ABSTRACT
Background
Healthcare administrative and pathological anatomy data were used to identify Italian patients with early breast cancer (EBC) with HR+/HER2- status at high risk of recurrence, evaluating drug utilization and other healthcare resource use in clinical practice.
Methods
This retrospective analysis, based on 9.4 million of Italian National Health Service beneficiaries, included adult patients with hospitalization discharge diagnosis for EBC in 01/2015–12/2020. Those with HR+/HER2- status were selected; among them, patients that underwent removal of lymph nodes (LN) were analyzed.
Results
Of 24,137 patients with EBC and HR+/HER2- status, 3619 patients (15%) had documented LN removal. Overall, 4.7% of HR+/HER2- patients and 9.9% of patients with LN removal experienced distant relapse over a median follow-up of 33.2 months (Q1-Q3: 17.0–50.6). Local relapse occurred in approximately 9.1–9.3% of patients in each group. Among the 1,175 patients with LN removal that had available pathological anatomy data, 399 (34.0%) had pathological high-risk characteristics and 13.3% experienced distant relapse.
Conclusions
One in ten patients with EBC who underwent LN removal experienced a relapse, highlighting the strong need to prevent early recurrence.
1. Introduction
Worldwide estimations indicate that breast cancer is the most commonly diagnosed cancer among females and the leading cause of cancer death [Citation1]. Similarly, the Italian Cancer Registry Association (Associazione Italiana Registri Tumori, AIRTUM) reports that breast cancer is the most frequent neoplasia in women, accounting for 30% of all female cancers in Italy [Citation2]. To date, thanks to screening campaigns and organized programs that have improved women’s awareness, most breast cancers are diagnosed at an early stage when surgical treatment is more often conservative and therapy is more effective [Citation2]. Indeed, about 90% of patients with breast cancer have early-stage disease (Early Breast Cancer, EBC) [Citation3]. Breast cancer can be classified into major subtypes based on the expression of estrogen or progesterone hormone receptors (HR) and human epidermal growth factor 2 receptor (HER2): the most frequent subtype is represented by HR-positive/HER2-negative (HR+/HER2-) breast cancer, which is observed in approximately 70% of patients, followed by HER2+ breast cancer (15–20%) and triple-negative breast cancer (TNBC 15%) [Citation3,Citation4].
Treatments of EBC involve a combination of local modalities, such as surgery and radiotherapy, and systemic treatments (neoadjuvant/adjuvant chemotherapy (CT) and endocrine therapy), with the aim of eradicating tumor cells and preventing local and metastatic recurrence [Citation5,Citation6]. Regarding the HR+/HER2- subtype, current guidelines indicate that adjuvant endocrine therapy (with or without CT) is the standard treatment and has been associated with a lower risk of recurrence and death [Citation5,Citation7,Citation8]. Nevertheless, a recent meta-analysis reported that 1 out of 6 women with node-positive HR+/HER2- EBC incurs recurrence or death within 5 years after the start of therapy [Citation9].
Interest in real-world data analysis in the field of oncology has been increasing in recent years. These data indeed allow for the generation of evidence to address many of the questions arising in cancer management by using information from daily clinical practice to evaluate the quality of care at a population level [Citation10,Citation11]. Moreover, using administrative data (which include pharmacy, outpatient and lab/test datasets) is an established approach to describe the clinical management of cancer patients, with the innovative contribution of pathological anatomy databases [Citation12].
In this context, our aim is to provide an overview of the current management of care of patients with EBC and HR+/HER2- status, that could be helpful from an organizational standpoint for the health policy decision-making. By using both administrative data and pathological anatomy databases, the real-world analysis herein reported described the characteristics of patients with EBC with HR+/HER2- status in the setting of clinical practice in Italy and identified, among them, those at high risk of recurrence following the definition used in the monarchE study [Citation7]; evaluated the drug utilization and treatment/procedure patterns in terms of therapies prescribed and provided a cost description for these patients in the perspective of the Italian National Health Service (INHS).
