ABSTRACT
Introduction
Cancer imposes a high economic burden with medical care and medication costs. We evaluate the costs, the use of resources, the administration time, and the patient preferences associated with the use of biotechnological drugs in SC and IV presentations.
Methodology
A systematic literature search was conducted in PubMed, Embase, and seven additional databases. The search was carried out in September 2021 and included only studies directly comparing SC and IV presentations. Evidence was synthesized narratively.
Results
34 references were included, which only analyzed bortezomib, daratumumab, rituximab, and trastuzumab. Reduction in preparation costs of SC compared to IV presentations ranged from 6.6% to 50.1%, and in administration costs from 4.5% to 95.3%. SC administration of rituximab and trastuzumab resulted in less productivity loss. More than 68% of patients reported greater satisfaction with the SC route. A reduction of time in the infusion chair, lower costs of resources for preparation, and health personnel for the administration process were identified with SC administration.
Conclusions
The use of SC daratumumab, rituximab, and trastuzumab in patients with cancer reduces direct and indirect costs and adverse events compared to IV use. Patients prefer the SC administration, perceiving more comfort, and less pain at the administration site.
1. Introduction
Cancer is considered by the World Health Organization (WHO) to be the second leading cause of death in the world [Citation1]. In 2018, 9.6 million deaths worldwide attributed to cancer were reported [Citation1]. In 2020, there were an estimated 19.2 million new cases and 9.9 million deaths from cancer worldwide, according to Global Burden of Cancer (GLOBOCAN) reports [Citation2]. Cancer has become one of the biggest challenges for healthcare systems worldwide [Citation3].
The economic burden attributed to cancer in the European Union (EU) was estimated to be approximately €199 billion in 2018 [Citation4]. This includes the cost of healthcare, informal care, and productivity losses due to cancer-related mortality and morbidity. The national cancer-attributed medical care costs in the United State in 2015 were $183 billion, and projected to increase 34% to $246 billion by 2030, based only on population growth [Citation5]. By 2030, the global incidence of cancer is expected to increase by 75%, leading to an increase in its economic burden [Citation6].
Currently, many treatments in patients with cancer are administered intravenously (IV); however, this route of administration is associated with longer infusion times that entail an additional burden on healthcare resources and an impact on the patient’s quality of life. For this reason, in recent years, several of these treatments have been developed to be administered subcutaneously (SC) to provide a faster and more satisfactory method of administration for patients, as well as a significantly reduced preparation and administration times and the use of medical resources [Citation7].
The different characteristics of the available treatments elicit an examination of the economic burden that chronic diseases such as cancer impose on the costs associated with medical care, the expenses of the procedures, as well as other aspects related to these interventions, such as loss of productivity due to work disability and lost workdays. Therefore, the objective of this systematic review (SR) was to evaluate the direct and indirect costs, the use of resources, the administration time and the patient preferences associated with the use of biotechnological drugs in SC and IV presentations in patients with cancer.
2. Materials and methods
A SR was conducted on the costs, use of resources, patient preferences, and administration time associated with the use of biotechnological drugs for cancer in IV presentations compared with SC presentation. This review was carried out following the guidelines of Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [Citation8].
The research protocol was registered in the PROSPERO database of the National Institute for Health Research with the code CRD42021277979.
A systematic and comprehensive literature search was performed in the following electronic databases: PubMed, Embase, LILACS, SCOPUS, EBSCO host, EconLit, Health Technology Assessment (HTA), DARE, and the NHS Economic Evaluation Database. The search was carried out in September 2021. The search strategy was adapted to each database’s requirements. Studies available as full-text publications of systematic reviews of economic literature, observational studies, health technology assessments, public policy documents, or clinical trials with direct comparison of biotechnological drugs with SC and IV presentations were included in this review. Only articles published in English or Spanish were included in this review; this criterion was implemented in the screening and not in the search. Not have publication year restrictions. Studies published only in abstract format were excluded because the reported information is incomplete to assess their methodological quality.The search strategy and its results are presented in Supplement 1 y 2. Likewise, the selection and inclusion process is detailed in Supplements 3 and the list of excluded studies and the reasons is detailed in Supplement 4.
After the identification of relevant studies and the removal of duplicates, four reviewers (AP, CH, LP, and DQ) independently performed reference screening based on the title and abstract. Subsequently, three reviewers (AP, CH, and LP) independently performed the full-text review, checking the eligibility criteria in the pre-selected references. Disagreements were solved by consensus.
Data extraction was carried out by a reviewer (AP) and quality control was implemented by a second reviewer (CH), comparing the results included in the evaluation report with those presented in the original document.
