ABSTRACT
Introduction
Cognitive behavior therapy (CBT) is effective for pathological health anxiety, but little is known about unwanted outcomes.
Areas covered
We investigated unwanted outcomes in the form of adverse events, overall symptom deterioration, and dropouts in CBT for pathological health anxiety based on a systematic review of 19 randomized controlled trials (PubMed, PsycInfo, and OATD; last updated 2 June 2023; pooled N = 2188), and then a secondary original study of two randomized controlled trials (pooled N = 336). In the systematic review, 10% of participants in CBT reported at least one adverse event and 17% dropped out. Heterogeneity was substantial. In the original investigation, 17% reported at least one adverse event, 0–10% met criteria for overall symptom deterioration, and 10–19% dropped out. In guided Internet-delivered CBT, dropouts were more common with lower education and lower credibility/expectancy ratings. Higher adherence was associated with a larger reduction in health anxiety.
Expert opinion
Unwanted effects are routinely seen in CBT for pathological health anxiety, but, under typical circumstances, appear to be acceptable in light of the treatment’s efficacy. There is a need for more consistent methods to improve our understanding adverse events, dropouts, and overall symptom deterioration, and how these outcomes can be prevented.
Plain languages summary
People who worry excessively about having or developing a serious disease are commonly offered cognitive behavior therapy (CBT). Little is known about unwanted outcomes in CBT for this patient group. This study had two parts. First, we conducted a systematic search of the existing literature where we found that about 10% of patients in CBT experience an event that they perceive as unwanted or negative. About 17% of patients drop out of treatment prematurely. Results differed substantially between studies. Second, we analyzed the outcome of two original studies and found that about 17% of patients in CBT experience an event that they perceive as unwanted or negative. Patients who experienced such an event reported, on average, a smaller reduction in health anxiety if CBT was delivered face-to-face, but not if it was delivered via the Internet. About 0–10% rated their health anxiety as having become worse after CBT, and 10–19% dropped out prematurely. In CBT delivered via the Internet, patients were more likely to drop out if their level of education was lower, and if they rated the treatment as less credible and expectancy-evoking during week 2. We conclude that unwanted effects are relatively common but typically mild and acceptable.
1. Introduction
Pathological health anxiety is characterized by an excessive and recurrent fear of, or preoccupation with, having or developing a serious health condition such as terminal cancer, a severe cardiovascular disease, or a progressive neurological disorder [Citation1–6]. The prevalence of pathological health anxiety is approximately 0.4–13.1% depending on the precise definition, with most estimates clustering around one to a few percent [Citation7]. If left untreated, pathological health anxiety is predictive not only of long-term distress and functional impairment, but also of increased healthcare consumption [Citation8–10]. Cognitive behavior therapy (CBT) that focuses on either cognitive restructuring techniques, behavioral experiments, or exposure and response prevention has a response rate of approximately 66% [Citation11]. Little is known, however, about unwanted outcomes such as adverse events, deterioration, and treatment dropouts.
‘Adverse events’ refers to events that occur during treatment, and that are perceived as unwanted or negative [Citation12]. A factor analysis of unwanted effects in CBT for social anxiety disorder found support for most events belonging to one of the following categories (factors): (1) an increase in illness severity or symptoms, (2) disappointment with the quality of the treatment, (3) dependency on the treatment or therapist, (4) stigma associated with being enrolled in treatment, (5) hopelessness, and (6) a sense of failure and lowered self-efficacy [Citation13]. In the case of pathological health anxiety, no such extensive analysis, nor a systematic review of the existing evidence pertaining to adverse events, has ever been conducted. It is therefore unclear how common adverse events are, and which variants that are more common than others. It is also unclear if adverse events are associated with worsened outcomes, or if there is a ‘no pain, no gain’ relationship, meaning that adverse events typically result from more engagement with the treatment, and therefore correlate with more beneficial effects [Citation14].
‘Overall symptom deterioration’ here refers to an increase in the main symptom domain (e.g. health anxiety) over the treatment phase; rates of which are rarely reported in psychotherapy trials. In therapist-guided Internet-delivered CBT (ICBT) for various anxiety disorders, rates of reliable symptom deterioration (α = 20%) have been reported in the range of 0–9%, as compared to 5–36% in the control groups of randomized controlled trials (RCTs) [Citation15]. In CBT for pathological health anxiety, symptom deterioration rates are unknown.
A ‘dropout’ is usually understood to imply the premature termination of a treatment. In psychological treatments in general, a typical dropout rate is 20% [Citation16]. In the treatment of anxiety disorders, CBT is the typical therapy and dropout rates have been reported anywhere from 17 to 85% in individual trials [Citation17–19], with pooled estimates ranging from 11 to 18% [Citation19,Citation20]. Early dropouts constitute a substantial proportion of these [Citation17,Citation21,Citation22]. For obsessive-compulsive disorder (OCD), pooled estimates have ranged from 10 to 15% [Citation23,Citation24], and have been found to be significantly lower than those for pharmacotherapy [Citation23]. An indirect way of estimating dropout rates is on the basis of missing data. In Internet-delivered, mostly CBT, treatments, one systematic review found that the pooled pathological health anxiety missing data rate was 10% (95% CI: 0–23, k = 10) [Citation25]. Another systematic review that concerned pathological health anxiety reported a missing data rate of 8% in ICBT (95% CI: −3–18, I2 = 93%, k = 4) and 15% in face-to-face CBT (95% CI: 8–22, I2 = 87%, k = 12) [Citation11]. More direct indicators of dropout rates have not been systematically reviewed with regard to CBT for pathological health anxiety. Causes of dropouts are also poorly understood. It is for example unclear whether, as in OCD [Citation26], early and late dropouts are differentially associated with symptoms and outcomes. In the prediction of dropouts, there is evidence from other psychotherapy research that the patient’s age, educational attainment, personality traits, overall symptom burden, initial adherence, expectations and beliefs about the treatment, and also therapist characteristics could be important [Citation17,Citation18,Citation22,Citation27,Citation28]. Structural factors such as whether the treatment is delivered in routine care, and the financial burden on the patient may also be predictive of dropouts [Citation22].