2. Methods
2.1. Study design and data source
A retrospective study was conducted across Italian Local Health Units (LHUs), based on administrative databases of healthcare resource consumption and pathological anatomy databases. According to the objectives of the analysis, the administrative databases queried, available since January 2010 to December 2020 (study period), were demographic database containing all the demographic data of patients in the analysis; the pharmaceutical database including data on all drugs prescribed and reimbursed by the INHS for outpatient use based on the Anatomical-Therapeutic Chemical (ATC) code, number of packages, number of units per package, unit cost per package, and prescription date; the hospitalization database containing data on all hospitalization along with discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), Diagnosis Related Group (DRG) and DRG related tariffs; and the outpatient specialist service database, i.e. laboratory tests and specialist visits database, containing all data about diagnostic tests and visits for patients included in the analysis. The data collected from the administrative databases were linked with those retrieved from the pathological anatomy database using the anonymous univocal numeric code assigned by the INHS. The pathological anatomy database is available in some LHUs only and contains the histopathological examination data of the patients.
To protect patient privacy, the electronic linkage across all databases can be performed through the anonymous univocal numeric code of each beneficiary (Patient ID). This is the only patient identifier adopted. The use of anonymous codes ensured the anonymity of the extracted data to maintain full compliance with UE Data Privacy Regulation 2016/679 (‘GDPR’) and Italian D.lgs. n. 196/2003, as amended by D. lgs. n. 101/2018 [Citation13]. Results were produced exclusively in aggregate, so it was not possible to identify from the extracted data, either directly or indirectly, the patients involved in the analyses. According to the pronouncement of the Data Privacy Guarantor Authority (General Authorization for personal data treatment for scientific research purposes – n.9/11 December 2014th – published on the Official Gazette n. 301 on 30 December 2014) data treatment is authorized without informed consent when the acquisition of patient consent is impossible due to organizational reasons. The project from which these analyses were drawn was approved by the Ethics Committee of each participating LHU (see Appendix 1).
2.2. Study population
All adult (≥18 years old) female patients with at least one hospitalization discharge diagnosis – main or secondary – for breast cancer (ICD-9-CM code: 174, malignant neoplasm of female breast) from January 2015 to December 2020 (index period) were included. The date of first hospitalization for breast cancer within the inclusion period was the index date. To identify EBC, patients with the presence of distant metastases (defined as at least a discharge diagnosis with ICD-9-CM codes 197, secondary malignant neoplasm of respiratory and digestive systems or 198, secondary malignant neoplasm of other specified sites) or a prescription for drugs indicated for advanced or metastatic disease [i.e. CDK 4/6 inhibitors palbociclib (ATC code: L01XE33), abemaciclib (ATC code: L01XE50), or ribociclib (ATC code: L01XE42), which were available in Italy from 2017] at the index date or before the index date were excluded.
Among the patients with EBC, those with at least one surgery code for quadrantectomy/lumpectomy (ICD-9-CM codes 85.2–85.3) or mastectomy (ICD-9.CM 85.4) were selected. Afterward, patients with HR+/HER2- status were identified (HR+/HER2- cohort). HR+/HER2- status was defined by the presence of HR+ therapy (endocrine therapy) and absence of HER2 targeted therapy. In detail, HR+ status was defined as the administration of endocrine therapy with tamoxifen (ATC code: L02BA01), aromatase inhibitors (ATC code: L02BG), and fulvestrant (ATC code: L02BA03) from the index date to all available follow-up dates. HER2- status was identified by the absence of trastuzumab (ATC code: L01XC03), lapatinib (ATC code: L01XE07), pertuzumab (ATC codes: L01XC13, L01XY02), and neratinib (ATC code: L01EH02) from the treatment plan during the entire available study period. The algorithm of identification of the HR+/HER2- status was previously used in literature [Citation14].
Among the HR+/HER2- patients, those that underwent lymph node (LN) removal were identified by presence of at least one surgery code for lymphadenectomy (including sentinel lymph nodes) (ICD-9-CM code 40.2–40.5).