Two reviewers (AP and CH) independently assessed the quality of the included studies using standardized and widely used tools for each included study design, and discrepancies were resolved by consensus. The Quality of Health Economic Studies tool (QHES) [Citation9] was used to assess systematic reviews of the economic literature, that uses a numeric scale from zero to 100; the higher the score, the better the quality. The Cochrane Collaboration’s bias risk tool [Citation10] was used to evaluate randomized clinical trials, which assesses selection, performance, detection, attrition, reporting, and notification bias, and classifies each article as high risk of bias, low risk of bias, or unclear risk of bias. The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies from the National Heart, Lung, and Blood Institute (NHI) [Citation11] was used to evaluate observational studies in terms of the risk of potential selection, information or measurement bias, and confounding. High risk of bias translates to a rating of poor quality. For cross-sectional studies, the Critical Appraisal Tool to Assess the Quality of Cross-Sectional Studies (AXIS) [Citation12] was used, which consists of 20 items that assess aspects related to the objective, methodology, results, and discussion. The result of the quality rating can be found in Supplement 5.
A narrative synthesis of the available evidence was made with the data obtained from each study on the outcomes of direct and indirect costs, use of resources, patient preferences and administration time. Due to the heterogeneity of the information presented in the included studies, it was considered appropriate to carry out a descriptive analysis and not a statistical analysis.
3. Results
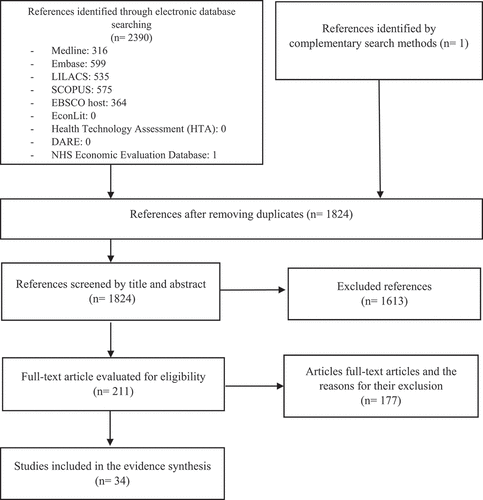
1824 references were identified by searching electronic databases after removing duplicates. After selection by title and abstract format, 221 full-text references were evaluated, of which 34 met the inclusion criteria and were included in the evidence synthesis (). Of the 34 included studies, 20 were economic evaluations (medium-high methodological quality), 8 were randomized clinical trials (high risk of bias due to the absence of double blinding, due to the impossibility of concealing the intervention; two of the trials did not report the method of randomization), 4 were cross-sectional studies (methodological quality of these studies was reduced by failure to report the number of non-responders or the measures used to address them, in two studies the sample size was not justified, and the precision of the reported estimators was not clear), and 2 were observational cohort studies (good methodological quality).
Supplement 6 describes the outcomes reported in each of the included studies, the medication used and the indication for which the drug was used.
Differences between the direct costs of SC vs IV presentations of biotechnological drugs for cancer treatment, such as daratumumab, rituximab and trastuzumab, were reported by 20 of the included studies [Citation13–32]. Six studies also noted differences in indirect costs between the SC and IV presentation of rituximab and trastuzumab in treating breast cancer and non-Hodgkin’s lymphoma [Citation13,Citation18,Citation21,Citation27,Citation29,Citation30]. Preferences for one of the two administration routes were evaluated by 12 of the studies [Citation21,Citation33–43], while 19 references reported the time required for the preparation and administration of drugs [Citation15–19,Citation21–25,Citation27,Citation29,Citation31,Citation32,Citation34,Citation39,Citation44–46].
3.1. Direct costs
Six studies reported the direct costs associated with drug preparation in cancer treatment settings [Citation14,Citation17–19,Citation26,Citation32]. In five of these studies, the pharmacy area was in charge of the preparation of biotechnological drugs for patients with cancer; this outcome was reported for daratumumab, rituximab and trastuzumab in the SC and IV presentations.
shows the summary of results of the studies that give information on the direct costs related to the preparation, administration and costs of biotechnological drugs.
Table 1. Percentage of direct cost reduction for preparation, administration, and drug costs of subcutaneous versus intravenous administration.
Compared with the IV presentation, the SC presentation represented a considerable difference in the costs associated with the drug preparation. According to Sánchez et al. [Citation14], the costs of the pharmacy nursing staff for IV and SC preparations of rituximab had a difference of €2.25 per cycle, representing a reduction of 50.1% in favor of the SC presentation. The dose of intravenous rituximab was at 375–500 mg/m, the SC route uses a fixed dose of 1400 mg. Every cycle of chemotherapy in IV administration considered 1 hr to 6 hrs, compared to 5 mins for every dose of SC rituximab injection.
