite of death from cardiovascular causes or hospitalization for HF (P = 0.009), and all-cause mortality compared with the placebo group (P = 0.004) [Citation15]. A pre-specified analysis of the DAPA-CKD study found that dapagliflozin treatment reduced the rate of eGFR decrease over time compared with placebo, with greater effects in patient subgroups with more rapid progression of eGFR decline [Citation21].
The DAPA-HF study showed that dapagliflozin treatment significantly reduced the risk of the primary outcome of worsening of HF (hospitalization or urgent visit resulting in intravenous therapy for HF) or cardiovascular death in patients with HF and reduced ejection fraction compared with that with placebo (P < 0.001) [Citation22]. Differences in the primary outcome and hospitalization for HF were seen as early as within 3 months after dapagliflozin treatment was initiated, with lower cumulative incidences in the dapagliflozin group [Citation22]. A prespecified renal analysis of DAPA-HF found that the effects of dapagliflozin on the primary outcome (cardiovascular death or worsening of HF) did not vary according to baseline kidney function (eGFR <60 and ≥60 mL/min/1.73 m2, P = 0.54 for interaction) [Citation16]. Similarly, the efficacy of dapagliflozin treatment was consistent when analyzed with eGFR as a continuous variable (P = 0.77) [Citation16]. Although there was no significant difference in the composite renal outcome [Citation22], patients treated with dapagliflozin had a slower rate of eGFR decline than that in patients in the placebo group, regardless of baseline kidney function [Citation16].
The DELIVER study demonstrated that dapagliflozin significantly (P < 0.001) reduced the risk of the primary outcome of time to first occurrence of any component of the primary composite outcome of cardiovascular death or worsening HF (defined as unplanned hospitalization for HF or an urgent HF visit) in patients with HF with preserved or mildly reduced ejection fraction [Citation23]. The benefit of dapagliflozin for the primary outcome was seen within 2 weeks of treatment initiation, with the first nominal statistical significance at day 13 (P = 0.046) [Citation24]. In DELIVER, nearly half of the participants (49.0%) had an eGFR of <60 mL/min/1.73 m2, and the effect of treatment on the primary outcome was consistent across the range of baseline eGFR values [Citation23]. Among patients with baseline eGFR <60 mL/min/1.73 m2, the hazard ratio for the primary outcome was 0.81 (95% confidence interval [CI] 0.69–0.94) [Citation23].
Based on the DAPA-CKD study findings, the US Food and Drug Administration (FDA) approved dapagliflozin for the treatment of CKD in patients with or without T2D in April 2021 [Citation18]. Dapagliflozin is also indicated for improving glycemic control in patients with T2D, reducing the risk of hospitalization for HF in patients with T2D and established cardiovascular disease or multiple risk factors, and for treating HF in patients with or without T2D [Citation18].
Although SGLT-2is such as dapagliflozin have demonstrated clinical efficacy, medication costs may affect prescribing in clinical practice [Citation25–27]. Analyses using models have found that dapagliflozin was cost-effective in the long term in patients with non-diabetic CKD [Citation28] and in patients with CKD with or without T2D [Citation29]. The slowing of eGFR decline in the DAPA-HF and DAPA-CKD studies [Citation16,Citation21] and the early reduction in hospitalization for HF in DAPA-HF [Citation22] and DELIVER [Citation23] suggest that there is also potential for short-term cost benefit with dapagliflozin treatment in patients with CKD. However, there is a lack of real-world evidence regarding the impact of dapagliflozin on short-term medical costs in patients with CKD. The aim of this study was to describe the impact of dapagliflozin on short-term medical costs in patients with stage 3 CKD.
2. Patients and methods
2.1. Study design
This was a non-interventional, retrospective, observational cohort study using real-world data from patients with stage 3 CKD treated with dapagliflozin 10 mg (dapagliflozin cohort) and matched patients who did not receive dapagliflozin (non-dapagliflozin cohort). The study used de-identified patient-level medical and pharmacy claims data from the IQVIA PharMetrics Plus database, which is representative of the commercially insured US national population for patients under 65 years of age. The database contains medical and pharmacy claims and enrollment data for patients with commercial health insurance (national and sub-national plans as well as self-insured employer groups); more than 150 million patients have been enrolled in these plans since 2006 [Citation30].