To identify the high risk of recurrence population close to monarchE inclusion criteria [Citation7], overcoming the limitations associated with missing information on number of positive lymph nodes (LN) in pathological reports, a proxy based on the administrative data was adopted: specifically, all patients with LN removal (ICD-9-CM code 40.3–5) were analyzed, considering this procedure as a proxy indicator of LN involvement and, therefore, associated with high risk of recurrence.
2.3. Sensitivity analysis
To sustain robustness of results, a sensitivity analysis was performed within patients with LN removal, identifying those with available data in the pathological anatomy database and, among them, the subgroup with pathological high-risk characteristics as patients ≥4 positive LN or, if 1–3 positive LN, either grade 3 disease, tumor size >5 cm or Ki-67 index >20% [Citation7].
All patients were characterized during 12-months before index-date (pre-index period) and followed-up from the index date to the most recent available date among date of death, date they exited the database or the end of data availability, whichever occurred first.
2.4. Study variables
2.4.1. Baseline characteristics
The age at the index date is expressed as the mean and standard deviation and by the distribution of patients in age ranges for HR+/HER2- patients overall and for those with LN removal only. Clinical characteristics are evaluated during pre-index period by using the Charlson comorbidity index (CCI) [Citation15], which assigns a score to each concomitant disease (assessed during the pre-index period in terms of drug treatment and hospitalizations [Citation15]; therefore, untreated/unhospitalized comorbidities are not captured). A modified version of the CCI not accounting for cancer was applied. The index score is the sum of the weights for all identified conditions. An index score of 0 indicates no comorbid conditions, while higher scores indicate a greater level of comorbidity. Usually, Charlson index scores of 1–2 indicate mild comorbidities; CCI scores of 3–4 indicate moderate comorbidities; and CCI scores ≥5 indicate severe comorbidities [Citation15].
2.4.2. Treatments in analysis
The treatments in the adjuvant setting were endocrine therapy, as reported in the previous section, and CT (identified by DRG code: 410 or hospital/ambulatory procedures 99.25, 99.28, 99.29 or ATC code L01, excluding CDK 4/6 inhibitors). The type of conservative surgery performed was retrieved using ICD-9-CM codes 85.2–85.3, and mastectomy was retrieved using surgery code ICD-9-CM 85.4. Distant relapse was defined as the presence of distant metastasis (identified by the presence of ICD-9-CM codes 197, 198) or the administration of CDK 4/6 inhibitors during follow-up. In a sub-analysis, the presence of nonspecific CT at 8 months postdiagnosis (post index-date) was also considered for the definition of distant relapse. In the absence of relapse criteria, local relapse was defined as undergoing another breast surgery with curative intent (ICD-9-CM codes 85.2, 85.3, 85.4) from the index date to all available follow-up dates.
2.4.3. Cost description
A cost description has been performed [Citation16,Citation17]. The mean annual direct healthcare costs were evaluated in Euros (€) among alive patients during the first year of follow-up in terms of the drug treatments prescribed (related and unrelated to EBC), all-cause hospitalizations, specialist visits, and diagnostic services. Healthcare cost description was performed from the perspective of the INHS by extrapolating costs from the administrative databases. Drug costs were evaluated using the INHS purchase price. Hospitalization costs were determined using DRG tariffs (regional), which represent the reimbursement levels by the INHS to healthcare providers. Healthcare costs related to specialist visits, and diagnostic services were defined according to the tariffs of each region (called Nomenclatore tariffario regionale).
2.5. Statistical analysis
The results are reported for the overall study cohort of patients with EBC (n = 38,837), the HR+/HER2- patients (n = 24,137) and, among them, those with LN removal (n = 3,619). The sensitivity analysis is reported for the cohort of patients with pathological high-risk characteristics (n = 399). Continuous variables are reported as the mean ± standard deviation (SD); categorical variables are expressed as count and percentages. All analyses were performed using STATA SE version 12.0/SE (StataCorp, College Station, TX, U.S.A.).