A better understanding of unwanted effects in CBT for pathological health anxiety could allow for more beneficial treatment decisions, and may provide the basis for improving the treatment format further. For example, if certain adverse events are found to be common, or if certain variables are found to be strong predictors of dropouts, this could be directly addressed in the treatment. We aimed to advance knowledge in this area by conducting two studies: First, a systematic review and meta-analysis of unwanted effects in RCTs of CBT for pathological health anxiety. Second, an original investigation of unwanted effects based on data pooled from two RCTs [Citation29,Citation30]. In the original investigation, we aimed to systematically determine the themes of all adverse events as based on the aforementioned taxonomy [Citation13]. Furthermore, we aimed to quantify the distress associated with events, and whether adverse events were associated with baseline variables, treatment adherence, and change in health anxiety. We also aimed to determine the rate of deterioration, the rate of dropouts, and whether dropouts were associated with baseline variables and change in health anxiety. Last, we also aimed to model the association between adverse events, deterioration, and dropouts. Overall, we expected outcomes similar to those seen in CBT for other anxiety and obsessive-compulsive spectrum disorders. Thus, we expected adverse events to be typically acceptable but associated with worse clinical outcomes [Citation14], we expected an incidence of overall symptom deterioration of a few percent [Citation15], and we expected dropout rates in the vicinity of 10–20% [Citation16,Citation19,Citation20,Citation23,Citation24].
2. Methods
2.1. Design
We explored unwanted outcomes – adverse events, overall symptom deterioration, and dropouts – in CBT for pathological health anxiety within two frameworks. First, we conducted a systematic review and meta-analysis of unwanted outcomes reported from published RCTs of CBT versus non-CBT controls for pathological health anxiety. For this review, we updated an existing literature search [Citation11] that was not preregistered. Second, we conducted an original investigation using data from two RCTs of CBT for pathological health anxiety [Citation29,Citation30], one of which was also included in the systematic review [Citation29]. Both trials were collaborations between Karolinska Institutet and Gustavsberg Primary Health Care Center, Stockholm, Sweden (pooled N = 336), were approved by the Regional Ethical Review Board in Stockholm (2013/375–31, 2014/1530–31), and all participants provided informed consent. The trials were preregistered at ClinicalTrials.gov (NCT01667822, NCT02314065) and this secondary study was preregistered via the Open Science Framework on 15 November 2022 (https://osf.io/pa89t/).
2.2. Method of the systematic review and meta-analysis
2.2.1. Search strategy and selection of studies
The completion and presentation of the systematic review adhered to PRISMA 2020 [Citation31]. On 6 February 2023, we updated our existing systematic search of PubMed, PsycInfo, and the Open Access Theses and Dissertations (OATD) resource (www.oatd.org). Searches combined terms pertaining to health anxiety with terms pertaining to RCTs. See the supplement of the original systematic review for the complete search terms [Citation11]. We de-duplicated search hits, last in Endnote X9 [Citation32], and then proceeded to independently assess all remaining search hits in parallel. This was first done for titles and abstracts, and then for full texts that had not been excluded by both assessors in the first phase. Reasons for exclusion were coded in consecutive order by the first author, so as to ensure that publications formally excluded on criterion b had passed criterion a, those excluded on c had passed a + b, and so on. For the updated search, a minor change to the method was the use of Rayyan [Citation33] to improve reliability. Though there were no cases of disagreemnet in the updated search (see below), in the original search, cases of disagreement were discussed and a decision was made in consensus.
2.2.2. Eligibility criteria
We included studies (a) published in English that were (b) RCTs versus at least one non-CBT control, (c) for adults (at least 18 years) (d) with pathological health anxiety, (e) and that focused on conventional CBT (cognitive restructuring, exposure-based techniques including response prevention, or both) and (f) reported treatment effects. Pathological health anxiety (criterion d) was defined as either (i) a clear-cut prototypical diagnosis such as hypochondriasis or illness anxiety disorder, (ii) all scores above a credible clinical cutoff, or (iii) somatic symptom disorder [Citation34] with recruitment clearly focusing on pathological health anxiety. For each RCT, (g) we formally included one publication only.
2.2.3. Data extraction
We extracted information about unwanted effects in electronic spreadsheets. For adverse effects, we tabulated frequencies and whether questions that concerned adverse events were open-ended or closed. For dropouts, we tabulated frequencies and the study-specific definitions. As an indirect indicator of dropout rates, we also tabulated the missing data rates at the post-treatment assessment. In addition to these main outcomes, we also tabulated basic study characteristics such as the author, year of publication, country of origin, overall sample size, main inclusion criterion, and the basic characteristics of CBT and the control conditions.