This analysis used data from September 2019 through December 2021 to allow for a 12-month baseline period before clinicians were first able to prescribe dapagliflozin for the treatment of CKD in September 2020. Because top-line results of the DAPA CKD study were made public in August 2020, dapagliflozin prescriptions for patients with CKD after that time were considered for this study.
The index dates were defined as the date of the first prescription fill for dapagliflozin 10 mg after diagnosis of stage 3 CKD (dapagliflozin cohort) and the diagnosis date of stage 3 CKD (non-dapagliflozin cohort). The baseline period was 12 months before the index date and the follow-up period was 6 months after the index date (Supplementary Figure S1).
This study used de-identified data and was therefore considered exempt from institutional review board approval as per Title 45 Code of Federal Regulations, part 46, specifically 45 CFR 46.101(b) (4).
2.2. Study population
Inclusion and exclusion criteria are listed in Supplemental Table S1. Patients were required to be aged ≥18 years and to have a diagnosis of stage 3 CKD, identified through medical claims with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10 CM) diagnosis codes for stage 3 CKD, from September 2020 through December 2021. Insurance coverage for ≥12 months before and ≥6 months after the index date was also required. Patients were excluded if they had type 1 diabetes, were on dialysis, had been diagnosed with ESKD or polycystic kidney disease, were on immunosuppressive treatment for kidney disease, or had a diagnosis of COVID-19 prior to the index date. Patients in the dapagliflozin cohort were required to have filled a prescription for dapagliflozin 10 mg after a stage 3 CKD diagnosis. Patients who filled any prescription for SGLT-2is during the follow-up period were excluded from the non-dapagliflozin cohort. At the time of this study, dapagliflozin was the only SGLT-2i indicated for CKD; exclusion of patients treated with other SGLT-2is was intended to avoid possible off-label use of other SGLT-2is for CKD treatment. Patients in the top 0.5% percentile of medical cost were excluded from all cohorts before conducting the matching procedure, because the analysis was focused on medical costs, and health plan paid costs in real-world settings are sensitive to outliers.
A prespecified analysis of dapagliflozin new-users (no SGLT-2i treatment during the baseline period) was conducted because the relative likelihood that dapagliflozin 10 mg therapy was initiated in response to the stage 3 CKD diagnosis was greater for this subgroup than for the overall population.
2.3. Study endpoints
The primary objective was to evaluate the impact of dapagliflozin on cardiorenal (CKD-related, including hospitalization with HF) medical costs paid by the health plan (payer) during the follow-up period. Cardiorenal medical costs were defined as the sum of claims for inpatient hospitalizations, emergency department (ED) visits, and outpatient visits with a diagnosis code for CKD in any diagnosis position and claims for inpatient hospitalizations with a diagnosis code for HF in any diagnosis position. The cardiorenal medical cost included costs for hospitalization with HF because the US FDA-approved CKD indication for dapagliflozin specifically includes reduction of hospitalization for HF. The secondary objective was to describe the patient out-of-pocket (OOP) costs for cardiorenal-related medical claims. This was defined as the sum of deductibles, coinsurances, and copays paid by the patient for cardiorenal-related medical claims (i.e. CKD-related inpatient hospitalizations, outpatient visits, and ED visits and HF-related inpatient hospitalizations). Exploratory objectives were to describe all-cause medical costs for payers, defined as the sum of costs for inpatient hospitalization, ED visits, and outpatient visits, and to describe all-cause pharmacy costs for payers. All-cause pharmacy cost is limited to outpatient pharmacy utilization and the all-cause pharmacy cost to payers does not exclude rebates and discounts given to payers.
For all study endpoints, mean costs per patient over the 6-month follow-up period were described and compared between the dapagliflozin and non-dapagliflozin cohorts. Medical and pharmacy payer costs were based on health plan paid amounts (i.e. not on charged or billed amounts) and are presented in 2021 United States dollars (USD). The health plans collected and fully adjudicated the costs related to medical and pharmacy claims. All study endpoints were assessed in the overall population and the dapagliflozin new-user population. The results for the new-user subgroup were considered the primary results because excluding patients with baseline exposure to SGLT-2is increases the relative likelihood that dapagliflozin was initiated in response to the diagnosis of stage 3 CKD.