3. Results
The population consisted of 9.4 million of beneficiaries of INHS (female population: 4.8 million), corresponding to approximately 15.7% of the entire Italian population, covered by the administrative databases used in this study.
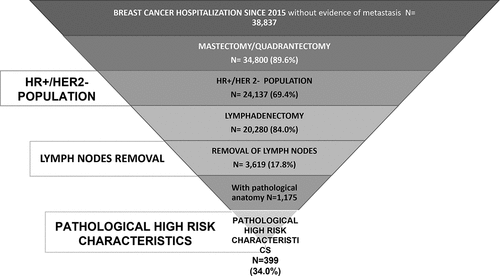
A total of 38,837 adult female patients with EBC were included (). Of them, approximately 90% underwent mastectomy/quadrantectomy. A total of 24,137 (69%) patients were deemed to have HR+/HER2- status, among whom 20,280 underwent lymphadenectomy.
A total of 3,619 Patients with LN removal were identified, representing 15% of all patients with HR+/HER2- EBC.
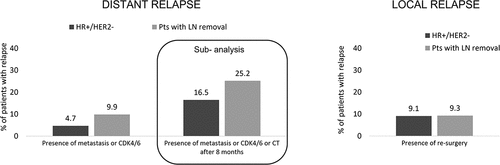
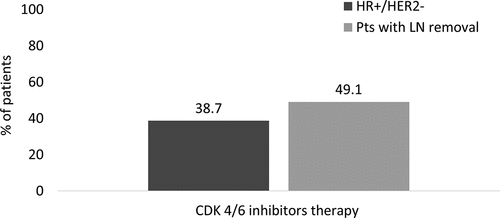
The demographic and clinical characteristics of the patients with EBC overall, of those included in the HR+/HER2- and in the LN removal cohorts are reported in . The mean age at the index date was 62–64 years among all cohorts considered, with a similar proportion of patients in each age range. Previous hospitalizations related to breast cancer (during pre-index period) were noted for 5.6% of all patients, 4.4% of HR+/HER2- patients and 7.2% of patients with LN removal. HR+/HER2- patients were followed up for a mean of 37 months [median (Q1-Q3), 36.9 (21.2–52.7) months], with those having LN removal being followed for 38.5 [38.7 (22.4–54.5) months]. Quadrantectomy was the most common conservative surgery performed among HR+/HER2- patients (63.7%); in patients with LN removal was 62.4% (). Approximately 64% of HR+/HER2- and 44.5% of patients with LN removal received endocrine therapy only in adjuvant settings, while concurrent CT (neoadjuvant/adjuvant) and endocrine therapy was administered to over half of the patients with LN removal (54.2%) and in 35.2% of the overall HR+/HER2- cohort (not-shown). Over a median follow-up of 33.2 months (Q1-Q3: 17.0–50.6), 4.7% of HR+/HER2- patients and 9.9% of patients with LN removal experienced distant relapse after a mean period of 1.9 years [median (Q1-Q3), 1.7 (0.7–2.9)] in HR+/HER2-)] and 1.9 years [1.7 (0.8–2.9)] respectively (). When the presence of CT at 8 months post index date was considered a proxy indicator for the definition of distant relapse, the proportion of relapsing patients increased to 16.5% (HR+/HER2-) and 25.2% (patients with LN removal). Local relapse occurred in approximately 9.1–9.3% of patients in each cohort. In both populations, the most frequent sites of distant relapse reported in the database were bone (1.6% HR+/HER2-, 3.4% in patients with LN removal), liver (1.3% HR+/HER2-, 3.0% p with LN removal) and respiratory organs (1.0% HR+/HER2-, 1.9% with LN removal) (). After distant relapse, 38.7% of HR+/HER2- and 49% patients with LN removal started a therapy with CDK 4/6 inhibitors, respectively ().