2.2.4. Statistical analysis
For the systematic review, descriptive data were compiled and are presented primarily in terms of frequencies on a per-condition and per-RCT basis. Such frequencies of adverse events, dropouts, and missing data were also pooled in meta-analysis. For this purpose, we used R 4.2.0 [Citation35] with the metafor 3.8–1 package [Citation36] and fitted random-effects models using the restricted maximum likelihood estimator. For within-group effects, we used percent as the effect measure. For between-group effects, we instead estimated the relative risk ratio (RR). Because the RR is undefined when the comparator rate is zero, for outcomes where at least one cell of the 2 × 2 table was zero, 0.5 was added to each cell before calculating the RR. In this study, RRs above 1 are indicative of higher frequencies of the unwanted outcome in CBT as compared to the control group. We quantified heterogeneity both in terms of the τ2, which stands for the absolute variance, and the I2, which stands for the proportion of the variance that is due to true study differences as opposed to sampling error. An I2 of 25% is typically regarded as indicative of low heterogeneity, 50% as moderate, and 75% as high [Citation37]. It should be noted, however, that the I2 also increases with the precision of the original studies pooled in the meta-analysis. For the adverse events and dropout rates, we assessed the risk of publication bias based on visual inspection of the funnel plot (CBT groups only), and also Egger’s test [Citation38] and the Duval and Tweedie trim and fill procedure [Citation39]. We did not, however, conduct the corresponding tests with regard to missing data rates because we suspected that these would be systematically smaller in larger studies due a more pronounced focus on intention-to-treat analyses.
2.3. Method of original study based on two randomized controlled trials
2.3.1. Recruitment and procedure
To the extent allowed by this journal, the original study is reported in accordance with the CONSORT harms 2022 statement [Citation40]. Both RCTs were advertised under the caption of ‘Do you worry a lot about your health?’, and information was also sent to the routine care services. All applicants, including those referred from routine care, gave informed consent for participation completed a screening battery of self-report questionnaires via the online study platform, before being contacted for an eligibility interview with a clinical psychologist or master-level resident psychologist. In both trials, it was made clear that the treatment was tailored for individuals with a fear of, or preoccupation with, having or developing a serious disease. We recruited adults with pathological health anxiety [Citation2] who met full criteria for DSM-5 somatic symptom disorder or illness anxiety disorder according to the Health Preoccupation Diagnostic Interview [Citation41] used in conjunction with the Mini-International Neuropsychiatric Interview [Citation42]. Key exclusion criteria were another primary psychiatric disorder, a psychotic or bipolar disorder, severe depression, and recurrent suicidal ideation. Applicants were also required to either not take antidepressant medication, or to express an intent to maintain a stable dosage of antidepressant medication, and not to take part in another psychological treatment, during the main (pre- to post-treatment) phase of the RCT.
Those who completed the pre-treatment assessment were included and randomized. In the first RCT, the conditions were therapist-guided ICBT (n = 32), unguided ICBT (n = 33), unguided cognitive-behavioral bibliotherapy (n = 34), and a waitlist condition (n = 33). In the second RCT, the conditions were face-to-face CBT (n = 102) and therapist-guided ICBT (n = 102). CBT followed variations on the same protocol which was 12 weeks long and based on exposure and response prevention with a strain of mindfulness exercises and instructions that emphasized not acting on negative thoughts and physical sensations. A combination of interoceptive exposure, exposure in vivo, and imaginary exposure exercises were employed [Citation43,Citation44]. In the first weeks of treatment, participants were expected to begin practicing mindfulness, and to begin identifying behaviors relevant for the intervention. They were then encouraged to work with exposure and response prevention on a daily basis. For a more detailed description of the treatment protocol, see the primary publication of each RCT [Citation29,Citation30].
2.3.2. Measures
Participants completed self-report questionnaires via the study web platform. We measured health anxiety using the 18-item Health Anxiety Inventory (HAI-18) [Citation45] before treatment, each week during treatment, and after treatment – resulting in 13 measurement points. At post treatment in both trials, participants completed a questionnaire about adverse events experienced during the 12-week main phase [Citation46], i.e. either during CBT or the WLC. Participants first reported whether they ‘had experienced any type of unwanted event which [they believed was] caused by [their] participation’. Up to three adverse events could be reported. For each adverse event, participants completed a free text field to provide a short description. The participants also indicated how negatively the event had impacted them, and whether the event still impacted them, on a scale from 0: ‘not at all’, to 3: ‘very negatively’. Baseline data on age, gender, educational attainment, depression symptoms, and functional impairment were derived from psychiatric interviews and self-report questionnaires, notably the Montgomery-Åsberg Depression Rating Scale – self report (MADRS-S) [Citation47] and the Sheehan Disability Scale (SDS) [Citation48]. At week 2 in treatment, we administered the C/E scale [Citation49] as a measure of treatment credibility and the expectancy of improvement.
