ABSTRACT
Objective
Exploratory analysis to conceptualize and evaluate the potential cost-effectiveness and economic drivers of using a novel tissue valve compared with mechanical heart valves for surgical aortic valve replacement (SAVR) in people aged 55–64 and 65+ with aortic stenosis (AS) from a National Health Service (NHS) UK perspective.
Methods
A decision-analytic model was developed using a partitioned survival model. Parameter inputs were obtained from published literature. Deterministic and probabilistic sensitivity analyses (DSA and PSA) were conducted to explore the uncertainty around the parameters.
Results
The novel tissue valve was potentially associated with higher quality-adjusted life years (QALYs) of 0.01 per person. Potential cost savings were greatest for those aged 55–64 (£408) versus those aged 65+(£53). DSA indicated the results to be most dependent on relative differences in general mortality, procedure costs, and reoperation rates. PSA estimated around 75% of the iterations to be cost-effective at £20,000 per QALY for those aged 55–64, and 57% for those aged 65+.
Conclusions
The exploratory analysis suggests that the novel tissue valve could be a cost-effective intervention for people over the age of 55 with AS who are suitable for SAVR in the UK.
1. Introduction
Aortic stenosis (AS) is a common valvular heart disease in England. It is caused by an obstruction of blood flow across the aortic valve, due to pathological narrowing, which can progress to heart failure and is associated with a poor prognostic outlook and reduced quality of life [Citation1,Citation2]. Treating AS can put a significant strain on health systems, with treatments for severe AS often involving surgery to replace the faulty valve with a mechanical or tissue valve [Citation3]. AS is a degenerative condition and incidence increases with age [Citation4–6]. It has been predicted that, due to the aging population in Western countries, prevalence of AS and other valvular heart disease will increase, representing a high burden on the health-care system [Citation7]. This could lead to capacity challenges for health services when faced with increasing demand for surgical and transcatheter interventions [Citation8].
Valve choice (mechanical or tissue) is indicated within NICE, EACTS/ESC, and AHA/ACC guidelines to be a shared-decision making process, depending on patient age, preference, lifestyle, environmental factors, and risk related to anticoagulants among other factors [Citation9–11]. A benefit of tissue valves over mechanical valves is that they do not require lifelong anticoagulant treatment and monitoring [Citation11]. Mechanical valves were found to be associated with a higher risk of bleeding, and potentially an increased risk of stroke or thromboembolic events compared with tissue valves [Citation12–14]. However, mechanical valves have also been shown to be more durable than tissue valves and require fewer reoperations. A number of factors are thought to contribute to prosthetic valve longevity; some are specific to prosthetic valve design [Citation15–17], while others relate to peoples’ predisposing factors such age. However, around half of porcine aortic valves suffer structural valve degeneration (SVD), a type of valve failure, within 15 years of implantation [Citation18].
The valve under study is a next-generation tissue valve for aortic valve replacement (INSPIRISTM) that is the same as the Perimount Magna-Ease valve, with the addition of a novel tissue treatment, RESILIA, and valve in valve (VFit) technology [Citation19]. The novel tissue valve has undergone initial testing and it is assumed that this new tissue treatment will reduce the risk of recalcification as a result of the anti-calcification technology, which may allow the valve to last longer than conventional bioprosthetic valves [Citation20].
The novel tissue has demonstrated improvements in hemodynamic and anticalcification properties when compared with standard of care valves in safety and early efficacy studies undertaken to date [Citation21]. Latest evidence from the COMMENCE aortic FDA clinical trial, which was designed to evaluate the safety and effectiveness of a bioprosthetic valve with RESILIA tissue, suggests that there are clinical benefits and no evidence of structural valve deterioration over 5 years [Citation22]. Evaluation is still ongoing, and a subsample of nearly 200 people will be followed up to the end of the 11th year to provide the medical community with longer term durability data, with results expected in 2027 [Citation23].
Only one known study has looked at the cost-effectiveness of different types of heart valves in aortic stenosis; it did not, however, analyze the key economic drivers by way of sensitivity analysis and was not from a UK perspective [Citation24]. This study took the perspective from Iran and concluded tissue valves were more effective and less expensive compared to mechanical valves [Citation25]. Key differences in study methodologies, assumptions, and geography limit the generalizability of this evidence to the UK NHS.
The objective of this study was to develop a robust framework for assessing the cost-effectiveness of tissue versus mechanical valves. This was then applied to provide an exploratory health-economic model to assess the potential cost-effectiveness of using the novel tissue valve versus generic mechanical valves for those with severe AS eligible for SAVR in the UK NHS. As part of this exploratory analysis, a range of sensitivity and scenario analyses were conducted to explore the potential impact of introducing the novel tissue valve based on the available clinical evidence and long-term durability expectations. This conceptual framework will be important for future analysis of other types of aortic valve replacements as more data become available. This framework will also be important for considering the importance of valve choice for different groups of people (such as age), which may become increasingly important as the burden of disease increases with an aging population in the UK.
2. Methods
2.1. Model structure
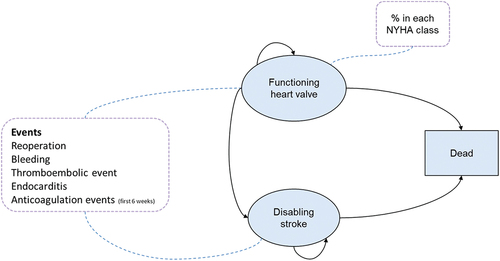
The analysis was aligned with the reference case of the English National Institute for Health and Care Excellence (NICE) [Citation26]. Costs were applied from the perspective of the English National Health Service (NHS), outcomes were quantified in terms of quality-adjusted life years (QALYs), and both costs and QALYs were discounted at 3.5% per annum. A lifetime time horizon was applied due to the chronic nature of the condition. The model was developed in Microsoft Excel® and combined with a decision tree structure and a partitioned-survival model. The decision tree structure was used to capture early events that may occur within the first 30 days of the valve being inserted, while a partitioned-survival model extrapolated the long-term survival data for the risk of mortality and disabling strokes, accounting for how these probabilities would develop over time, rather than assuming constant transitions as per a Markov framework.