Figure 2. The most frequent type of conservative surgery and mastectomy among HR+/HER2- patients and those with LN removal.
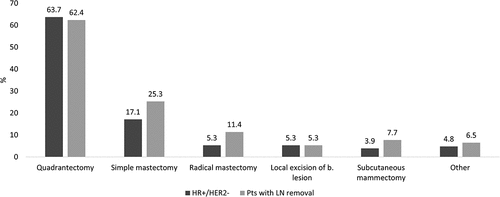
Table 1. Demographic and clinical characteristics of the study population.
Table 2. Presence of metastasis at distant relapse.
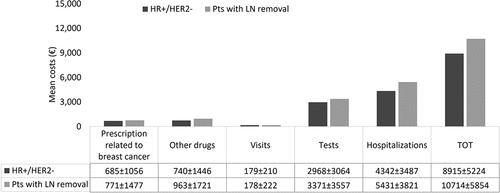
During the first year of follow-up, the mean total annual healthcare cost per HR+/HER2- alive patient (N = 21,635, corresponding to 89.6% of the overall HR+/HER2- cohort) was €8,915, which was mainly related to the all-cause hospitalization cost (€4,342) and medical tests (€2,968) (). Among the patients with LN removal the mean total cost was €10,714 (N = 3,539, corresponding to 98% of the overall group) (). Hospitalization was also the major driver in these cohorts (€ 5,431), followed by medical tests (€ 3,371).
Figure 5. Mean annual direct costs during the first year of the follow-up period among alive patients with HR+/HER2- status (N = 21,365) and among those with LN removal (N = 3,539).
3.1. Sensitivity analysis
A sensitivity analysis was done utilizing the pathological anatomy database. At the time of the analysis, it covered 6.3 million beneficiaries. Compared to administrative databases in which all data are coded, the reports from pathological anatomy databases are often reported as text requiring visual inspection and may not include all the information. For the purpose of the study, the pathological anatomy databases with the greatest availability of coded data of interest consisted of 4 million beneficiaries.
Among patients with LN removal, 1,175 patients had available pathological anatomy data. Within 1,175 patients previously defined, 399 (34.0%) patients had pathological high-risk characteristics: about one-third of patients with LN removal represent the subgroup with pathological high-risk characteristics.
Patients with pathological high-risk characteristics have similar mean age at the index date compared to the overall EBC population, with 6.3% of them reporting a previous hospitalization for BC (Suplementary Table S1). They were followed up for a mean of 33.5 [32.8 (19.4–45.0) months], quadrantectomy was performed among 39.3% of them; 29.6% received endocrine therapy only in adjuvant settings, while concurrent CT and endocrine therapy was administered to 68.4%; 13.3% experienced distant relapse after a mean period of 1.4 years [median (Q1-Q3), 1.0 (0.4–1.8)] after the index date; after distant relapse, 44% of patients started a therapy with CDK 4/6 inhibitors, respectively. During the first year of follow-up, the mean total annual healthcare cost was €12,675 among alive patients (N = 388, corresponding to 97.2% of the group) (Supplementary Figure S1).
4. Discussion
The present study aimed to identify patients at high-risk of recurrence with HR+/HER2- EBC, providing an overview of their treatments and disease patterns. During the past 20 years, there have been limited advancements to standard adjuvant therapies, and an unmet need exists for patients with EBC at high risk of recurrence [Citation18]. The usage of CDK4/6 inhibitors in adjuvant settings opens new therapeutic options for these patients. For example, the recent monarchE trial demonstrated a significant improvement in invasive disease-free survival in patients with HR+, HER2- node-positive EBC at high risk of early recurrence treated with the CDK4/6 inhibitor abemaciclib combined with ET [Citation7].
This retrospective secondary data usage analysis has been conducted to study the resource utilization and real-world outcomes, selecting as main data sources the healthcare reimbursement databases, enriched with clinical information from pathological anatomy database for some LHUs.