2.3.3. Classification of unwanted effects
For adverse events, the authors independently matched each free text description to one or several of the following pre-defined factors: (1) an increase in illness severity or symptoms, (2) disappointment with the quality of the treatment, (3) dependency on the treatment or therapist, (4) stigma associated with being enrolled in treatment, (5) a sense of hopelessness, and (6) a sense of failure and lowered self-efficacy [Citation13]. We also assessed the probability that the event was related to the treatment on a 1-5 Likert scale derived from Boettcher et al. [Citation50], where 1 stood for ‘unrelated’, 2 ‘probably unrelated’, 3 ‘possibly related’, 4 ‘probably related’, and 5 ‘related’. For overall symptom deterioration, the primary classification was based on whether there had been a reliable pre- to post-treatment increase in the HAI-18 as per the Jacobson and Truax criteria [Citation51], assuming a test-retest correlation of 0.87 [Citation52] and an α of 5%. Because there is no widesly agreed upon method of establishing overall symptom deterioration rates, we also conducted four sensitivity analyses based on the following criteria: (1) reliable deterioration but instead assuming that the true value for the test-retest reliability could be as low as 0.70, (2) reliable deterioration but instead with relaxed criteria in terms of an SD multiplied by 0.84 and an α of 20% as used by previous investigators [Citation15], (3) an increase of 30% or more [Citation53], and (4) an increase of 3 points or more (a 2.25 point change being clinically significant [Citation30]). For dropout status, we originally planned to classify participants on the basis of having missed at least 3 sessions without resuming treatment in face-to-face CBT, and on the basis of not having replied for at least 3 weeks without resuming treatment, or having informed the therapist or researchers that they would discontinue the treatment and then did so in guided ICBT. However, based on the available data, this could only be done for the guided ICBT group from one of the two RCTs (n = 102) [Citation30]. In a sensitivity analysis, dropout status in all CBT groups was instead determined on the basis of having initiated no more than 5 (out of 12) sessions or modules. We also tabulated missing data rates at the post-treatment assessment as a crude indicator of dropout, primarily in order to study how well this would correspond to more direct measures.
2.3.4. Statistical analysis
For the original secondary study, we conducted all statistical analyses in Stata 15.1. We classified the participants’ severity of pathological health anxiety based on the first 14 items of the HAI-18 [Citation54]. Rates of adverse events, overall symptom deterioration, and dropouts are presented both as absolute and relative frequencies. For adverse events, the inter-rater reliability of the categorization is presented in terms of Cohen’s κ, and the inter-rater reliability of the relatedness-to-treatment scores is presented in terms of the Pearson correlation. Prior to all inferential tests, missing data were imputed using predictive mean matching (5 neighbors) within a multiple imputation by chained equations framework (20 samples). Due to limited power, we did not test for significant differences in rates of unwanted effects between the treatment formats, or versus the waitlist condition. For the same reason, we only tested for predictors in face-to-face CBT and therapist-guided ICBT, which were the treatment formats with by far the largest sample sizes. Such analyses were based on logistic regression and focused on seven preregistered potential predictors: age, female (1/0), post-secondary education (1/0), baseline health anxiety (HAI-18), baseline depression symptoms (MADRS-S), no previous experience of psychological treatment (1/0), and the perceived treatment credibility and expectancy of improvement at week 2 (C/E scale). For dropout rates, our analyses focused on the guided ICBT group because this was the only treatment format for which we could model the preregistered primary measure (see above). We tested the effect of adverse events and dropout status on change in health anxiety using linear mixed effects regression models.
3. Results
3.1. Systematic review and meta-analysis
3.1.1. Search hits and study characteristics
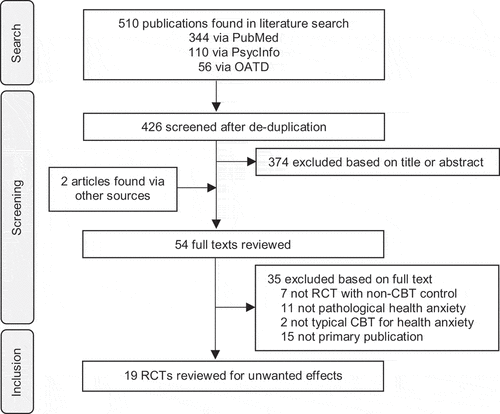
As can be seen in , we reviewed 54 publications in full text. Agreement was almost perfect (κ = 0.91) in the original search, and perfect in the updated search. We identified 19 RCTs from the original search, 1 additional RCT in the update [Citation55], and also revised 1 decision of the original search to exclude one RCT on the basis of subclinical symptoms [Citation56]. This resulted in a final sample of 19 RCTs with 1103 participants randomized to CBT and 1085 to non-CBT controls [Citation29,Citation55,Citation57–73]. The pooled mean age was 40 (SD = 14) and approximately 63% were female. The most common RCT main eligibility criterion was a diagnosis of DSM-III, DSM-IV, or ICD-10 hypochondriasis (11 RCTs), followed by a cutoff on a health anxiety scale (5 RCTs), and a diagnosis of DSM-5 somatic symptom disorder or illness anxiety disorder (3 RCTs). All RCTs reported pathological levels of health anxiety, usually with a mean in the mild to moderate clinically significant range [Citation54]. See the online supplement for details. None of the RCTs reported overall symptom deterioration rates.
3.1.2. Meta-analysis of adverse events
Adverse events were reported in 8/19 RCTs. The pooled rate of participants reporting at least one adverse event in CBT was 10%, though with substantial heterogeneity (95% CI: 3–16; 6 RCTs; τ2 = 7, I2 = 85%). Out of 589 participants at risk in 7 RCTs, one participant in CBT reported an adverse event that was described as serious (0.1%). This was a participant with gastrointestinal problems [Citation71]. One RCT reported adverse events but not whether these were serious. The control groups were too disparate to be pooled in meta-analysis but are tabulated in .
Table 1. Adverse events and dropout rates reported in randomized controlled trials of cognitive behavior therapy for pathological health anxiety as compared to controls other than cognitive behavior therapy, sorted by year of publication.