The decision tree captured the adverse events and complications associated with each intervention in the first 30 days following the procedure. The adverse events captured were re-operations, major bleeding, endocarditis, permanent pacemaker implantation, thromboembolic events, and disabling strokes. The cohort was split into discrete health states at the end of the decision tree, which defined the starting health states for the longer-term, partitioned-survival portion of the model. These health states were functioning heart valve, disabling stroke, and dead. The functioning heart valve health state was defined as those who have undergone the procedure suffering either no adverse events or short-term adverse events. The disabling stroke health state was defined as those who had suffered a severe stroke which is expected to impact both long term medical costs and health-related quality of life (HRQoL).
A partitioned survival model was used to capture the longer-term outcomes of people with AS (). The model cycle length was equivalent to 1 month. The model splits those in the functioning valve state by the NYHA class, to account for heart failure symptoms among people with AS (which NYHA is a key measure of) that may be worse than others; due to a paucity of published data, however, the proportions in each treatment arm were assumed to be equal. NYHA is regularly collected as part of clinical trials, but the data is not always published beyond baseline; hence, it was still considered useful to have in the conceptual framework for the economic model, as future economic evaluations may have access to complete trial data. The overarching structure of the model and the events captured were discussed with UK cardiac surgeons. This was done to verify the plausibility of the model structure as well as to confirm whether key potential differences between valve types were captured, based on the available published data.
Figure 1. Model structure.
Bleeding, endocarditis, and thromboembolic events (that were not considered disabling) were captured by applying monthly rates to those who were alive in each cycle with a one-off cost and utility decrement applied. The use of anticoagulant medication was captured by applying a monthly cost in each model cycle; for the novel tissue valve, this was applied for the first 3 months postprocedure only, based on clinical guidelines [Citation11]. For those with a mechanical valve, anticoagulation costs were applied for the rest of their life.
Requiring a re-operation was an ongoing risk with both treatments and was included in the model for those who survived without a disabling stroke. This was based on clinical opinion which indicated those who have a disabling stroke are unlikely to be healthy enough to undergo a re-operation. Importantly, reoperations in the model only incorporated the cost of the procedure, without capturing any effect the new valve may have on adverse events e.g. risk of stroke. This assumption was made due to insufficient data for modeling treatment sequences.
It was assumed that people with AS with symptomatic structural valve deterioration (SVD) undergo reoperation soon after initial symptoms present and were therefore not included as a health state in the model.
2.2. Model inputs
The key clinical studies used to populate the model were the COMMENCE trial, matched observational studies for generic tissue and generic mechanical valves, as well as previous studies on specific tissue valves split by age groups, which were identified from a pragmatic literature review [Citation12–14,Citation27–30].
2.2.1. Population
All people entering the model were eligible to receive an aortic valve replacement. Given that the exact proportion of men versus women receiving aortic valve replacement is unknown, it was assumed in the base case that 60% of the population was male, as indicated by previous trials and observational studies [Citation12,Citation14,Citation30]. The starting age of the population was 55 or 65 years, depending on the choice of analysis. The 65+ age group was of interest because of the current guideline recommendations [Citation9,Citation11]. The 55–64 age group was chosen to assess the potential benefits in a younger population because it is anticipated the new valve may be an alternative choice for younger people who do not wish to be on anticoagulant medication for the rest of their life [Citation9,Citation11].
2.2.2. Mortality
The impact of each treatment on mortality was estimated using parametric survival analysis. Published Kaplan–Meier (KM) curves from Glaser et al. were utilized for the analysis of the generic mechanical valve [Citation30]. The Kaplan–Meier (KM) method provides a representation of survival over the time period in which data was collected, allowing for incomplete patient records where patients are lost to follow up [Citation31]. The survival curves were digitized using WebPlotDigitizer. The digitized data were then analyzed using RStudio using an algorithm developed by Guyot et al., whereby digitized KM curves were used to recreate individual patient-level data [Citation32]. A Gompertz function was used to extrapolate the data because this was found to be the best fit using standard statistical methods (AIC and BIC). The plausibility of the long-term extrapolation was validated by clinical experts. A proportional hazards assumption was used for the novel tissue valve based on a constant hazard ratio of 1, therefore it was assumed in the base case that there was no difference in mortality between valves, as indicated by Attia et al. [Citation33], but the model retains the functionality to investigate potential differences in mortality rates. Mortality rates in the model were adjusted to those in the general population in England, to calibrate mortality so that the rates in each model cycle never drop below the population average [Citation34].
2.2.3. Adverse events
2.2.3.1. Thromboembolic events and disabling stroke
Thromboembolic events and disabling stroke data were captured using parametric survival curve analysis, with a constant proportion of all thromboembolic events classified as a disabling stroke, i.e. events which were more severe and therefore incur longer term costs and outcomes. The constant proportions were based on a previous study which defined the proportion of these events as disabling Long-term probabilities were estimated from published KM curves, as described for mortality, with a Weibull function used to extrapolate the data. AIC and BIC statistics were used to give an initial indication of the best fitting curves, with the final curve selection undertaking following validation by clinical experts. For the novel tissue valve, they were based on previous studies on specific tissue valves split by age group [Citation28–30]. A hazard ratio was then used to determine the mechanical valve thromboembolic events and disabling strokes, based on a previous paper reporting the hazard (0.78 in favor of bioprosthetic valves) [Citation13]. Similar papers have also reported an improvement in thromboembolic events or stroke from using bioprosthetic valves [Citation12]. This is a naïve comparison, given it is assuming that the novel tissue valve will be as effective as older generic bioprosthetic valves. This limitation is detailed further in Section 4.1.