Since administrative databases do not collect data on clinical features, the HR+/HER2- status was defined by the presence/absence of specific therapies, as already utilized in other studies [Citation14]. In recent publications approximately 70% of BC patients have been reported with HR+/HER2- status [Citation19], therefore, the proportion estimated in the present study (69.4% of patient with hospitalization and surgery were HR+/HER2-) sustains the robustness of the adopted approach.
Regarding the identification of population with high-risk of recurrence, two limitations have been managed: the availability of pathology database for a subgroup of the analyzed population and missing information about LN involvement in pathological reports. The population at high risk of recurrence was defined as patients who underwent LN removal, based on the assumption that surgical lymphadenectomy is strongly associated with LN involvement; indeed, in the literature, approximately two-third of patients with lymph node dissection have been reported to undergo this procedure, which is indicated for ≥1 positive sentinel nodes [Citation20]. The sensitivity analysis includes all patients from the cohort of patients with LN removal, with available data in the pathological anatomy database, and meeting MonrchE criteria.
The demographic characteristics of patients with EBC with HR+/HER2- show a mean age of 62–64 years old, slightly older than the age range reported in other real-world studies on the same population, which is between 57 and 62 years old [Citation21]. However, such a difference could be associated with the applied methodology: while those studies reported the age at EBC diagnosis, in the present study age was based on the hospitalization discharge diagnosis from 2015, potentially including incident as well as a minor proportion of prevalent patients.
In patients with a high-risk profile, a peak of early recurrence has been clearly described within 2 years after surgery [Citation22,Citation23]. The most frequently reported sites of distant metastasis in this were bone, liver and lung metastases, however metastatic patients that did not require hospitalization might be under-traced. In analyzing the treatment patterns of patients with LN removal, we do acknowledge that the therapeutic approach is an everchanging scenario, especially after the AMAROS, SINODAR-ONE and ACOSOG Z0011 trials, that suggested the axillary lymph node dissection could be safely omitted in case of low involvement of sentinel nodes. However, to date, there is no clear direct evidence and further data are needed [Citation24–26].
In patients with LN removal, hospitalizations were the major cost-driver, accounting for approximately 50% of total direct costs. This figure appears to be consistent with Piccinni et al. [Citation14], showing that overall hospitalization over 1 year (index and other admissions) accounted for 49% of total cost (index included).
The study has limitations due to its observational nature and due to the utilized data sources: the restricted availability of pathology database and the presence of free text fields in pathological reports, which could determine a non-comprehensive capture of clinical data, such as staging data, number of involved LN, or luminal A or B type that could have an impact on prognosis. Moreover, the use of fulvestrant prior to the index date was not considered exclusionary, although it could indicate patients with advanced disease; nevertheless, this was found in very few patients and did not significantly alter the results (data not shown). The limited proportion of patients that started CDK4/6i at distant relapse herein observed may be due to the timing of Italian Medicines Agency (AIFA) approval for these drugs, from 2017 onwards, while our analysis started at 2015. CT could be under-estimated since it may not have been captured in the hospitalization database. Cost description reflected the clinical practice of the study period, ie the time patients actually consumed the resources reimbursed by the INHS. The main strength of this Italian study is providing a real-word burden of EBC, with a focus on the identification of population at high-risk of recurrence by synergically using both administrative and clinical data.
5. Conclusions
In oncology, data from real-world have been gaining increasing interest in recent years. The present analysis evaluated the burden of patients with EBC in clinical practice, with particular reference to high-risk of recurrence subgroups. Around 10% of patients with HR+/HER2- with EBC that underwent LN removal experienced a relapse over the period in analysis, highlighting the strong need to prevent recurrence.
The data of the present analysis confirm the current international literature and suggest that the integration between different data sources (administrative and clinical) could represent a valuable approach to generate evidence in oncological diseases clinical practice.
Article highlights
The analysis combined administrative and pathological anatomy data to provide insights on early breast cancer in real-world settings in Italy;
HR+/HER2- patients and, among them, those with lymph node removal were described in terms of characteristics, treatments, recurrence and healthcare resource consumption; a sub- cohort with pathological high-risk characteristics was identified as well.