3.1.3. Meta-analysis of dropout rates and missing data rates
Dropout rates were reported in 17/19 RCTs. As can be seen in , definitions varied widely and were often vague. The pooled dropout rate in CBT was 17%, though again with substantial heterogeneity (95% CI: 12–22; τ2 = 9, I2 = 87%). There was no significant difference in dropout rates as compared to other psychological treatments (RR = 1.09, 95% CI: 0.72–1.67; 4 RCTs). Two RCTs reported drop-out rates from antidepressant and pill placebo control groups [Citation62,Citation71] which are tabulated in . Remote CBT, i.e. delivered without face-to-face meetings, did not result in significantly different dropout rates (17% vs. 16%; P = 0.807).
Missing data rates were reported in 16/19 RCTs. The pooled rate of missing data in CBT was 12%, and heterogeneity was high (95% CI: 7–17; τ2 = 10, I2 = 92%). There was no significant difference in missing data rates as compared to the waitlists (RR = 1.05, 95% CI: 0.71–1.53; 8 RCTs), treatment-as-usual controls (RR = 0.82, 95% CI: 0.53–1.26; 3 RCTs), or other psychological treatments (RR = 1.44, 95% CI: 0.90–2.32; 4 RCTs). Remote CBT, i.e. delivered without face-to-face meetings, did not result in significantly different missing data rates (10% vs. 13%; P = 0.551). In the 14 RCTs that reported both dropout rates and missing data rates in CBT, the pooled CBT dropout rate was 16% and the pooled CBT missing data rate was 13%.
3.1.4. Risk of publication bias
Though indicators were conflicting (see the online supplement), if there was publication bias, this probably led to a negligible overestimation, and not an underestimation, of unwanted effects.
3.2. Original study based on two randomized controlled trials
3.2.1. Sample characteristics
In the original study based on two RCTs (N = 336) [Citation29,Citation30], the average participant was a 39 year old female (72%) with a tertiary education and pathological health anxiety in the mild to moderate (82%) clinically significant range (; ). Ninety-nine percent (334/336) of the sample scored above the commonly cited cutoff of 18 on the HAI-18, and 96% (321/336) scored at least 22 [Citation54].
Figure 2. Flowchart pertaining to the two randomized controlled trials of cognitive behavior therapy for pathological health anxiety on which the secondary original study was based.
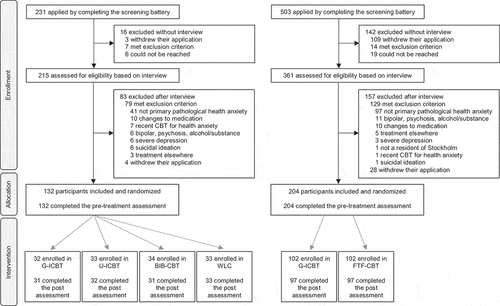
Table 2. Baseline characteristics in two randomized controlled trials of cognitive behavior therapy for pathological health anxiety.
3.2.2. Adverse events and their predictors
The inter-rater reliability was almost perfect in the assessment of adverse event types (κ = 0.91) and moderate in the assessment of relatedness to treatment (r = 0.65). Approximately 17% of participants who completed the post-treatment assessment (48/288) reported at least one adverse event via the open-ended questionnaire. No serious adverse event, i.e. leading to death or requiring hospitalization or immediate care, was observed. Based on the systematic coding of adverse events, the most common type was distress/deterioration (). In our sample, on average, this type of adverse event appeared to be rated as less likely to be related to the treatment as compared to other types of adverse events. For all types of adverse events, overall mean distress ratings were 2.0 or lower on a scale from 0 to 5 at the post-treatment assessment (). None of the planned analyses identified significant predictors of adverse events (). In a non-planned analysis, we tested if guided treatments (face-to-face CBT and guided ICBT) were associated with more adverse events than unguided treatments (unguided ICBT and bibliotherapy) but this difference was not statistically significant (OR = 2.30, 95% CI: 0.93–5.66).
Table 3. Adverse events in two randomized controlled trials of cognitive behavior therapy for pathological health anxiety.
Table 4. Deterioration and dropouts in two randomized controlled trials of cognitive behavior therapy for pathological health anxiety.
3.2.3. Overall symptom deterioration
Overall symptom deterioration was seen in 0–10% of participants, usually toward the lower bound of the range, depending on the definition and the CBT format (). This incidence was too low, and apparent differences in the sample were too small, to allow for inferential tests versus the WLC or between the CBT formats. The distribution of change scores appeared to be symmetrical in all groups and can be found in the online supplement.
3.2.4. Dropouts and their predictors
For reasons elaborated on in the methods section, most analyses of dropout rates focused on guided ICBT. In guided ICBT, 19% of the participants (19/102) were inactive for at least 3 weeks and did not return. Almost two thirds of these dropouts (12/19; 63%) took place during the first half of treatment. See for rates based on other definitions. In the planned analyses, two variables were significant and robust predictors of dropout in guided ICBT: (i) not having a post-secondary education, and (ii) having given a lower rating of treatment credibility and expectancy at week 2 ().
Table 5. Potential predictors of adverse events and dropouts in two randomized controlled trials of cognitive behavior therapy for pathological health anxiety.
3.2.5. Relationship between adverse events, dropouts, and treatment efficacy
In guided ICBT, dropout status defined as at least 3 weeks of inactivity without resuming treatment was not predicted by having reported at least one adverse event (OR = 0.73, 95% CI: 0.17–3.12). Furthermore, across all CBT formats, the number of completed sessions or initiated modules was not significantly predicted by having reported at least one adverse event (b = 0.6, 95% CI: −0.5–1.6). Overall symptom deterioration was too rare to be analyzed in relation to the other unwanted outcomes.