2.2.3.2. Reoperation and bleeding events
Reoperation and bleeding rates differ between valve types and by age subgroup in the relevant clinical evidence [Citation27–30]. These are presented in the supplementary material. Data were extracted using WebPlotDigitizer from published KM curves, with the annual rates calculated from these curves. Time varying rates of reoperation and bleeding were used in the model with both increasing over time, as aligned with expert clinical opinion sought as part of the analysis. This is also confirmed in published guidelines that over time people are at higher risk of these events [Citation9].
2.2.3.3. Endocarditis
The risk of endocarditis was assumed to be the same across all valve types, given that endocarditis is reported to be independent of valve type [Citation35]. To capture the short-term occurrence of this event (within 30 days of the procedure) a rate of 0.4% and 0.1% for age 55–64 and 65+ subgroups, respectively, was applied both based on available data for a specific tissue valve [Citation28,Citation29]. The endocarditis rate in the long term is estimated to be 0.3–1.2% per year [Citation35]. The midpoint of these long-term estimates was taken (0.75%) and assumed to be constant over the time horizon of the model beyond 30 days.
2.2.3.4. NYHA classification
NYHA classes were used to assign health state costs and utility scores to people in the functioning heart valve health state. Due to the paucity of data on NYHA class, this was assumed not to differ by valve type. Five-year data were available from the COMMENCE trial, which was used for both valve types [Citation27]. It was assumed that the NYHA class distribution for those still alive beyond 5 years remains constant, consistent with other heart failure models [Citation36,Citation37]. Future evidence collection should look to capture greater detail on NYHA classification over time, given this can be used to show differences in quality of life in this population.
2.2.3.5. Pacemaker implantation
The risk of having a new permanent pacemaker fitted was only applied in the first 30 days, since future pacemaker implantation may not be related to the aortic valve, but wider heart failure is already present. However, complications associated with pacemakers such as infections and replacements were captured in the longer-term. Data relating to the proportion of people with AS having a permanent pacemaker was limited, with only the COMMENCE trial providing data for this parameter, which reported 5.3% of the individuals had a pacemaker implanted within 30 days [Citation27]. This proportion was used for all valves in the model.
2.2.3.6. Anticoagulation complications
Complications related to anticoagulation within the first 6 weeks were captured for those with mechanical valves using results from an observational study by Lopez et al. [Citation38]. These percentages were adjusted to account for the 8.7% of the people with AS already on anticoagulation due to previously diagnosed atrial fibrillation in previous observational study data for mechanical valves [Citation30]. The percentages used in the model were 1.8% of the people requiring re-admission and 7.3% of the people requiring additional visits to the primary cardiac center ward. This included admission to treat subtherapeutic INR and appointments to adjust dosage. These events were only applied for those people who were new to warfarin in the mechanical valve arm because they relate to dose stabilization, which was verified by clinical experts.
2.2.4. Costs and resource use
The costs were in British pounds sterling. The majority of the costs used in the model were taken from NHS reference costs, with the others sourced from a pragmatic review of the available literature [Citation39]. All costs used 2019/2020 prices, with older costs inflated using personal social services and research unit (PSSRU) inflation indices [Citation40]. Staff costs were sourced from the PSSRU [Citation40]. Costs used in the model are outlined and listed in .
Table 1. Costs used in the model.
2.2.4.1. Procedure costs
Procedure costs were micro-costed and included preprocedural costs, periprocedural costs, and post-procedure costs. Preprocedural costs were the same for all valves; however, the periprocedural costs and postprocedure costs varied depending on the heart valve type. The key differences were the cost of the valve, with the novel tissue valve being more expensive, and the length of stay postprocedure, which was longer with the mechanical valve [Citation38]. It was assumed, due to paucity of data, that those undergoing a re-operation would incur the same cost regardless of the initial valve type because, according to expert clinical opinion, the choice of valve may vary and will be dependent on several factors, such as age, co-morbidities, and patient preference.
2.2.4.2. Anticoagulation complications
Background heart failure costs were also captured in the model. They included outpatient visits and medications classified by NYHA status. People with AS in the functioning heart valve health state required a follow-up consultation at 1 month, 6 months and 12 months postprocedure and annually thereafter, based on NICE guidelines and discussion with clinical experts [Citation9]. Each appointment was costed as a cardiology outpatient appointment and applied as a monthly cost of £259 [Citation39]. The medication costs were dependent upon NYHA classification, which was informed by previous NICE guidance for heart failure and costed using the British National Formulary (BNF) [Citation45,Citation46]. The number of outpatient appointments such as GP visits by NYHA classification is based on a previous heart failure study in Germany, with the costs applied from PSSRU and NHS England reference costs [Citation39,Citation40,Citation44].
2.2.4.3. Medication costs
Associated costs such as warfarin medication and monitoring costs were applied at a total cost of £14 per month for 3 months for a small minority of people (4.7%) who required warfarin on the novel tissue valve and for the remainder of a patient’s life with a mechanical valve [Citation40,Citation45]. Regular quarterly checkups were also captured for INR monitoring for those with a mechanical valve. Novel oral anticoagulants were not considered in the model, because warfarin was expected to be the dominant medication used despite recent evaluations comparing the two anticoagulant types.
The majority of people with the novel tissue valve received aspirin if they were not on warfarin, with the cost taken from the BNF [Citation45]. Those with a novel tissue valve required an echocardiogram annually starting 3 years after valve replacement. This acknowledges the variability in clinical practice and opens guidance from NICE that monitoring may range from 1 to 5 years based on individual patient characteristics and clinical judgment [Citation9]. This variability was also explored in the sensitivity analysis. The unit cost of £85 for an echocardiogram was applied monthly at £7.08 per month from 3 years [Citation39].
2.2.5. Health-related quality of life
Utility values used in the economic model were calculated using age-related UK population norms from Szende et al. [Citation47]. presents the utility values used in the model.
Table 2. Utilities used in the model.