In the study of Farolfi et al. [Citation19], the mean cost of trastuzumab preparation per patient had a significant difference of €35.14 between SC and IV presentations, representing a cost reduction of 36.9% using SC administration. In addition, Franken et al. and Mihajlović et al. [Citation17,Citation18] reported differences between the SC and IV presentations of rituximab of 18% (€0.11) and 6.6% (€0.19) in the cost of premedication and the mean cost of preparation in the pharmacy, respectively, in favor of the SC presentation [Citation17,Citation18].
Administration costs were reported by five publications [Citation17,Citation22,Citation24,Citation28,Citation31]. These studies documented the costs of IV administration compared with the costs of SC administration of rituximab and trastuzumab for treating breast cancer and lymphoma patients. The differences between the mean costs of administering one cycle per patient of IV trastuzumab versus SC administration ranged from €80.00 to €213.88, representing a percentage reduction between 4.5% and 95.3% when trastuzumab is administered via the SC route [Citation22,Citation24,Citation28,Citation31]. The breadth of this range of costs is attributed to certain aspects of the administration, such as the costs of the antineoplastic drug, health personnel (doctor, nurse, and pharmacist), use of the catheter, additional resources required for the administration, possible drugs waste, and structural costs.
The comparative analysis of IV and SC costs of daratumumab, rituximab, and trastuzumab used in the treatment of lymphoma, multiple myeloma, and breast cancer, was reported in 10 studies [Citation14,Citation17,Citation18,Citation20,Citation21,Citation26–30]. The mean cost per cycle of rituximab for patients with lymphoma was reported by three studies [Citation14,Citation17,Citation18], with reductions between 4.5% and 8.5% in the drug cost in its SC presentation compared with the IV presentation; this specifies savings when considering only the drug cost.
Seven studies documented the cost of trastuzumab in its SC and IV presentations for treating patients with breast cancer [Citation18,Citation20,Citation21,Citation27–30]. In two of the publications [Citation20,Citation30] the cost of treatment given by IV administration was lower than the cost of treatment given by SC administration. However, in the study of Rojas et al. [Citation30] the cost was estimated using a weight of 73 kg to calculate the dose per kilogram. If the patient’s weight were larger, the cost of trastuzumab in its IV presentation would be higher than that of the SC presentation since two additional maintenance vials would be required compared to the fixed dose of the SC presentation. Likewise, in the study of Simoens et al. [Citation20] IV medication costs are directly proportional to the patient’s body weight.
Regarding the cost of the resources required for the preparation and administration of rituximab and trastuzumab in patients with breast cancer and lymphoma, these were reported in 10 studies included in the systematic review [Citation16–18,Citation21,Citation22,Citation27–30,Citation32]. In general, the costs of these resources were considerably lower with the SC administration than with the IV administration. Some of the resources considered for IV administration included the materials for administration in the treatment room, and for drug reconstitution, the surgeries to implant permanent venous access devices, and the cost per order for IV reconstitution in the pharmacy, among others. For the SC application, less expensive materials are assessed, such as the materials for SC administration in the treatment room, the cost of loading the needle, the cost of the injection needle, and the cost of the syringe.
In addition, 10 studies provided information on the cost associated with the active time of health care providers necessary for the preparation, dispensing, and administration of biotechnological drugs such as rituximab and trastuzumab [Citation16–18,Citation21,Citation22,Citation27–30,Citation32]. Regarding the mean time employed by health personnel for the preparation, distribution, and supply of the drug in patients with cancer, SC administration of trastuzumab is less expensive than IV administration. When analyzing this outcome for rituximab, in two studies [Citation17,Citation23], this difference associated with personnel costs favored IV administration.
Regarding other direct costs, it was found that the costs associated with the mean hospital occupation time per day were lower for the administration of medications by the SC route in patients with cancer compared with the time required to administer drugs by the IV route [Citation14,Citation17–19].
3.2. Indirect costs
summarizes the results of the studies that give information on indirect costs.
Table 2. Percentage of indirect cost reduction of subcutaneous versus intravenous administration.
Indirect costs such as loss of productivity, measured by time in the hospital ward and hospitalization time [Citation13,Citation21,Citation27,Citation29,Citation30], the travel expenses [Citation18], and the cost of the informal caregiver [Citation18] were described by six studies. Consistent with reported findings for patients with breast cancer and non-Hodgkin’s lymphoma, the indirect costs associated with productivity loss were substantially lower with the SC administration of rituximab and trastuzumab than with IV administration since the time required by the patients to receive IV therapy was higher. Additionally, the most significant difference between the costs associated with SC administration and IV administration was travel expenses and the time of informal caregivers. Although the costs of productivity loss due to paid and unpaid work had a smaller effect, these costs were relevant in patients who received SC rituximab [Citation18].