In linear mixed effects regression, having reported at least one adverse event was significantly predictive of a smaller reduction in health anxiety over the treatment phase in face-to-face CBT (HAI-18: b = 7.0, 95% CI: 2.4–11.6) but not in guided ICBT (b = 2.6, 95% CI: −0.8–5.9). Having dropped out defined as at least 3 weeks of inactivity without resuming, at any point in the treatment, was not significantly associated with the treatment outcome in guided ICBT (b = 3.3, 95% CI: −0.7–7.2). However, when a distinction was made between early and late dropouts (week 1–6 vs. 7–12), early dropout in guided ICBT was significantly predictive of a smaller reduction in health anxiety (b = 5.9, 95% CI: 1.0–10.8) whereas late dropout was not (b = −1.1, 95% CI: −7.1–4.9). Across all CBT formats, having completed less sessions or initiated less modules was significantly associated with a smaller reduction in health anxiety over the treatment phase (b = −0.4, 95% CI: −0.7–0.1).
4. Discussion
4.1. Main findings
This study combined (i) a systematic review and meta-analysis with (ii) an original secondary analysis of two RCTs to investigate the incidence and characteristics of unwanted outcomes in cognitive behavior therapy for pathological health anxiety. We found that adverse events were reported by 10% of patients in the systematic review, and 17% in the original study. It appears that these adverse events rarely necessitated immediate care. Most adverse events appeared to be variations on increased distress, which can be high in the short term, but tends to be low at the end of treatment. There were, however, also other adverse events reported in this treatment. For example, 3 out of 51 adverse events in the original study (6%) were classified as patients having experienced a sense of failure (). Notably, in the original study, because there was no way for us to confirm with certainty that the adverse events reported were caused by the treatment, we listed all adverse events reported, and some of these were deemed unlikely to have been caused by CBT. We found that adverse events were associated with worse outcome in face-to-face CBT, but perhaps not in guided ICBT. Similar to previous findings in OCD [Citation14], the ‘no pain, no gain’ hypothesis of adverse events typically being associated with higher CBT efficacy was not supported in our data. In summary, adverse events in CBT for pathological health anxiety appear to be relatively common and associated with distress which subsides in the long term.
Overall symptom deterioration appears to occur only in a single-digit number percent of patients. This is similar to the median overall symptom deterioration rate of 4% reported previously for ICBT in general [Citation15], and the 0% reported for acceptance and commitment therapy for pathological health anxiety specifically [Citation74]. Power was limited in all studies which means that small differences in percentages could be entirely due to sampling error. An important point is also that even though cases of deterioration can be documented in CBT, this does not necessarily mean that this deterioration was caused by the treatment itself. For that to be established, it would be necessary to test, using randomized controlled designs, if higher deterioration rates are seen in CBT than in the relevant control groups. As is evident from and previous research [Citation15], deterioration rates in CBT may not differ from those of waiting lists, which highlights the importance of not interpreting within-group deterioration rates as necessarily having been caused by the treatment.
A typical dropout rate appears to be 17%, and similar in remote and non-remote treatments, as based on the systematic review, and 19% in ICBT as based on the original study. As expected, this was close to the average psychotherapy dropout rate of 20% [Citation16], and the pooled dropout rates of 10 to 18% in psychotherapies for the anxiety disorders and OCD specifically [Citation19,Citation20,Citation23,Citation24]. We suspect that a prerequisite for low dropout rates in ICBT is a highly structured format [Citation75] such as that evaluated here in the original study [Citation29,Citation30]. Here, all trial applicants underwent a structured diagnostic assessment where they could discuss the treatment format with a clinician, and all participants were subsequently encouraged to set aside time for the treatment so that they could work with it on a daily basis. The treatment content was well thought through and comprised previously used worksheets and questions intended to prompt reflection and – in therapist-guided ICBT – relevant feedback to the therapist, from whom the patient received continuous text-based, including on-demand, and sometimes telephone support. There was a prespecified end date for the treatment. There were also weekly SMS reminders to log in to the treatment platform, and weekly assessments of symptoms that required patients to log in to their personal account at the same webpage that was used to convey the treatment modules. In guided ICBT for pathological health anxiety, similar to findings pertaining to OCD [Citation26], early but not late dropouts appear to be associated with worse treatment outcome. Tentatively, dropouts in guided ICBT are more frequent when patients do not have a post-secondary education and when ratings of treatment credibility and expectancy are lower early in treatment. Overall, about 1 in 5 patients appear to drop out of treatment, and this can be tied to educational attainment and treatment credibility, with seemingly detrimental effects for the treatment if it occurs at an early stage.
4.2. Limitations
This study had several noteworthy limitations. With regard to the systematic review, this was based on outcomes reported in the original publications. Therefore, certain outcomes, most notably overall symptom deterioration, could not be tabulated or analyzed, even when relevant data had been collected as part of the trial. An independent patient data meta-analysis could potentially address this shortcoming.
Most RCTs discussed here were not based in routine care. This is likely to have had implications for recruitment, such that participants were more highly educated and also more motivated than had otherwise been the case [Citation76]. Notably, this may have led to lower dropout rates [Citation77]. This said, it is also worth pointing out that one of the two RCTs in the original study was based at a primary care clinic and employed a mixed recruitment strategy where participants fared about as well regardless of whether they had been recruited via routine care or not [Citation30].