Baseline population norms were applied according to the starting age of the model and adjusted over time as the mean age of the cohort moved through the age categories, in order to derive the functioning heart valve utility. The constant disutility value reported for each NYHA class (without hospitalization) from Griffiths et al. was then subtracted from the population norm at a given time to estimate the utility for each NYHA class health state [Citation49].
The same approach was used to determine the utility value for the disabling stroke health state. A constant decrement based on a weighted average of modified Rankin Scale (mRS) scores 3 to 5 reported by Rivero-Arias et al. was applied [Citation50].
A separate utility decrement for each event included in the model was also applied to capture the short-term impact of hospitalization on quality of life. These were taken from two utility studies. The first captured utilities from questionnaires in the USA but mapped to EQ-5D using UK preference values, and the second captured utilities from questionnaires conducted in Germany [Citation51,Citation52]. Each of these was applied for a duration of 1 month.
2.3. Economic analysis
Primary outcomes generated from the cost-effectiveness model were incremental costs, incremental QALYs, ICERs, net monetary benefit (NMB), and net health benefit (NHB) (). A breakdown of the incremental costs by cost category is given in the supplementary material. ICERs are a summary measure representing the economic value of an intervention, compared with an alternative [Citation31]. An ICER is calculated by dividing the incremental cost by the difference in the chosen measure of health outcome or effect (incremental effect) to provide a ratio of ‘extra cost per extra unit of health effect.’ NMB is a summary statistic that represents the value of an intervention in monetary terms when a willingness to pay threshold for a unit of benefit (for example, a measure of health outcome or QALY) is known. Net health benefit (NHB) is a summary statistic that represents the impact on population health of introducing a new intervention. If NMB and NHB are above zero, this suggests that the intervention being evaluated is cost-effective.
Table 3. A summary of the base case results.
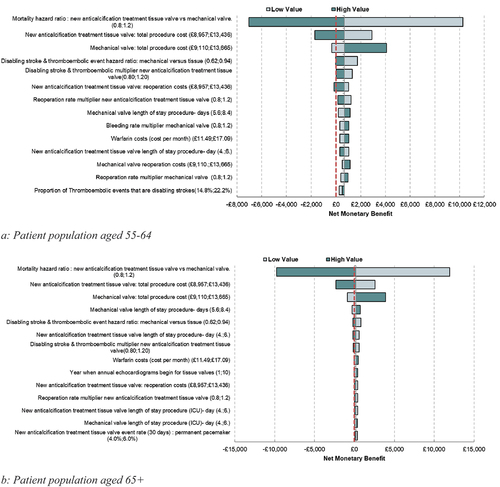
Deterministic sensitivity analysis (DSA) around all parameters was undertaken to determine the key parameters driving the results of the model. This is one of the key objectives of the paper, which is used to and highlight where future evidence generation would be most worthwhile. This is presented in a tornado diagram (). The tornado diagrams are presented using NMB, to avoid any difficulty in the interpretation of a negative ICER.
Figure 2. Deterministic sensitivity analysis.
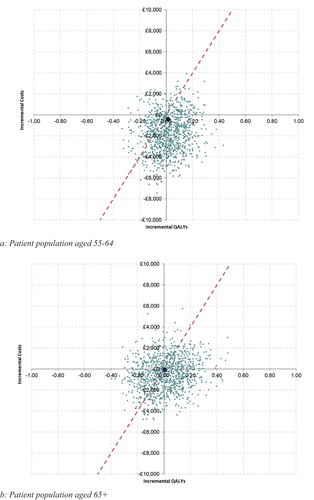
Probabilistic sensitivity analysis (PSA) was undertaken to quantify the level of confidence in the results of the model, in relation to uncertainty in the model inputs. Inputs were varied by the reported standard errors where available, or the standard error was assumed to be 10% of the mean value where standard errors were not reported. The model was run for 1,000 iterations, and results are presented using a cost-effectiveness plane and a cost-effectiveness acceptability curve (CEAC).
Several exploratory scenarios were run in order to investigate clinical experts’ suggestions and the initial evidence that the novel tissue valve may have a higher durability than traditional tissue valves. In the absence of long-term durability data for the RESILIA tissue, a conservative assumption is made to extrapolate long-term reoperation rates (5+ years), such that they are assumed to be equal to generic tissue valves. The results were run with an assumed 10%, 20%, and 30% improvement in reoperation rates (a proxy for durability as a whole) vs the other tissue valves over time to determine the impact this may have on the results of the model. This was run for both age groups 55–64 and 65+. Other scenarios include running the model at different time horizons, to see how the model results change when there is longer extrapolation. These results are presented in the supplementary material.
3. Results
3.1. Base case results
Compared with generic mechanical valves, use of the novel tissue valve is estimated to result in an additional 0.01 QALYs and a decrease in costs of £408 per patient over a lifetime time horizon for those aged 55–64. For those aged 65+, the novel tissue valve is estimated to result in an additional 0.01 QALYs and a decrease in costs of £53 per patient. The estimated ICER per QALY gained is dominant for both those aged 55–64 and 65+; lower costs and higher QALYs. These results are summarized in .
The small difference in QALYs between arms is primarily driven by an increased number of people having a disabling stroke in those with a generic mechanical valve, which has a long-term impact on quality of life. The difference in costs between the novel tissue valve and mechanical valves was driven by the cost of warfarin use associated with mechanical valves. There were also cost savings from a reduction in disabling stroke and bleeding events. These outweighed cost increases associated with the novel tissue valve from the initial procedure, increased reoperations, and additional monitoring costs. Further detail on the differences in costs between the two valve types is provided in . Further information on the difference between events is provided in the supplementary material.
Table 4. Cost breakdown per patient.