2.4. Statistical analysis
Sample size was calculated to provide 80% power to detect a statistically significant $2000 difference in cardiorenal medical costs between cohorts. The estimated required sample size was 524 patients treated with dapagliflozin and 524 matched control patients.
Eligible patients in the overall population were 1:1 propensity score matched on baseline characteristics to control for variables that may be strongly associated with filling a prescription for dapagliflozin and/or independently associated with medical cost outcomes; only the baseline variables in were used for propensity score matching. Patients who filled dapagliflozin prescriptions (dapagliflozin cohort) and patients with no dapagliflozin prescription fills (non-dapagliflozin cohort) were 1:1 matched using a caliper of 0.02 on the propensity score scale without replacement. Because dapagliflozin is indicated for treatment of T2D and HF in addition to CKD, patients were exact matched on baseline T2D and HF status before applying propensity score matching to reduce confounding by indications. Patients were also exact matched on new-user status to facilitate the new-user subgroup analyses. Therefore, the dapagliflozin new-user subgroup and corresponding non-dapagliflozin cohort represented a subset of the overall matched population. Both the dapagliflozin and non-dapagliflozin cohorts met the pre-defined study inclusion and exclusion criteria before exact and propensity score matching was conducted. All patients that met the study criteria were included, and the analyses were not based on a random sample of patients.
Table 1. Baseline patient demographics, clinical characteristics, medications, and healthcare resource utilization after propensity score matching.
Continuous variables were summarized as mean ± standard deviation (SD) and categorical variables were summarized using count and proportion. Medians were also reported for per-patient costs. Patient demographic, medical, and treatment characteristics during the 12-month baseline period were described and compared between cohorts. Covariate balance between matched cohorts was assessed using standardized differences; standardized mean differences of <0.2 (20%) indicated that the multivariate regression adjustment was adequate.
For all study endpoint assessments, mean per-patient costs were calculated for the 6-month follow-up period and compared between matched cohorts. All study endpoints were compared between the matched cohorts using one-way ANOVA (analysis of variance) tests to calculate P-values. Multivariate adjustment was achieved through 1:1 matching within each matched set of patients; therefore, no further multivariate regression adjustment was necessary to detect statistically significant differences in costs.
All analyses were performed within the Instant Health Data (IHD) platform (Panalgo, Boston, MA) that was integrated with R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patients
Claims data were available for 2353 patients with a dapagliflozin prescription fill and 114,175 patients with no dapagliflozin prescription fills. Of these, 1149 patients with a dapagliflozin prescription fill and 31,560 patients with no dapagliflozin prescription met all inclusion criteria (). After propensity score matching, the overall population comprised 783 patients treated with dapagliflozin and 783 matched patients not treated with dapagliflozin; the new-user subgroup included 503 patients who were treated with dapagliflozin and 503 matched patients who were not ().
Figure 1. Patient disposition before propensity score matching (overall population and new-user subgroup).
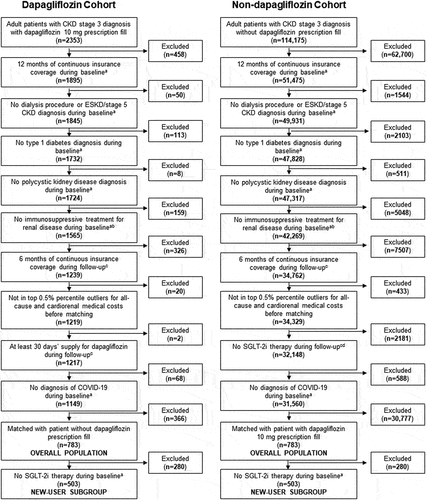
Baseline patient demographic and clinical characteristics, medication use, and healthcare resource utilization are summarized in . The dapagliflozin and non-dapagliflozin cohorts were well balanced after propensity score matching. The standardized mean differences between cohorts were <0.2 for all variables in the overall population and new-user subgroup, indicating good balance between matched pairs.