In the original study, the power of our statistical tests was sufficient to model dichotomous outcome differences of approximately 15% and above with 80% power with regard to face-to-face CBT and guided ICBT. The unguided ICBT and bibliotherapy groups were considerably smaller. Therefore, the precise percentages derived from these analyses are highly tentative.
Another limitation is that we had insufficient documentation to classify participants in face-to-face CBT as dropouts in terms of successive weeks of nonattendance. Dropouts in face-to-face CBT could only be approximated in terms of the number of sessions attended, which was not our preregistered definition of what it meant to be a dropout. This required us to focus on guided ICBT in our analyses of dropouts in the original study.
Last, as part of the original study, we did not collect descriptions of adverse events or dropouts at the time when these occurred. Rather, we asked participants about adverse events at the post-treatment assessment only. Considering our finding that the distress associated with adverse events typically subsides over time, it is conceivable that adverse events were underreported. Also, because participants who dropped out were more likely not to complete this post-treatment adverse events questionnaire, in the observed data, adverse events were most likely systematically underestimated in dropouts. This said, inferential analyses were probably relatively robust because these were based on imputed data.
4.3. Conclusion
CBT for pathological health anxiety is associated with unwanted effects. About 1 in 10 patients report experiencing at least one adverse event. Most commonly, such events come in the form of increased distress of moderate to high intensity, with a reduction to the end of treatment. Overall symptom deterioration appears to be an unusual outcome, perhaps affecting a few percent of patients. About 1 in 5 patients drop out, which appears to be more common with lower educational attainment, and when patients do not find the treatment credible or expect to become better. The existing literature is limited and heterogeneous.
4.4. Expert opinion
4.4.1. Clinical implications
For any treatment, potential harms need be weighed against potential benefits. In the case of CBT for pathological health anxiety, based on published RCTs, the treatment is typically about 10–12 weeks long, after which 66% of patients respond and 48% achieve remission. The true response and remission rates may be slightly lower due to probable publication bias [Citation11]. As judged based on the present study, about 1 in 10 patients report at least one adverse event, and very few deteriorate. To determine whether these are acceptable outcomes, the most informative approach is probably to look at patient behavior. Given that 4 in 5 patients stay in treatment, and there appears to be no clear relationship between adverse events and dropout status, our conclusion is that most adverse events appear to be tolerated. The clinician would be advised to inform the patient that CBT for pathological health anxiety can be demanding, and that it is relatively common to experience discomfort in the short term, but that such experiences are typically transient and that most patients benefit from treatment.
Should our findings withstand replication, there could be a need to monitor patients with low educational attainment, and those who express low confidence in the treatment, as these groups could be more likely to drop out. Considering that most dropouts are seen during the first half of treatment, clinicians working with CBT for pathological health anxiety would be advised to provide particular support at an early stage. This highlights the importance of the initial assessment as an opportunity to address concerns with the treatment, and to educate patients about potential benefits in a manner suitable for the individual. We also advise clinicians to address contextual variables that may influence the patent’s ability to stay in treatment.
Many clinicians are reluctant introduce exposure or behavioral experiments in the treatment of anxiety due to a concern for adverse events or overall symptom deterioration [Citation78,Citation79]. Such negative beliefs of unwanted outcomes can cause clinicians to deliver CBT in an overly protective manner that, paradoxically, results in lower treatment effectiveness [Citation80]. The present study indicates that long-term distress due to adverse events is an unusual outcome. This was the case even though participants in the original study were encouraged to work with exposure and response prevention on a daily basis, every week, from about week 3 onwards. In order words, the rates reported here are those that would be expected of a high-intensity exposure-based treatment. This underscores the importance of promoting exposure or behavioral experiments to achieve beneficial effects of CBT for pathological health anxiety. The promotion of best practice CBT – which commonly includes exposure or behavioral experiments – requires clinicians to be informed about both the benefits and harms of treatment, as well as policy makers being explicit about the components of evidence-based CBT [Citation81].
4.4.2. Comparison with antidepressant medication
In routine care for pathological health anxiety, the alternative to CBT is often the use of antidepressant medication (2 RCTs included here [Citation62,Citation71]). Unwanted effects of such medications remains a controversial topic, not least in the long term and at discontinuation [Citation82,Citation83]. Common side effects include sexual dysfunction, gastrointestinal symptoms, sleep disturbance, and fatigue [Citation84]. One RCT (N = 195) directly compared adverse events in CBT to those of antidepressants, with measurements at week 12 and 24 [Citation71]. At week 12, rates of participants reporting at least one adverse event were 17% for CBT, 22% for antidepressant medication, 13% for combination therapy, and 14% for pill placebo. At week 24, rates were 4% for CBT, 9% for antidepressant medication, 8% for combination therapy, and 5% for pill placebo. As to acceptability and dropouts, 248 applicants were excluded from participation in the trial on the basis of not being willing to be allocated to antidepressant medication, while only 1 applicant was excluded on the basis of not being willing to be allocated to CBT. Subsequent dropout rates were 21/53 (40%) for CBT, 13/45 (29%) for antidepressant medication, 17/53 (32%) for combination therapy, and 24/44 (55%) for pill placebo. In another RCT, the dropout rate was 10/40 (25%) for CBT and 11/37 (30%) for antidepressant medication [Citation62]. It is still too early to say with certainty how either beneficial or unwanted effects typically differ between CBT and antidepressants. There is thus a need for future RCTs that compare both the efficacy and unwanted effects of CBT and antidepressant medication in the treatment of pathological health anxiety.