3.3. Use of resources
Two of the included studies reported the use of medical resources for administering IV trastuzumab compared with SC trastuzumab in patients with breast cancer [Citation16,Citation27]. The total cost of the resources for IV administration of trastuzumab was €856.80 and €440.50 for the SC formulation, representing savings of €516.30% saving of 60.2%) in favor of the SC route. Trastuzumab treatment demands considerable healthcare resources, including the insertion of a permanent venous catheter required for prolonged IV infusion. This consequently generates an increase in costs for the operating room, necessary for insertion and anesthesia time, among others; for this reason, the authors of this study conclude that SC administration optimizes medical resources [Citation16].
3.3.1. Patient preferences
Patient preferences were documented in 12 publications between 2013 and 2021 [Citation21,Citation33–42], in which bortezomib, daratumumab, rituximab, and trastuzumab in their IV and SC presentations were evaluated. The measurement of this outcome was assessed through general measurement tools or scales for patients with cancer, such as the Patient Preference Questionnaire (PPQ) and Cancer Therapy Satisfaction Questionnaire (CTSQ), or specific scales such as Rituximab Administration Satisfaction Questionnaire (RASQ). For interpreting these tools, a greater patient satisfaction or preference is defined as the higher the score is on the scale. Face-to-face or telephone surveys were other strategies used to assess the level of satisfaction or the patients’ preferences. The results obtained with the PPQ, CTSQ and RASQ scales or tools were similar to the answers provided by the patients interviewed, showing that between 68% and 94% of the patients prefer or have greater satisfaction with the SC route than with the IV route [Citation21,Citation34,Citation40,Citation42,Citation43].
Among the reasons expressed by patients for choosing the SC route over the IV route are shorter administration time [Citation34,Citation39,Citation40,Citation42], less pain at the injection site [Citation39,Citation40], more comfort [Citation34,Citation39,Citation40,Citation42], less anxiety [Citation39,Citation40], no need for venous access or exploring the possibility of it [Citation40,Citation42], no adverse events or no apparent adverse effects [Citation34,Citation40,Citation42], and less feeling of being sick [Citation34]. However, patients who prefer IV administration state that they perceive greater efficacy and fewer adverse reactions [Citation40], feel they can interact with other people with the same health condition [Citation40], and perceive a greater benefit from the resources when they are used for a longer time [Citation40]; other reasons reported were the absence of hematomas [Citation34], less pain [Citation34,Citation42], and less fatigue [Citation42].
3.4. Administration time
shows the results of the studies that give information on preparation and administration times.
Table 3. Percentage of preparation and administration time reduction of subcutaneous versus intravenous administration.
The time required for the preparation and administration of biotechnological drugs via SC and IV routes for patients with lymphoma and breast cancer was recorded by 11 studies [Citation16–19,Citation25,Citation27,Citation32,Citation39,Citation44–46]. They describe a shorter preparation time for rituximab and trastuzumab by the SC route than the IV route in patients with breast cancer and non-Hodgkin’s lymphoma [Citation17–19,Citation25]. Similar results were reported for the drug administration time; findings from eight studies demonstrated a shorter time for SC administration of daratumumab, rituximab and trastuzumab compared with IV administration [Citation16–19,Citation27,Citation32,Citation39,Citation45].
In their time and motion study of 2016, De Cock et al. [Citation46] assessed the administration time of SC rituximab compared with that of IV rituximab in patients with non-Hodgkin’s lymphoma in eight countries. The mean duration of IV infusion of rituximab was 180.9 minutes. In comparison, the time for SC administration was 8.3 minutes, representing a main reduction in infusion chair time with the SC route, close to 95%.
4. Discussion
In their IV and SC presentations, the direct and indirect costs, the use of resources, the patient preferences, and the administration time required for rituximab and trastuzumab in patients with breast cancer and lymphoma respectively, were described in this systematic review. Also included were studies that reported preparation and administration time and patient satisfaction related to the use of SC or IV daratumumab [Citation35,Citation41,Citation45]. In addition, the difference between the direct costs associated with the administration of IV and SC daratumumab was reported by Kading et al. 2021 [Citation26], while the preferences of patients with multiple myeloma between SC or IV treatment with bortezomib were reported by one of the publications [Citation34].