In the dapagliflozin and non-dapagliflozin cohorts of the overall population and the new-user subgroup, the majority of the patients were male, and the mean age in each cohort ranged from 63.8–66.5 years (). The majority of the patients had commercial insurance (59.6%–71.5%) and the remainder had Medicare Advantage coverage. In both the overall population and the new-user subgroup, the most common comorbidities were hypertension (94.5%–96.4%), T2D (79.7%–86.7%), and dyslipidemia (81.5%–86.2%). Because of exact matching for T2D and HF, the proportions of patients with these conditions were the same in the dapagliflozin and non-dapagliflozin cohorts. In the overall population, 86.7% of patients had T2D and 33.5% of patients had HF; in the new-user subgroup, 79.7% of patients had T2D and 43.1% had HF. At baseline, 71.2%–76.1% of patients in the dapagliflozin and non-dapagliflozin cohorts of the overall population and new-user subgroup were on renin-angiotensin-aldosterone system inhibitor (RAASi) therapy. During the follow-up period, 62.1%–66.8% of patients in the dapagliflozin and non-dapagliflozin cohorts of the overall population and new-user subgroup were on RAASi therapy.
3.2. Cardiorenal (CKD-related, including hospitalization with HF) medical costs
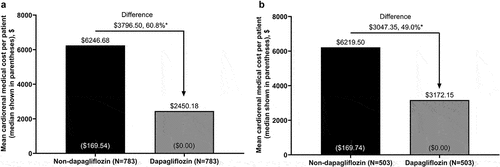
In the overall population, there was a significant reduction of $3796.50 (95% CI $2083.99–$5509.91; P < 0.001; 60.8% reduction) in the mean per-patient cardiorenal medical cost over 6 months for the dapagliflozin versus non-dapagliflozin cohort (). In the new-user subgroup, there was a significant reduction in the mean per-patient cardiorenal medical cost of $3047.35 (95% CI $838.28–$5256.43; P < 0.001; 49.0% reduction) for the dapagliflozin versus non-dapagliflozin cohort ().
Figure 2. Cardiorenal medical costa to payers during 6 months’ follow-up for propensity score-matched patients with stage 3 CKD in the dapagliflozin and non-dapagliflozin cohorts in the (a) overall population and (b) new-user subgroup.
3.3. Patient OOP (out-of-pocket) costs
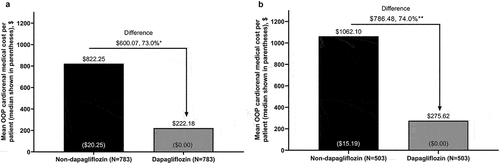
Patient OOP cardiorenal medical costs were significantly reduced for the dapagliflozin versus non-dapagliflozin cohort in the overall population and the new-user subgroup. Mean per patient OOP cardiorenal medical cost was reduced by 73.0% (reduction of $600.07, 95% CI $182.95–$1017.18; P = 0.005) in the overall population () and by 74.0% (reduction of $786.48, 95% CI $150.44–$1,422.53; P = 0.02) in the new-user subgroup ().
Figure 3. OOP cardiorenal medical costa to patients during 6 months’ follow-up for propensity score-matched patients with stage 3 CKD in the dapagliflozin and non-dapagliflozin cohorts in the (a) overall population and (b) new-user subgroup.
3.4. All-cause medical and pharmacy costs
In the overall population, all-cause medical costs (sum of costs for inpatient hospitalizations, ED visits, and outpatient visits) were significantly reduced for patients treated with dapagliflozin versus non-dapagliflozin-treated patients, with a 40.0% reduction in mean per-patient cost of $4742.72 (95% CI $2597.51–$6887.92; P < 0.001) over 6 months (). All-cause pharmacy costs were higher for the dapagliflozin versus non-dapagliflozin cohort, with a 36.4% increase in mean per-patient cost of $2401.53 (95% CI $705.90–$4097.15; P = 0.01) ().