3.2. Scenario analysis
Scenario analyses were run with an assumed 10%, 20%, and 30% improvement in reoperation rates over time. The results of this analysis are summarized in . This analysis indicated that the variation in reoperation rates had a significant impact on the costs and NMB, particularly in the age 55–64 group. This could potentially lead to savings of around £800 per patient for those aged 55–64 and around £200 per patient for those aged 65+, assuming a 30% improvement in reoperation rates. Given that the risk of reoperation is one of the biggest limitations for people with AS opting for a tissue valve, the results align with the expectation that improvements in reoperation rates would impact the results when compared to mechanical valves. Further scenario analysis is presented in the supplementary material on the time horizon. This analysis suggests that for both age groups, the benefit over time potentially increases from using the novel tissue valve, with the lifetime horizon providing the highest NMB. This is because the benefits of reducing disabling strokes will accrue over time from quality-of-life differences, while the cost of using warfarin associated with mechanical valves will also continue to accrue into the future.
Table 5. Scenario analysis- improvement in reoperation rate for novel tissue valve from the base case.
3.3. Sensitivity analysis
Results from the DSA showing the key drivers in the model are shown in both for those aged 55–64 and those aged 65+.
shows the impact of varying individual parameters one at a time on the NMB in the model, with a negative value corresponding to an ICER above £20,000 per QALY gained and a positive value to an ICER below this threshold. Based on the sensitivity analysis, the key drivers of the model identified in this analysis are mortality, procedure costs (including length of stay postprocedure), warfarin costs, thromboembolic event and disabling stroke rates, as well as reoperation and bleeding rates.
The results of the DSA indicate that mortality, reoperation costs and rates, thromboembolic events, and disabling stroke rates have a substantial impact on the model results. Therefore, any improvement in reoperation rates (through reductions in the reoperation rates and costs) with the novel tissue valve is likely to see improved cost-effectiveness results when compared with the base case.
PSA was conducted to highlight the uncertainty of the model results. Around 57% of the iterations were cost-effective at a willingness to pay threshold of £20,000 per QALY gained when comparing the novel tissue versus generic mechanical valves for those aged 65+, and this increased to 75% of the iterations for those aged 55–64. This analysis is presented in . The results tables and the CEACs for the PSA are presented in the supplementary material.
4. Discussion
4.1 Summary of findings
The exploratory analysis highlights that the key drivers of the model are relative mortality between valve types, procedure costs, reoperation rates, warfarin costs, and disabling stroke and thromboembolic event rates. By highlighting these key economic drivers, the model can help explore the impact of extrapolated evidence on long-term valve-related outcomes and may also shape future evidence generation so that adjusted and relevant comparisons can be made surrounding key parameters in the development of any study protocol.
The exploratory analysis provided cost-effectiveness results for a novel tissue valve across different time horizons. The analysis highlights that the novel tissue valve can potentially be cost saving and improve health outcomes versus generic mechanical valves at a reimbursement threshold of £20,000 per QALY gained for people with AS aged 55 and over, when considering a lifetime horizon. The disaggregated results highlight how the benefits of the intervention are accrued over time, given the chronic nature of the condition. This is the first known cost-effectiveness analysis to compare valve types for surgical aortic valve replacement for a population in the UK with AS.
Previous cost-effectiveness analysis of tissue valves compared with mechanical valves is limited, with only one previous study identified from Iran, which presented similar results to this paper, indicating tissue valves have the potential to be cost saving, with an improvement to health [Citation15]. The framework and modeling approach set out in this paper can therefore be used to compare and synthesize future evidence and provide decision makers with a platform to evaluate differences in aortic valve types.
However, there remains uncertainty in the results due to the exploratory nature of the analysis. This uncertainty is primarily due to a lack of long-term data concerning the efficacy of the novel tissue valve, which can be explored in the future analysis. The novel tissue valve is an updated iteration of previous tissue valves Magna Ease and Perimount, incorporating technology from these valves with an updated durable tissue. The base case assumption is that the novel tissue valve will be at least as good as the previous generation tissue valves, given the technology is based on these valves, but early indications are that it will perform better, particularly in terms of durability [Citation19].
Several conservative assumptions were made in the analysis due to data availability for the new valve. Firstly, the main disadvantage of tissue valves is durability when compared with mechanical valves, meaning tissue valves are more likely to require reintervention [Citation12,Citation14,Citation34]. However, the novel tissue valve is expected to have improved durability compared with older generation valves (such as Magna Ease products). The scenario analysis conducted around reoperation rates highlights that there is the potential for greater cost savings if reoperation rates with the new valve improve on its previous iterations [Citation19]. Longer term durability data is expected in the coming years which will allow this analysis to be updated.
Furthermore, there may be other benefits associated with the novel tissue valve which have not been investigated in the current analysis. Relative to other types of tissue valves, the novel tissue valve has been specifically designed for future valve-in-valve procedures i.e. transcatheter aortic valve replacement (TAVR), at a time when people are older and potentially at a higher risk for complications [Citation53]. A TAVR is a minimally invasive heart procedure to treat AS, useful for people who are at high-risk of complications from a SAVR. This may therefore provide additional relevant treatment options for those who initially received the tissue valve.
However, these features are based on bench data and have yet to be evaluated in clinical studies to establish the safety and effectiveness of the device for use in valve-in-valve procedures. This potential benefit could be added to future analysis once the efficacy is confirmed, which could potentially show further cost savings from the novel tissue valve. In addition to this, the valve-in-valve feature is likely to provide important benefits that cannot be captured in an economic model relating to patient choice. The fact that current guidelines encourage shared decision-making and an individualized approach to valve choice based on peoples’ values and preference highlights the importance of ensuring people with AS be made aware of such design differences among valve types, so as to enable a fully informed decision on behalf of the patient [Citation9–11].
The choice between the use of mechanical valves and tissue valves is one that has been debated within the literature, and previous guidelines have tended to base some of the recommendations around age, with tissue valves advised in older age groups, however patient preference remains a key determinant [Citation3,Citation9,Citation54]. Hence, it is important to reflect the results for different age groups, given younger age groups may have their own preferences for a particular valve type. Additionally, the need for lifetime anticoagulation medication associated with mechanical valves could strongly factor into patient preference for a tissue valve, resonating as a potential benefit in the younger, 55–64 age group [Citation9,Citation11].