Overall, 4.7% of HR+/HER2- patients and 9.9% of patients with LN removal experienced distant relapse over a median follow-up of 33.2 months (Q1-Q3: 17.0-50.6) and reached up to 13% in patients with pathological high-risk characteristics. Local relapse occurred in approximately 9% of patients.
Our results can support further evidence into the need of therapies for early stages and can help the health decision makers by showing how patients were managed, and their burden for the Italian National Health Service.
Given the growing interest for real-world data in oncology, the integration between different data sources (administrative and clinical) herein provided could be a useful tool for evidence generation from clinical practice.
Declaration of interest
V Perrone, E Giacomini, D Sangiorgi, L Degli Esposti are employees of CliCon S.r.l. Società Benefit and report no conflicts of interest related to this work. A Tamma and C Buzzoni are employees of Eli Lilly Italy S.p.a., Italy; M Giovannitti is an employee of Eli Lilly S.p.a. & Company Roma, Italy. The agreement signed by Clicon S.r.l. Società Benefit and Eli Lilly does not create any entity, joint venture or any similar relationship between parties. Clicon S.r.l. Società Benefit is an independent company. Neither CliCon S.r.l. Società Benefit nor any of their representatives are employees of Eli Lilly for any purpose. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Study conception: C Buzzoni, V Perrone, L Degli Esposti. Acquisition/analysis/interpretation of data: V Perrone, E Giacomini, D Sangiorgi, L Degli Esposti. Validation: A Tamma, M Giovannitti, C Buzzoni, L Degli Esposti. Writing first draft: E Giacomini, V Perrone. Revision and editing of the draft: all authors. Final approval of the manuscript for publication: all authors.
Data sharing statement
All data used for the current study are available upon reasonable request next to CliCon s.r.l. which is the body entitled of data treatment and analysis by Local Health Units.
Supplemental Material
Download MS Word (34.9 KB)Acknowledgments
The present manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by Courey A. from American Journal Experts (AJE).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2246652.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492
- I numeri del cancro in Italia 2021 [Internet]. AIOM. [cited 2022 Feb 2]. Available from: https://www.aiom.it/i-numeri-del-cancro-in-italia/
- Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014;5:465–477. doi: 10.5306/wjco.v5.i3.465
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173
- MAMMELLA AND. 2021;p. 663.
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–3998. doi: 10.1200/JCO.20.02514
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: asco clinical practice guideline focused update. J Clin Oncol. 2018. [cited 2021 Dec 8]; Available from. Internet https://ascopubs.org/doi/pdf/10.1200/JCO.18.01160
- Salvo EM, Ramirez AO, Cueto J, et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: a systematic review and meta-analysis. Breast Edinb Scotl. 2021;57:5–17. doi: 10.1016/j.breast.2021.02.009
- Di Maio M, Perrone F, Conte P. Real‐world evidence in oncology: Opportunities and limitations. Oncology. 2020;25(5):e746–e752. doi: 10.1634/theoncologist.2019-0647
- Guarneri V, Pronzato P, Bertetto O, et al. Use of electronic administrative databases to measure quality indicators of breast cancer care: experience of five regional oncology networks in Italy. JCO Oncol Pract. 2020;16(2):e211–e220. doi: 10.1200/JOP.19.00466
- McKay DR, Nguyen P, Wang A, et al. A population-based study of administrative data linkage to measure melanoma surgical and pathology quality. PLoS One. 2022;17(2):e0263713. doi: 10.1371/journal.pone.0263713
- DECRETO LEGISLATIVO 10 agosto 2018, n. 101 [Internet]. [cited 2023 Jun 29]. Available from. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2018-09-04&atto.codiceRedazionale=18G00129&elenco30giorni=true
- Piccinni C, Dondi L, Ronconi G, et al. HR+/HER2- metastatic breast cancer: Epidemiology, prescription patterns, healthcare resource utilisation and costs from a large Italian real-world database. Clin Drug Investig. 2019;39:945–951. doi: 10.1007/s40261-019-00822-4
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8
- Drummond M. Methods for the economic evaluation of health care programmes. Fourth ed. Oxford, (UK) ; New York, NY, USA: Oxford University Press; 2015.