Figure 4. All-cause medical and pharmacy cost to payers during 6 months’ follow-up for propensity score-matched patients with stage 3 CKD in the (a) overall population and (b) new-user subgroup.
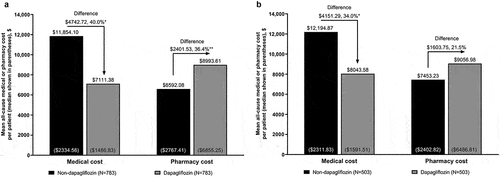
In the new-user subgroup, there was a significant reduction of $4151.29 in the mean all-cause medical cost per patient (95% CI $1338.73–$6963.85; P < 0.001; 34.0% reduction) (). The mean all-cause pharmacy cost per patient increased by 21.5% (increase of $1603.75; 95% CI −$950.41–$4157.91; P = 0.22) in the dapagliflozin versus non-dapagliflozin cohort (); however, this increase was not statistically significant.
4. Discussion
SGLT-2is have become increasingly important in the management of CKD and HF, including in patients without T2D. At the time of this publication, dapagliflozin is the only US FDA-approved treatment for CKD in patients with or without T2D after demonstrating efficacy in the DAPA-CKD study [Citation15,Citation18].
To our knowledge, this is the first study focused on the short-term medical cost impact of dapagliflozin in a real-world population of US patients with stage 3 CKD. This real-world study analyzed costs for commercially insured patients with stage 3 CKD with or without dapagliflozin 10 mg treatment. In our retrospective study, treatment with dapagliflozin 10 mg was associated with significant reductions in cardiorenal medical costs to payers over a 6-month follow-up period, irrespective of SGLT-2i use during the baseline period (P < 0.001 for both the overall and new-user populations). Patient OOP cardiorenal medical costs were also significantly reduced in the overall population (P = 0.005) and in patients without baseline SGLT-2i use (P = 0.02).
Our study also included an exploratory analysis of all-cause medical costs to provide context for the cardiorenal medical cost results. All-cause medical costs to the payer were significantly reduced in the dapagliflozin versus non-dapagliflozin cohorts in the overall population and new-user population (P < 0.001 in both). In both the overall population and the new-user subgroup, there was an increase in mean all-cause pharmacy costs for the dapagliflozin versus non-dapagliflozin cohort. These increases in mean all-cause pharmacy costs were smaller than the reductions in mean all-cause medical costs. Actual all-cause pharmacy costs were lower than those reported in this study because rebates and discounts to the payer were not excluded. Therefore, the medical cost offset to payers can be larger than that reported in this study.
The results of our study suggest that treatment with dapagliflozin 10 mg is associated with short-term medical cost savings in patients with stage 3 CKD. Because CKD is a relatively new indication for dapagliflozin, there are few published studies investigating its impact on treatment costs. Previous studies have used models to analyze the long-term effects of dapagliflozin on costs in patients with CKD. A study of dapagliflozin in patients in the US with non-diabetic CKD, which used a model based on results from the subgroup of patients without T2D in the DAPA-CKD study, determined that dapagliflozin given in addition to standard care was cost-effective [Citation28]. The addition of dapagliflozin increased total discounted lifetime healthcare costs by $79,000 (corresponding to $60,000 per quality-adjusted life-year [QALY] gained), which was below the willingness-to-pay threshold of $100,000 per QALY gained [Citation28].
Although the periodic monitoring of a patient’s basic metabolic panel (including serum creatinine and eGFR) is recommended following RAASi initiation, guidelines do not recommend routine additional assessment of renal function following initiation of SGLT-2i therapy [Citation31,Citation32]. Dose-adjustment or titration of dapagliflozin therapy for CKD is not required, as shown in the pivotal DAPA-CKD clinical trial and the FDA-approved CKD indication for dapagliflozin [Citation15,Citation18]. In addition, initiation of SGLT-2i therapies, including dapagliflozin, does not contribute to electrolyte imbalances (e.g. hyperkalemia) [Citation32–34]. Therefore, monitoring of basic metabolic panel and fluid status is not typically required following the initiation of SGLT-2i therapy [Citation32]. These are some of the key reasons why the median cardiorenal medical cost paid by payers for patients treated with dapagliflozin was zero dollars, whereas patients with diagnosed CKD and not treated with SGLT-2i therapy had a small median cardiorenal medical cost (<$170 per patient over 6 months) that was commensurate to outpatient expenses related to monitoring. The need to avoid excessive monitoring of patients and overburdening of clinicians and their care teams where patient access is typically limited is another advantage of dapagliflozin therapy initiation in patients with early-stage CKD (i.e. stage 3 CKD).