Over time, there have been considerable improvements in tissue valves, aiming to reduce calcification in these types of valves [Citation55,Citation56]. Assuming the new generation tissue valve may be more durable versus existing ones, this should heighten the interest of clinicians in considering tissue valves for those under the age of 60, with new clinical evidence continuing to be monitored. Presently, the NICE, EACTS/ESC, and AHA/ACC guidelines indicate that it is reasonable to individualize the choice of either a mechanical or tissue aortic valve replacement (AVR) for people with AS aged 50–65 years and that valve selection should be a shared decision-making process that considers the patient’s values and preferences [Citation9–11].
Currently, tissue valves are standard of care for older patients, but due to durability concerns, younger patients are often implanted with a mechanical valve. It is important to bear in mind patient choice in decision-making when deciding between a tissue or mechanical valve. Factors which could influence the choice of valve include the patient’s own lifestyle choices. Further evidence collection with RESILIA is ongoing and can be used to improve the certainty of this analysis in the future as data become available [Citation57]. These real-world data, once available, can be propensity score matched to offer a comparative analysis versus mechanical valves, providing greater robustness to the current conclusions.
4.1. Limitations
There are several assumptions and data limitations underpinning the analysis. Firstly, a proportion of the data used in this analysis was taken from retrospective matched observation studies which compared generic tissue with generic mechanical valves. Despite being controlled for certain observed population characteristics, this is not as robust as data from head-to-head randomized control trials (RCTs) [Citation58]. Hence, there is likely to be more heterogeneity in unobservable characteristics, which may have some explanatory factor in the difference in events between valves. Furthermore, some of the data for the novel tissue valves were taken from a single-arm study, which have been compared naïvely with the data on mechanical valves. Specifically, event rates for bleeding at 30 days, longer term reoperation rates, thromboembolic events, and disabling stroke were based on single-arm data. These are all explored in sensitivity analysis.
Secondly, due to the lack of comparative evidence for the novel tissue valve, other events such as bleeding, mortality, thromboembolic events, and disabling strokes use longer term data from either generic tissue valves or perimount (an earlier iteration of the novel tissue valve). However, there is potential that the novel tissue valve could lead to a reduction in these events, relative to other types of tissue valves, which would mean an improvement in the cost-effectiveness of the new valve, although the expected impact of the novel tissue valve is unknown. Given that the novel tissue valve is an updated version of previous technology, then it is unlikely that the novel tissue will perform worse than these previous valve types. However, the results should be interpreted with caution until comparative evidence is produced.
Thirdly, data has been used from multiple studies, which may all define clinical endpoints slightly differently. This may mean that some of the differences in data could be accounted for by slightly different definitions, which are not regularly published alongside the corresponding clinical papers [Citation12,Citation22,Citation28–30]. The Value Academic Research Consortium published updated clinical endpoint definitions for aortic valve research most recently in 2021 [Citation59]. Future clinical evidence collected in relevant studies should look to align clinical endpoint definitions with these suggestions to provide consistency and to allow clearer robust comparisons between evidence sources.
Fourthly, where data were limited, several assumptions were made in the analysis. These include NYHA classification and pacemaker implantation, which are assumed to not differ by valve type, while those requiring a reoperation incurred the same cost, given they are assumed to have an equal chance of receiving any valve type. NYHA classification and pacemaker implantation are not expected to significantly impact the results of the model, while it is uncertain which valve people would receive in a reoperation, given this is likely to depend on the individual's clinical background and any co-morbidities they may have [Citation60]. Clinical opinion was sought to verify the assumption for reoperations, and in the absence of any reliable data, this was used to determine the costs of reoperations. Future research should look to replace the assumptions made where there is a paucity of data, with a view to using any robust data sources to aid the certainty of the analysis.
Additionally, mortality is expected to not differ by valve type in the model, based on previous literature and discussion with clinical experts [Citation33]. However, there is some variation in the literature, with some studies indicating that there may be difference in mortality, although many of these studies are based on much older tissue valve types [Citation12,Citation14]. Deterministic sensitivity analysis was conducted to test this assumption, highlighting that mortality may have a large impact on the results.
Finally, there are multiple guidelines which cover recommended anticoagulation medication and echocardiography monitoring for tissue valves [Citation9,Citation54]. This may vary based on a person’s own characteristics and co-morbidities, further increasing variation in clinical practice. Echocardiography monitoring for tissue valves was explored in sensitivity analysis and appeared to have some impact on results, more so in those aged 65+, with improved cost-effectiveness results where a longer time period between the procedure and commencement of monitoring was used. It is expected most people on tissue valves are not expected to be on anticoagulation medication, with most receiving aspirin. This is not likely to have a significant impact on the results but should still be confirmed with any new available data. If a higher proportion of people with the new tissue valve were on anticoagulation medication, then costs would increase, and this would reduce the cost-effectiveness estimates for the new valve.
5. Conclusion
The exploratory cost-effectiveness analysis set out in this paper creates a framework to evaluate future aortic valve types, as more evidence becomes available. As part of the analysis, the framework was used to understand the key drivers of the results, which were mortality, procedure costs, durability, warfarin costs, and disabling stroke and thromboembolic event rates. An exploratory analysis was conducted which suggests that the novel tissue valve has the potential to save costs and improve health outcomes in the UK NHS when compared with generic mechanical valves for those aged between 55 and 64 and those aged 65+. These results highlight the further importance of patient choice within the valve type for AVR, as younger populations with AS may consider newer tissue valves as an alternative to mechanical valves.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
R Malcolm, C Buckley, and J Shore were involved in the conceptualization and design of the economic model, with input from A White, B Marti. N Nikolaidis, A Lopez, and O Wendler provided critical input from a clinical perspective for the model design. The analyses were undertaken and guided by R Malcolm, J Shore, C Buckley, A Stainthorpe, and J Carapinha. Review was provided by M Vernia, J Deckert, A White, B Marti, and J Carapinha. The manuscript was initially drafted by R Malcolm, C Buckley, J Shore, and A Stainthorpe. Critical revision was provided by all other authors. All authors read and approved the final version of the manuscript for publication.