- Cortesi PA, Paolicelli D, Capobianco M, et al. The value and sustainability of ocrelizumab in relapsing multiple sclerosis: a cost-effectiveness and budget impact analysis. Farmeconomia Health Econ Ther Pathw. 2019 [[cited 2023 Jun 29]];20. InternetAvailable from: http://journals.seedmedicalpublishers.com/index.php/FE/article/view/1435
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(12):1571–1581. doi: 10.1016/j.annonc.2021.09.015
- Cognetti F, Masetti R, Fabi A, et al. Pondx: real-life utilization and decision impact of the 21-gene assay on clinical practice in Italy. NPJ Breast Cancer. 2021;7(1):1–8. doi: 10.1038/s41523-021-00246-4
- Dengel L, Van Zee KJ, King TA, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014;21(1):22–27. doi: 10.1245/s10434-013-3200-6
- Tarantino P, Jin Q, Mittendorf EA, et al. Clinical and pathological features of breast cancer patients eligible for adjuvant abemaciclib. Ann Oncol Off J Eur Soc Med Oncol. 2022;33(8):845–847. doi: 10.1016/j.annonc.2022.04.069
- Karacin C, Ergun Y, Oksuzoglu OB. Saying goodbye to primary endocrine resistance for advanced breast cancer? Med Oncol Northwood Lond Engl. 2021;38(1):5. doi: 10.1007/s12032-020-01449-8
- Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol Lond Engl. 2022;18(21):2667–2682. doi: 10.2217/fon-2022-0310
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7
- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi: 10.1001/jama.2017.11470
- Tinterri C, Gentile D, Gatzemeier W, et al. Preservation of axillary lymph nodes compared with complete dissection in T1-2 breast cancer patients Presenting one or two metastatic sentinel lymph nodes: the SINODAR-ONE multicenter randomized clinical trial. Ann Surg Oncol. 2022;29:5732–5744. doi: 10.1245/s10434-022-11866-w
Appendix 1.
List of Ethics Commettee
The project from which these analyses were drawn was approved by the Ethics Committee of each participating LHU: Comitato Etico Regionale Umbria (protocol number 19414/20/ON, approval date 16/09/2020); Comitato Indipendente di Etica Medica (protocol number 48144, approval date 28/05/2021); Comitato Etico Inter-aziendale Campania Sud (protocol number 64, approval date 03/11/2020); Comitato Etico Inter-aziendale Campania Sud (protocol number 51, approval date 02/09/2020); Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana (protocol number 20190211, approval date 12/09/2019); Comitato Etico per le Sperimentazioni Cliniche (CESC) della Provincia di Vicenza (protocol number 1627, approval date 28/10/2020); Comitato Etico Interaziendale A.O. “SS. Antonio e Biagio e Cesare Arrigo” – Alessandria (protocol number 0008668, approval date 20/04/2021); Comitato Etico “Lazio 1” (protocol number 1079/CE Lazio 1, approval date 23/09/2020); Comitato Etico “Lazio 2” (protocol number 0216084/2020, approval date 16/12/2020); Comitato Etico “Lazio 1” (protocol number 1080/CE Lazio 1, approval date 23/09/2020); Comitato etico interprovinciale Area I (protocol number 63/CE/20, approval date 3/12/2020); Comitato Etico Regionale Liguria (protocol number 0179046/2020, approval date 14/06/2021); Comitato Etico per le Sperimentazioni Cliniche (CESC) della Provincia di Vicenza (protocol number 0036999, approval date 28/04/2021); Comitato Etico Delle Province Di Chieti E Pescara (protocol number 07, approval date 18/03/2021).