A strength of our study is that it included a real-world population of insured patients with stage 3 CKD who would have been eligible for dapagliflozin treatment, which could include patients not typically included in clinical trials. In addition, the IQVIA PharMetrics Plus database captures claims for a large proportion of the US commercially insured population [Citation30]. Therefore, the study results may be more reflective of real-world clinical practice and associated short-term medical costs than results of analyses using modeling based on clinical trial data. Another strength is the use of propensity score matching to minimize potential treatment influences and ensure tight control of baseline variables (i.e. patient demographics, baseline comorbidities, healthcare resource use, and medications) between the dapagliflozin and non-dapagliflozin cohorts.
This study has potential limitations inherent in the retrospective design and the use of claims data. Dapagliflozin has multiple approved indications (T2D, HF, and CKD) in the US [Citation18]. Because pharmacy claims cannot provide the diagnosis or indications for written prescriptions, it is not possible to confirm from claims data that dapagliflozin 10 mg was prescribed specifically for the treatment of CKD. To increase the relative likelihood that dapagliflozin 10 mg had been prescribed to treat stage 3 CKD, we also analyzed the study endpoints in patients with no SGLT-2i prescriptions during the baseline period (new-user subgroup).
Claims data can be affected by coding limitations; therefore, it is possible to misidentify disease status, study outcomes, and covariates. Furthermore, the use of pharmacy-filled generic medications or samples of branded medications from physicians that was not billed to insurers is not captured in claims data. The claims data source used in this study is not generalizable to the Medicare (age ≥65 years) population; therefore, caution is required when applying the study results to older patients.
Given the recent approval of dapagliflozin 10 mg for the treatment of CKD, only 6 months of patient follow-up through claims data was feasible. Future research with a longer follow-up period may become possible as the real-world uptake of dapagliflozin in patients with CKD is growing over time. Because this study was based on insurance claims and continuous coverage was required throughout the baseline and follow-up periods, the results do not capture uninsured patients and may not be generalizable to those who lost or had a change in coverage. This study was limited to patients with stage 3 CKD. Although some patients with a stage 4 CKD diagnosis would meet the eGFR criteria for dapagliflozin 10 mg treatment (≥25 mL/min/1.73 m2) [Citation18], these patients could not be captured because claims data do not include eGFR values. The lack of eGFR values in claims data has also limited the options to identify alternative index dates for patients not treated with dapagliflozin and is another study limitation. Because the diagnosis code for stage 3 CKD covers a broad eGFR range (between 30 and 59 mL/min/1.73 m2), relying on the timing of diagnosis and treatment to define index dates for the non-dapagliflozin and dapagliflozin cohorts, respectively, can introduce differential bias in exposure between the two cohorts. Therefore, future research that can index and match on eGFR values is suggested. Additional areas for future research include active comparator designs.
Cardiorenal medical cost was defined as medical claims for hospitalization, ED visit, or outpatient visit with a diagnosis of CKD or hospitalization with a diagnosis of HF where the diagnosis was in any claim position. Given the reimbursement landscape in the US, CKD or HF may not be always in the primary diagnosis position within claims data; thus, the cost analysis is not restricted to encounters with CKD or HF in the primary diagnosis positions. Moreover, clinicians who are more likely to prescribe dapagliflozin may also be those who are more likely to attain lower cardiorenal medical cost. These provider-level aspects may lead to overestimation of the cardiorenal medical cost reduction attributable to the initiation of dapagliflozin. Future research can explore cardiorenal medical costs in different care settings (inpatient admissions, ED visits, and outpatient visits).