Among the reported outcomes included in the review were direct medical costs associated with the preparation and administration of biotechnical drugs in patients with cancer, costs of the active time of healthcare providers, and indirect costs due to lost productivity. The general administration costs, both per session and for complete cycles, were lower when they were administered by the SC route [Citation15,Citation18,Citation27]. This saving is mainly attributed to the decrease in the use of resources required for the administration [Citation16,Citation17,Citation32], a shorter time in the infusion chair [Citation16,Citation17], a shorter time in the use of the oncology unit [Citation18,Citation19], and less wastage of the drug via the SC route [Citation25]. These results concur with publications that support the SC administration of rituximab [Citation47] and trastuzumab [Citation48], since they are cheaper and provide efficacy and safety profiles similar to IV therapy.
The main variable estimated in the sensitivity analyses of the economic studies, and considered relevant in the cost difference between the IV and SC formulations, was the patient’s body weight, which defines the dose necessary for administration in the IV presentation [Citation20,Citation26,Citation28,Citation30]. IV infusion of drugs such as daratumumab and trastuzumab may then be less expensive, depending on the mean weight of the target population. Even then, considering the direct and indirect medical costs associated with drug preparation and administration, SC treatment remains a cost-saving option.
The time required by patients with cancer undergoing IV administration treatments is considerably longer compared to SC administration therapies. Time-and-motion studies carried out in patients treated with rituximab and trastuzumab, which assessed parameters such as the patient’s time in the hospital and the health professional’s time required for the preparation and administration of the drug, showed significant reductions in time, both in the infusion chair during treatment and of the healthcare personnel active during therapy [Citation44,Citation46]. Thus, less time in the treatment room could result in a greater number of patients treated in oncology units, which translates into the optimization of resources, improvements in the quality of care and the efficiency of medical centers since the same number of patients could be treated with fewer resources than those required by the drug in its IV presentation or which could be administered on an outpatient basis, thus increasing the capacity of medical centers, the efficiency in assigning appointments, a better service opportunity, and significant time savings for patients.
Patient-reported outcomes can provide valuable insights into the real-world use, and effectiveness of biotechnological medicines administered subcutaneously and intravenously, and can help identify factors that influence treatment adherence. A greater patient satisfaction or preference for the SC presentation of the drugs analyzed was documented in 12 publications, with correlation between the results of clinical trials and real-world studies [Citation21,Citation33–42]. By leveraging this information, healthcare providers can make more informed treatment decisions and improve patient outcomes.
Despite the heterogeneity of the published results and the diversity of methods used, there is a consensus on the advantages offered and the favorability of the SC administration of drugs such as daratumumab, rituximab, and trastuzumab in patients with cancer, compared with the IV infusion, in the scenarios of less cost required for preparation and supply, the time needed for administration, and patient preferences.
In the evaluation of the methodological quality of the studies, the eight randomized clinical trials showed a high risk of bias, mainly due to the impossibility of blinding researchers and participants concerning the study intervention. Considering the nature of the interventions (comparing routes of administration of oncological treatments) blinding was not feasible, however, it is recognized that studies could have adopted methodologies to reduce this performance and detection bias [Citation49]. However, the outcomes evaluated in these clinical trials were patient preferences and were analyzed qualitatively. That is, quantitative estimates that might be overestimated were not made; instead, a narrative description of the direction of the effect was made. Therefore, the findings of this review on patients’ preferences are useful to know the reasons for choosing one of the routes of administration despite the high risk of bias.
For other outcomes related to costs and resource utilization, the studies presented a better methodological quality, which generates confidence in the reported findings. The two cohort studies included were of good quality, considering only the items that apply to the studies evaluated. The four cross-sectional studies did not report data on non-responders or internal consistency, which, beyond the expected representativeness, limits confidence in the results.
Concerning the 20 cost studies evaluated with the QHES scale, it was found that there was mainly an absence in the reporting of the perspective and the time horizon. These criteria enable delimiting and contextualizing the economic results. Because they were obtained mainly within observational studies or time and motion studies in specific healthcare institutions, they were not explicitly reported. In the same way, the studies also lack a detailed discussion of the potential risk of bias, its magnitude and its impact on the results. However, the reporting of specific data on costs and units was sufficiently detailed, allowing the extraction of a large amount of information.
One limitation that should be mentioned is the inability to perform an analysis considering the costs of off-patent IV preparations compared to the costs of proprietary SC preparations.
No studies reported patients’ economic benefits or preferences of patients regarding the use of biotechnological drugs in their subcutaneous and intravenous presentations at the local level, so the final analysis did not include national or Latin American results. The evidence for daratumumab was limited and lacked results to assess the costs associated with administering this drug.
This review synthesizes and describes the results of different studies on costs, resource use, administration time, and patient preferences associated with the use of biotechnological drugs in subcutaneous (SC) and intravenous (IV) presentations. Within our search for information, we did not find other systematic reviews that will address this topic in oncology, so this review provides a valuable resource for the scientific community and health systems o contribute to the development of more efficient and effective strategies for the treatment of patients with cancer.