Supplemental Material
Download MS Word (78.9 KB)Acknowledgments
Michelle Green gave advice in the design of the economic model to evaluate using the bioprosthetic valve with a novel tissue in the UK.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2249611
Additional information
Funding
References
- Bonow RO, Greenland P. Population-wide trends in aortic stenosis incidence and outcomes. Circulation. 2015;131(11):969–971. doi: 10.1161/CIRCULATIONAHA.115.014846
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373(9667):956–966. doi: 10.1016/S0140-6736(09)60211-7
- Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38(28):2183–2191. doi: 10.1093/eurheartj/ehx141
- Andell P, Li X, Martinsson A, et al. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017 Nov;103(21):1696–1703. doi: 10.1136/heartjnl-2016-310894
- Eveborn GW, Schirmer H, Heggelund G, et al. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99(6):396–400. doi: 10.1136/heartjnl-2012-302265
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8
- d’Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE population cohort study. Eur Heart J. 2016;37(47):3515–3522. doi: 10.1093/eurheartj/ehw229
- Strange GA, Stewart S, Curzen N, et al. Uncovering the treatable burden of severe aortic stenosis in the UK. Open Heart. 2022;9(1):e001783. doi: 10.1136/openhrt-2021-001783
- National Institute for Health and Care Excellence (NICE). Heart valve disease presenting in adults: investigation and management 2021. Available from: https://www.nice.org.uk/guidance/ng208
- Otto CM, Nishimura RA, Bonow RO, et al. ACC/AHA Guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Joint Committee on clinical practice guidelines. Circulation. 2020 [2021 Feb 2];143(5):e35–e71.
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic surgery (EACTS). Rev Esp Cardiol (Engl Ed). 2022 Jun;75(6):524. doi: 10.1016/j.rec.2022.05.006
- Brennan JM, Edwards FH, Zhao Y, et al. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the society of thoracic surgeons adult cardiac surgery national database. Circulation. 2013;127(16):1647–1655. doi: 10.1161/CIRCULATIONAHA.113.002003
- Kytö V, Ahtela E, Sipilä J, et al. Mechanical versus biological valve prosthesis for surgical aortic valve replacement in patients with infective endocarditis. Interact Cardiovasc Thorac Surg. 2019 Sep 1;29(3):386–392. doi: 10.1093/icvts/ivz122
- Zhao DF, Seco M, Wu JJ, et al. Mechanical versus bioprosthetic aortic valve replacement in middle-aged adults: a systematic review and meta-analysis. Ann Thorac Surg. 2016;102(1):315–327. doi: 10.1016/j.athoracsur.2015.10.092
- Biancari F, Valtola A, Juvonen T, et al. Trifecta versus perimount magna ease aortic valve prostheses. Ann Thorac Surg. 2020;110(3):879–888. doi: 10.1016/j.athoracsur.2019.12.071
- Lam KY, Koene B, Timmermans N, et al. Reintervention after aortic valve replacement: comparison of 3 aortic bioprostheses. Ann Thorac Surg. 2020;110(2):615–621. doi: 10.1016/j.athoracsur.2019.10.060
- Theologou T, Harky A, Shaw M, et al. Mitroflow and Perimount Magna 10 years outcomes a direct propensity match analysis to assess reintervention rates and long follow‐up mortality. J Card Surg. 2019;34(11):1279–1287. doi: 10.1111/jocs.14250
- Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79(3):1072–1080. doi: 10.1016/j.athoracsur.2004.06.033
- Shala M, Niclauss L. Early results of the Resilia Inspiris aortic valve in the old age patients-a retrospective comparison with the Carpentier Edwards Magna ease. J Cardiovasc Thorac Res. 2020;12(3):229–233. doi: 10.34172/jcvtr.2020.38
- Johnston DR, Griffith BP, Puskas JD, et al. Intermediate-term outcomes of aortic valve replacement using a bioprosthesis with a novel tissue. J Thorac Cardiovasc Surg. 2021;162(5):1478–1485. doi: 10.1016/j.jtcvs.2020.01.095
- Sadowski J, Bartuś K, Kapelak B, et al. Aortic valve replacement with a novel anti-calcification technology platform. Kardiol Pol. 2015;73(5):317–322. doi: 10.5603/KP.a2014.0214
- Bartus K, Litwinowicz R, Bilewska A, et al. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur J Cardiothorac Surg. 2021 Jan 29;59(2):434–441. doi: 10.1093/ejcts/ezaa311
- Pibarot P, Borger MA, Clavel M-A, et al. Study design of the prospective non-randomized single-arm multicenter evaluation of the durability of aortic bioprosthetic valves with RESILIA tissue in subjects under 65 years old (RESILIENCE trial). Structural Heart. 2020;4(1):46–52. doi: 10.1080/24748706.2019.1686554
- Azari S, Rezapour A, Omidi N, et al. A systematic review of the cost-effectiveness of heart valve replacement with a mechanical versus biological prosthesis in patients with heart valvular disease. Heart Fail Rev. 2020;25(3):495–503. doi: 10.1007/s10741-019-09897-9
- Yaghoubi M, Aghayan HR, Arjmand B, et al. Cost-effectiveness of homograft heart valve replacement surgery: an introductory study. Cell Tissue Bank. 2011;12(2):153–158. doi: 10.1007/s10561-009-9165-9
- National Institute for Health and Care Excellence (NICE). Developing NICE guidelines: the manual 2022. Available from: https://www.nice.org.uk/process/pmg20/chapter/introduction
- Bavaria JG, Griffith B, Heimansohn DA, et al. Five-year outcomes of the COMMENCE trial Investigating aortic valve replacement with a novel tissue bioprosthesis. Annals Thoracic Surgery. 2023 Jun 1;115(6):1429–1436.
- Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–837. doi: 10.1016/j.athoracsur.2014.09.030
- Bourguignon T, Lhommet P, El Khoury R, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50–65 years. Eur J Cardiothorac Surg. 2015;49(5):1462–1468. doi: 10.1093/ejcts/ezv384
- Glaser N, Jackson V, Holzmann MJ, et al. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. 2016;37(34):2658–2667. doi: 10.1093/eurheartj/ehv580
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford university press; 2015.
- Guyot P, Ades A, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):1–13. doi: 10.1186/1471-2288-12-9
- Attia T, Yang Y, Svensson LG, et al. Similar long-term survival after isolated bioprosthetic versus mechanical aortic valve replacement: A propensity-matched analysis. J Thorac Cardiovasc Surg. 2021 Jan 20;164:1444–1455.e4. doi: 10.1016/j.jtcvs.2020.11.181
- Office of National Statisics. National life tables UK (2017-19) 2020.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319
- Mealing S, Feldman T, Eaton J, et al. EVEREST II high risk study based UK cost-effectiveness analysis of MitraClip® in patients with severe mitral regurgitation ineligible for conventional repair/replacement surgery. J Med Econ. 2013 Nov;16(11):1317–1326. doi: 10.3111/13696998.2013.834823
- Shore J, Russell J, Frankenstein L, et al. An analysis of the cost-effectiveness of transcatheter mitral valve repair for people with secondary mitral valve regurgitation in the UK. J Med Econ. 2020 Dec;23(12):1425–1434. doi: 10.1080/13696998.2020.1854769
- Lopez-Marco A, Grant SW, Mohamed S, et al. Impact of mechanical aortic prostheses in hospital stay and anticoagulation related complications. J Surg Res. 2021;4:187–196. doi: 10.26502/jsr.10020125
- NHS. 2018/19 National cost collection data. 2018 Available from: https://www.england.nhs.uk/national-cost-collection/
- Curtis L, Burns A. Personal Social Services Research Unit (PSSRU). Unit Costs of Health and Social Care 2020. Canterbury: University of Kent; 2020. p. 1–188.
- Luengo-Fernandez R, Li L, Rothwell PM, et al. Costs of bleeding on long-term antiplatelet treatment without routine co-prescription of proton-pump inhibitors. Int J Stroke. 2021;16(6):719–726. doi: 10.1177/1747493019879658
- Xu X-M, Vestesson E, Paley L, et al. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J. 2018;3(1):82–91. doi: 10.1177/2396987317746516
- Fox M, Mealing S, Anderson R, et al. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess. 2007;11(47):iii–iv. (Winchester, England) ix. doi: 10.3310/hta11470
- Biermann J, Neumann T, Angermann CE, et al. Resource use and costs in systolic heart failure according to disease severity: a pooled analysis from the German Competence Network heart failure. J Public Health. 2012;20(1):23–30. doi: 10.1007/s10389-011-0452-0
- National Institute of Health and Care Excellence. BNF British National Formulary, 2021. Available from: https://bnf.nice.org.uk/
- National Institute of Health and Care Excellence. NICE guidance:TA314. Assessment Report, Table 112. 2013. Available from: https://www.nice.org.uk/guidance/ta314/documents/arrythmias-icds-heart-failure-cardiac-resynchronisation-assessment-report2
- Szende A, Janssen B, Cabases J Self-reported population health: an international perspective based on EQ-5D. 2014.
- Szende A, Janssen B, Cabases J, et al. Self-reported population health: an international perspective based on EQ-5D. Springer Nature: 2014. doi: 10.1007/978-94-007-7596-1
- Griffiths A, Paracha N, Davies A, et al. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the UK National health Service perspective. Heart. 2014;100(13):1031–1036. doi: 10.1136/heartjnl-2013-304598
- Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Mak. 2010;30(3):341–354. doi: 10.1177/0272989X09349961
- Kaier K, Gutmann A, Baumbach H, et al. Quality of life among elderly patients undergoing transcatheter or surgical aortic valve replacement–a model-based longitudinal data analysis. Health Qual Life Outcomes. 2016;14(1):1–10. doi: 10.1186/s12955-016-0512-9
- Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Mak. 2011;31(6):800–804. doi: 10.1177/0272989X11401031
- Saxon JT, Allen KB, Cohen DJ, et al. Bioprosthetic valve Fracture During valve-in-valve TAVR: Bench to Bedside. Interv Cardiol. 2018 Jan;13(1):20–26. doi: 10.15420/icr.2017:29:1
- Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394
- Manji RA, Ekser B, Menkis AH, et al. Bioprosthetic heart valves of the future. Xenotransplantation. 2014;21(1):1–10. doi: 10.1111/xen.12080
- Manji RA, Lee W, Cooper DK. Xenograft bioprosthetic heart valves: Past, present and future. Int J Surg. 2015;23:280–284. doi: 10.1016/j.ijsu.2015.07.009
- Meuris B, Borger MA, Bourguignon T, et al. Durability of bioprosthetic aortic valves in patients under the age of 60 years–rationale and design of the international INDURE registry. J Cardiothorac Surg. 2020;15(1):1–9. doi: 10.1186/s13019-020-01155-6
- Elamin MB, Montori VM. The hierarchy of evidence: from unsystematic clinical observations to systematic reviews. Neurology: Springer New York; 2012. p. 11–24. doi: 10.1007/978-0-387-88555-1_2
- Committee V-W, Généreux P, Piazza N, et al. Valve Academic research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42(19):1825–1857. doi: 10.1093/eurheartj/ehaa799
- El Oakley R, Kleine P, Bach DS. Choice of prosthetic heart valve in today’s practice. Circulation. 2008;117(2):253–256. doi: 10.1161/CIRCULATIONAHA.107.736819