Because the diagnosis code for stage 3 CKD does not distinguish between stage 3a CKD and stage 3b CKD, the lack of eGFR data can lead to residual confounding that can result in differences in the quality of care being delivered. If dapagliflozin was preferentially prescribed to patients with stage 3a CKD (eGFR between 45 and 59 mL/min/1.73 m2), there is potential for overestimation of the treatment effect of dapagliflozin compared with the non-dapagliflozin group. This would be anticipated to bias the study results in favor of showing medical cost savings with dapagliflozin treatment. Alternatively, if dapagliflozin was preferentially prescribed for stage 3b CKD (eGFR between 30 and 44 mL/min/1.73 m2), there is potential for underestimation of the treatment effect of dapagliflozin compared with the non-dapagliflozin group. This would be expected to favor the non-dapagliflozin cohort showing lower medical costs than in the dapagliflozin cohort.
5. Conclusions
This real-world retrospective cohort study found that dapagliflozin 10 mg was associated with reduced cardiorenal medical costs over 6 months in patients with stage 3 CKD, including in patients with no prior SGLT-2i treatment during the baseline period. Dapagliflozin 10 mg was also associated with significant reductions in all-cause medical costs in the overall population and the new-user subgroup. Although there was an increase in all-cause pharmacy costs in the dapagliflozin versus non-dapagliflozin cohorts of both populations, this was offset by the reduction in all-cause medical costs. This research study demonstrates medical cost savings with dapagliflozin therapy in patients with stage 3 CKD and shows that the effectiveness of dapagliflozin in real-world clinical practice can be realized in medical cost savings.
Declaration of interest
J Dwyer has acted as a scientific consultant for AstraZeneca and has received fees from AstraZeneca for the conduct of this study; has received fees from Sanofi and CSL Behring as part of a steering committee; has received fees from Novo Nordisk for outcome adjudication for a trial; has received fees from Boehringer-Ingelheim and Lilly for study design; and received personal fees from Bayer. A Agiro and P Desai are employees and stockholders of AstraZeneca. H Cremisi was an employee and stockholder of AstraZeneca at the time of the study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Study concept and design: J Dwyer, A Agiro, P Desai, H Cremisi. Data analysis: A Agiro. Data interpretation: J Dwyer, A Agiro, P Desai, H Cremisi. Drafting of the manuscript and/or revising it for critical intellectual content: J Dwyer, A Agiro, P Desai, H Cremisi. Final approval of the manuscript version to be published: J Dwyer, A Agiro, P Desai, H Cremisi. All authors agree to be accountable for all aspects of the work. All authors read and approved the final version of the manuscript to be published.
Ethical conduct of research
This study used de-identified data and was therefore considered exempt from institutional review board approval as per Title 45 Code of Federal Regulations, part 46, specifically 45 CFR 46.101(b) (4).
Prior presentation
Jamie P. Dwyer, Abiy Agiro, Pooja Desai, and Henry Cremisi. Short-term cost impact of dapagliflozin in chronic kidney disease. Poster presentation at the National Kidney Foundation (NKF) 2023 Spring Clinical Meeting, April 11–15, Austin, TX.
Supplemental Material
Download MS Word (51.3 KB)Acknowledgments
Daniel R. Turkewitz, PhD, and Raewyn M. Poole, MSc, of inScience Communications, Springer Healthcare, provided medical writing support in accordance with Good Publication Practice, funded by AstraZeneca. The authors wish to thank Joanna Huang and Richard Kraft, of AstraZeneca (Wilmington, Delaware, United States) for their contributions to the study concept and design.