5. Conclusions
The studies included in this review consider that the SC formulation of drugs such as daratumumab, rituximab, and trastuzumab in patients with cancer reduces the overall burden of costs, the time required for administration, the use of medical resources, and the occupancy of oncology units. The use of these therapies with the SC route could potentially impact health systems since they would mean the optimization of resources, the time of the personnel necessary for care, economic savings, and improvements in the quality of care.
Indirect costs such as productivity loss, lost workdays, travel expenses, and the expenses of informal caregivers are reduced with the use of SC therapies. The administration of biotechnological drugs through the SC route in patients with cancer was associated with a shorter administration time, more comfort, less pain at the administration site, less anxiety, and a reduction in adverse events, which resulted in the route of administration preferred by patients.
Article highlights
Cancer results in a high economic burden, including medical care and medication costs, and has become one of the biggest challenges for healthcare systems worldwide.
This systematic review (SR) presents the available evidence on the direct and indirect costs, the use of resources, the administration time, and the patient preferences associated with the use of biotechnological drugs in SC and IV presentations in patients with cancer.
Only studies available as full-text publications with direct comparisons of biotechnological drugs with SC and IV presentations were included in this review.
34 references were included and the drugs analyzed in the included studies were bortezomib, daratumumab, rituximab, and trastuzumab; no information was found for other treatments.
The use of SC daratumumab, rituximab, and trastuzumab in patients with cancer reduces direct and indirect costs compared to IV use. Patients prefer the SC administration, perceiving more comfort, less pain at the administration site, and reduced adverse events.
Declaration of interest
L Prieto is an employee of Roche, and received no remuneration for research or authorship of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
A Parra and C Hernández participated in the design and execution of the SR and were in charge of the synthesis of the results obtained and in the approach of the discussion and the conclusions. L Prieto-Pinto participated in the design of the SR and actively contributed to the selection of studies, as well as to the discussion and conclusions of the document. All authors read and approved the final version of the manuscript for publication.
Ethical aspects
Our study follows the principles of the Declaration of Helsinki and national and international guidelines for good research practices. This is a documentary study, therefore it does not require approval by the ethics committee.
Supplemental Material
Download (77.7 KB)Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2249232.
Additional information
Funding
References
- Organización Mundial de la Salud/OMS. Cáncer [Internet]. 2018. p. 1–7. Available from: https://www.who.int/es/news-room/fact-sheets/detail/cancer
- World Health Organization. Globocan 2020 [Internet]. WHO Chron. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- Knaul FM, Garcia PJ, Gospodarowicz M, et al. The Lancet commission on cancer and health systems: harnessing synergies to achieve solutions. Lancet. 2021;398(10306):1114–1116. doi: 10.1016/S0140-6736(21)01895-X
- Hofmarcher T, Lindgren P, Wilking N, et al. The cost of cancer in Europe 2018. Eur J Cancer. 2020;129:41–49. doi: 10.1016/j.ejca.2020.01.011
- Mariotto AB, Enewold L, Zhao J, et al. Medical care costs associated with cancer survivorship in the United States. Cancer epidemiology biomarkers and prevention;2020; 29(7): 1304–1312. doi: 10.1158/1055-9965
- Jackisch C, Kim SB, Semiglazov V, et al. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26(2):320–325. doi: 10.1093/annonc/mdu524
- Dent S, Ammendolea C, Christofides A, et al. A multidisciplinary perspective on the subcutaneous administration of trastuzumab in HER2-positive breast cancer. Curr Oncol. 2019;26:e70–e80. doi: 10.3747/co.26.4220
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. BMJ Publishing Group; 2021 372:n71. doi: 10.1136/bmj.n71
- Ofman JJ, Sullivan SD, Neumann PJ. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Managed Care Pharm. 2003;9(1):53–61. doi: 10.18553/jmcp.2003.9.1.53