Data availability statement
This was a claims database analysis using IQVIA PharMetrics Plus closed claims data obtained under license from IQVIA Inc. The raw data cannot be publicly shared since it was obtained from IQVIA and as per signed agreement between AstraZeneca and IQVIA Inc. However, we have provided all relevant data in the manuscript that supports the research objectives and conclusions. We confirm that interested researchers can reach out to IQVIA Inc. to access the data. For further information on data access, please contact IQVIA.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2237679.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2021 [Internet]. 2021. [cited 2022 Dec 9]. Available from: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2011;12(1):7–11. doi: 10.1016/j.kisu.2021.11.003
- U.S. Renal Data System. 2022 USRDS annual data report: epidemiology of kidney disease in the United States [Internet]. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2022 [cited 2022 Dec 9]. Available from. https://usrds-adr.niddk.nih.gov/2022
- Centers for Disease Control and Prevention. Chronic kidney disease (CKD) surveillance system [Internet]. [cited 2023 Mar 7]. Available from: https://nccd.cdc.gov/ckd/FactorsOfInterest.aspx?type=Age
- Bansal N, Zelnick L, Bhat Z, et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73(21):2691–2700. doi: 10.1016/j.jacc.2019.02.071
- Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–617. doi: 10.1016/S2213-8587(19)30180-9
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925
- Neuen BL, Ohkuma T, Neal B, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol. 2019;30(11):2229–2242. doi: 10.1681/ASN.2019010064
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744
- The Empa-Kidney Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388(2):117–127.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816
- Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143(4):298–309. doi: 10.1161/CIRCULATIONAHA.120.050391
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190
- AstraZeneca. Farxiga® (dapagliflozin) tablets, for oral use [prescribing information]. [Internet]. 2023. [cited 2023 Jul 18]. Available from: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/0be9cb1b-3b33-41c7-bfc2-04c9f718e442/0be9cb1b-3b33-41c7-bfc2-04c9f718e442_viewable_rendition__v.pdf
- Boehringer Ingelheim. Jardiance® (empagliflozin tablets), for oral use [prescribing information] [Internet]. 2022. [cited 2022 Dec 9]. Available from: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf
- Janssen Pharmaceuticals. Invokana (canagliflozin) tablets, for oral use [prescribing information] [Internet]. 2022 [cited 2022 Dec 9]. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVOKANA-pi.pdf
- Heerspink HJL, Jongs N, Chertow GM, et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):743–754. doi: 10.1016/S2213-8587(21)00242-4
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303
- Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–1098. doi: 10.1056/NEJMoa2206286
- Vaduganathan M, Claggett BL, Jhund P, et al. Time to clinical benefit of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified secondary analysis of the DELIVER randomized clinical trial. JAMA Cardiol. 2022;7(12):1259–1263. doi: 10.1001/jamacardio.2022.3750
- Rossing P, Caramori ML, Chan JC, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5):S1–S127. doi: 10.1016/j.kint.2022.06.008
- Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876–e894.
- ElSayed NA, Aleppo G, Aroda VR, et al. 11. Chronic kidney disease and risk management: standards of care in diabetes—2023. Diabetes Care. 2023;46(Suppl 1):S191–S202. doi: 10.2337/dc23-S011
- Tisdale RL, Cusick MM, Aluri KZ, et al. Cost-effectiveness of dapagliflozin for non-diabetic chronic kidney disease. J Gen Intern Med. 2022;37(13):3380–3387. doi: 10.1007/s11606-021-07311-5
- McEwan P, Darlington O, Miller R, et al. Cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: a health-economic analysis of DAPA-CKD. Clin J Am Soc Nephrol. 2022;17(12):1730–1741. doi: 10.2215/CJN.03790322
- IQVIA. IQVIA PharMetrics® Plus Fact Sheet [Internet]. 2020 [cited 2022 Dec 2]. Available from: https://www.greygreenmedia.com/app/uploads/sites/2/2020/09/IQVIA-PharMetrics-Plus-US-Fact-Sheet.pdf
- de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–3090. doi: 10.2337/dci22-0027
- Evans M, Morgan AR, Whyte MB, et al. New therapeutic horizons in chronic kidney disease: the role of SGLT2 inhibitors in clinical practice. Drugs. 2022;82(2):97–108. doi: 10.1007/s40265-021-01655-2
- Tang H, Zhang X, Zhang J, et al. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546–2551. doi: 10.1007/s00125-016-4101-6
- Yavin Y, Mansfield TA, Ptaszynska A, et al. Effect of the SGLT2 inhibitor dapagliflozin on potassium levels in patients with type 2 diabetes mellitus: a pooled analysis. Diabetes Ther. 2016;7(1):125–137. doi: 10.1007/s13300-015-0150-y