- Higgins J, Green S. Manual Cochrane de Revisiones Sistemáticas de Intervenciones, versión 5.1.0 [actualizada en marzo de 2011]. In: Higgins J, Green S, editors. Barcelona: Centro Cochrane Iberoamericano; 2012. https://es.cochrane.org/sites/es.cochrane.org/files/uploads/Manual_Cochrane_510_reduit.pdf
- National Heart Lung and BI (NHLBI). Quality assessment tool for observational cohort and cross-sectional studies [Internet]. Bethesda, MD: National Institutes of Health, Department of Health and Human Services. 2014. p. 1–4. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6(12):1–7. doi: 10.1136/bmjopen-2016-011458
- Elsamany S, Elsisi GH, Hassanin F, et al. Budget impact analysis of subcutaneous trastuzumab compared to intravenous trastuzumab in Saudi HER2-positive breast cancer patients. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):511–518. doi: 10.1080/14737167.2021.1860024
- Sánchez OD, Gutiérrez A, Do Pazo F, et al. Comparative cost analysis of intravenous and subcutaneous administration of rituximab in lymphoma patients. Clinicoecon Outcomes Res. 2019;11:695–701. doi: 10.2147/CEOR.S212257
- Stewart D, Boudreault J, Maturi B, et al. Evaluation of subcutaneous rituximab administration impacts on Canadian systemic therapy suites. Value Health. 2018;21:S250. doi: 10.1016/j.jval.2018.04.1692
- North RT, Harvey VJ, Cox LC, et al. Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study. Clinicoecon Outcomes Res. 2015;7:423–430. doi: 10.2147/CEOR.S85599
- Mihajlovic J, Bax P, van Breugel E, et al. Microcosting study of rituximab subcutaneous injection versus intravenous infusion. Clin Ther. 2017;39(6):1221–1232.e4. doi: 10.1016/j.clinthera.2017.05.342
- Franken MG, Kanters TA, Coenen JL, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791–801. doi: 10.1097/CAD.0000000000000648
- Farolfi A, Silimbani P, Gallegati D, et al. Resource utilization and cost saving analysis of subcutaneous versus intravenous trastuzumab in early breast cancer patients. Oncotarget. 2017;8(46):81343–81349. doi: 10.18632/oncotarget.18527
- Simoens S, Vulto AG, Dylst P. Simulating costs of intravenous biosimilar trastuzumab vs. subcutaneous reference trastuzumab in adjuvant HER2-positive breast cancer: a Belgian case study. Pharmaceuticals (Basel). 2021;14(5):450. doi: 10.3390/ph14050450
- Olofsson S, Norrlid H, Karlsson E, et al. Societal cost of subcutaneous and intravenous trastuzumab for HER2-positive breast cancer - an observational study prospectively recording resource utilization in a Swedish healthcare setting. Breast. 2016;29:140–146.
- Tjalma WAA, Van den Mooter T, Mertens T, et al. Subcutaneous trastuzumab (Herceptin) versus intravenous trastuzumab for the treatment of patients with HER2-positive breast cancer: a time, motion and cost assessment study in a lean operating day care oncology unit. Eur J Obstet Gynecol Reprod Biol. 2018;221:46–51. doi: 10.1016/j.ejogrb.2017.12.006
- Rule S, Collins GP, Samanta K. Subcutaneous vs intravenous rituximab in patients with non-Hodgkin lymphoma: a time and motion study in the United Kingdom. J Med Econ. 2014;17(7):459–468. doi: 10.3111/13696998.2014.914033
- Altini M, Gentili N, Balzi W, et al. The challenge of sustainability in healthcare systems: economic and organizational impact of subcutaneous formulations for rituximab and trastuzumab in onco-hematology. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):503–509. doi: 10.1080/14737167.2020.1764353
- Ponzetti C, Canciani M, Farina M, et al. Potential resource and cost saving analysis of subcutaneous versus intravenous administration for rituximab in non-Hodgkin’s lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey. Clinicoecon Outcomes Res. 2016;8:227–233. doi: 10.2147/CEOR.S97319
- Kading M, Beck B. Cost analysis of daratumumab therapy: is there a cost benefit to using the recently approved subcutaneous product versus the IV product? J Oncol Pharm Pract. 2021;27(4):978–979. doi: 10.1177/1078155221993218
- O’Brien GL, Cooke K, O’Mahony C, et al. Cost minimisation analysis of intravenous or subcutaneous trastuzumab treatment in patients with HER2-positive breast cancer in Ireland. J Oncol Pharm Pract. 2019;25:63–64. doi: 10.1016/j.clbc.2019.01.011
- Mylonas C, Skroumpelos A, Fountzilas G, et al. Cost minimization analysis of herceptin subcutaneous versus herceptin intravenous treatment for patients with HER2+ breast cancer in Greece. J Cancer Policy. 2017;13:11–17. doi: 10.1016/j.jcpo.2017.05.001
- Lopez-Vivanco G, Salvador J, Diez R, et al. Cost minimization analysis of treatment with intravenous or subcutaneous trastuzumab in patients with HER2-positive breast cancer in Spain. Clin Transl Oncol. 2017;19(12):1454–1461. doi: 10.1007/s12094-017-1684-4
- Rojas L, Muñiz S, Medina L, et al. Cost-minimization analysis of subcutaneous versus intravenous trastuzumab administration in Chilean patients with HER2-positive early breast cancer. Plos One. 2020;15(2):1–12. doi: 10.1371/journal.pone.0227961
- Olsen J, Jensen KF, Olesen DS, et al. Costs of subcutaneous and intravenous administration of trastuzumab for patients with HER2-positive breast cancer. J Comp Eff Res. 2018;7(5):411–419. doi: 10.2217/cer-2017-0048
- Hedayati E, Fracheboud L, Srikant V, et al. Economic benefits of subcutaneous trastuzumab administration: a single institutional study from Karolinska University Hospital in Sweden. Plos One. 2019;14(2):e0211783. doi: 10.1371/journal.pone.0211783
- Lugtenburg P, Avivi I, Berenschot H, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102(11):1913–1922. doi: 10.3324/haematol.2017.173583
- Barbee MS, Harvey RD, Lonial S, et al. Subcutaneous versus intravenous bortezomib: efficiency practice variables and patient preferences. Ann Pharmacother. 2013;47(9):1136–1142. doi: 10.1177/1060028013503122
- Usmani S, Mateos M-V, Hungria V, et al. Greater treatment satisfaction in patients Receiving subcutaneous (SC) versus intravenous (IV) daratumumab (DARA) for Relapsed or Refractory multiple myeloma (RRMM): COLUMBA. Clin Lymphoma Myeloma Leuk. 2019;19(10):e247–e248. doi: 10.1016/j.clml.2019.09.410
- Pivot X, Spano JP, Espie M, et al. Patients’ preference of trastuzumab administration (subcutaneous versus intravenous) in HER2-positive metastatic breast cancer: results of the randomised MetaspHer study. Eur J Cancer. 2017;82:230–236. doi: 10.1016/j.ejca.2017.05.009
- Assouline S, Buccheri V, Delmer A, et al. Pharmacokinetics and safety of subcutaneous rituximab plus fludarabine and cyclophosphamide for patients with chronic lymphocytic leukaemia. Br J Clin Pharmacol. 2015;80(5):1001–1009. doi: 10.1111/bcp.12662
- Ciruelos EM, Montano A, Rodriguez CA, et al. Phase III study to evaluate patient’s preference of subcutaneous versus intravenous trastuzumab in HER2-positive metastatic breast cancer patients: results from the ChangHER study (GEICAM/2012-07). Eur J Cancer Care (Engl). 2020;29(4):e13253. doi: 10.1111/ecc.13253
- Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836–842. doi: 10.1093/annonc/mdw685
- Pivot X, Gligorov J, Muller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979–1987. doi: 10.1093/annonc/mdu364
- Iida S, Ishikawa T, Min CK, et al. Subcutaneous daratumumab in Asian patients with heavily pretreated multiple myeloma: subgroup analyses of the noninferiority, phase 3 COLUMBA study. Ann Hematol. 2021;100(4):1065–1077. doi: 10.1007/s00277-021-04405-2
- Lazaro Cebas A, Cortijo Cascajares S, Pablos Bravo S, et al. Subcutaneous versus intravenous administration of trastuzumab: preference of HER2+ breast cancer patients and financial impact of its use. J Buon. 2017;22(2):334–339.
- Pivot X, Gligorov J, Müller V, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–970. doi: 10.1016/S1470-2045(13)70383-8
- De Cock E, Pivot X, Hauser N, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5(3):389–397. doi: 10.1002/cam4.573
- Slavcev M, Spinelli A, Absalon E, et al. Results of a time and motion survey regarding subcutaneous versus intravenous administration of daratumumab in patients with relapsed or refractory multiple myeloma. Clinicoecon Outcomes Res. 2021;13:465–473. doi: 10.2147/CEOR.S302682
- De Cock E, Kritikou P, Sandoval M, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. Plos One. 2016;11(6):e0157957. doi: 10.1371/journal.pone.0157957
- Harvey MJ, Zhong Y, Morris E, et al. Assessing the transition from intravenous to subcutaneous delivery of rituximab: benefits for payers, health care professionals, and patients with lymphoma. Plos One. 2022;17(1):e0261336. doi: 10.1371/journal.pone.0261336
- Olivera Changra H, Robles Díaz JF. Costos de la administración intravenosa vs. subcutánea del trastuzumab en pacientes peruanas con cáncer de mama HER2 positivo. Un análisis observacional de los costos directos e indirectos. J Healthc Qual Res. 2022;37(3):147–154. doi: 10.1016/j.jhqr.2021.10.008
- Kahan BC, Cro S, Doré CJ, et al. Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials. Trials. 2014;15(1):1–6. doi: 10.1186/1745-6215-15-456