4.4.3. Suggestions for the future measurement of unwanted outcomes
There exists no widely agreed-upon method of measuring adverse events, overall symptom deterioration, or dropout rates in psychotherapy. Questions about adverse events may be either open-ended (such as ‘Which if any negative effects did you experience?’) or closed (such as ‘Did you ever feel stressed out?’). Furthermore, patients may or may not be instructed to determine whether events were caused by the treatment. Depending on the method of measurement, the reporting of an adverse event could have different implications. For example, open-ended questions are probably less likely to elicit certain adverse events, as compared to when these are asked for explicitly. In order to report an adverse event, it is necessary to interpret the event as relevant to report, in addition to other factors that contribute to the reporting behavior. CBT for pathological health anxiety usually involves behavioral experiments or exposure, which are interventions that give rise short-term discomfort per definition. The fact that only 1-2 in 10 patents in this treatment reported at least one adverse events may illustrate that whether or not a temporary increase in discomfort is perceived as problematic has to do with factors such as whether this discomfort served a meaningful purpose, and perhaps also whether the patient had intended to purposely cause discomfort. In the original study, we saw several examples of patients being aware that experiencing discomfort was part of CBT and therefore expressing uncertainty as to whether their experiences ‘counted’ as adverse events, and should be reported. For this reason, we recommend future trials to combine the use of open-ended questions to approximate what events patients deem problematic, and closed questions (e.g. the Negative Effects Questionnaire [Citation85]) to ensure that rates are exhaustive and can be compared over treatment conditions.
An interesting challenge to the measurement of overall symptom deterioration rates is that even though practically all clinical trials measure change in symptoms, it is not always clear how much an individual needs to deteriorate for this to be considered relevant. Certain studies, including this one, have quantified the rate of patients who experienced a reliable deterioration, i.e. worsened to an extent where it is reasonable to assume that this was not merely the result of measurement error. However, and importantly, rates of reliable deterioration are a function not only of the true rate of deterioration, but also of the alpha level and the reliability of the outcome measure. With a less stringent alpha, a more reliable outcome, or a reduction in the standard deviation, higher rates of reliable deterioration are seen, which we demonstrated in this study (). This means that it is usually not meaningful to compare rates of reliable deterioration over studies unless these employ the same alpha, the same outcome measure, and the same reliability estimate. For this reason, reliable deterioration is an excellent method of determining whether change has occurred on the level of the individual, but it is not the ideal method of approximating rates of deterioration on the level of groups in clinical trials. An alternative could be to report the rate of patients deteriorating by a minimally important difference (MID): the smallest effect of clinical relevance [Citation86]. However, to our knowledge, no MID other than the HAI-18 2.25- or 3-point criterion suggested here has been established for any of the most widely used health anxiety measures. This is an important venture of future research.
Dropout rates can also be estimated in several ways, and one of several challenges is to define dropout in a manner that is meaningful also for the minimal-contact formats such as unguided bibliotherapy. An argument can be made that, at least in many scenarios, dropout status is a dichotomized proxy measure of the degree to which the patient has engaged in the treatment, and that a more nuanced, continuous or count variable could be more informative. On the other hand, quantifying adherence or treatment engagement as a continuous variable is not easily done in a reliable manner. The most common approach is to count the number of sessions in face-to-face CBT, or modules/chapters in ICBT or bibliotherapy. This has the drawback that even though patients attend the same number of sessions, or complete the same number of modules or chapters, they may have devoted very different amounts of time to treatment strategies such as exposure and response prevention. Most probably, there exists no easy ‘catch all’ solution suitable for measuring engagement in all treatment formats. Ideally, we believe that most clinical trials benefit from reporting report one or several continuous or count variables pertaining to adherence and engagement over and above dropout rates.
4.4.4. Need for larger studies, and studies in routine care
Unwanted effects are low-incidence outcomes for which very few psychological treatment trials have been adequately powered. In order to learn more about unwanted effects in pathological health anxiety, we encourage further work based on larger samples. Such samples may be derived either through cooperation and the harmonization of variables and pooling of datasets, or via the use of data from routine care.
Article highlights
We investigated unwanted outcomes in cognitive behavior therapy (CBT) for pathological health anxiety based on a systematic review and an original study.
Adverse events were reported by 10% of participants in CBT in the systematic review, and 17% in the original study.
Overall symptom deterioration was not reported in any trial in the systematic review, but the rate was 0-10% in the original study.
Dropout rates were 17% in the systematic review, and 10-19% in the original study.
Adverse events were associated with worse outcome in face-to-face CBT, but not in therapist-guided Internet-delivered CBT.
In therapist-guided Internet-delivered CBT, dropouts were predicted by lower education attainment and lower week two credibility/expectancy ratings.
Declaration of interest
The authors have developed and evaluated CBT protocols for the treatment of pathological health anxiety. E Axelsson was an original author in 2 trials, and E Hedman-Lagerlof in 3 trials, included in the systematic review. The authors have also coauthored books and book chapters on the topic of pathological health anxiety, which are available in the public marketplace. E Axelsson regularly lectures on CBT for pathological health anxiety for which he receives payment. E Hedman-Lagerlof is a shareholder of Hedman-Lagerlöf och Ljótsson psykologi AB: a company that licenses CBT manuals. E Axelsson contributed to the completion of this study within his position at Liljeholmen Primary Health Care Center, funded by the Swedish Research Council (EA; 2021–06496) which had no any role in the design, execution, or publication process other than to require the study to be published in an open access journal article. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
E Axelsson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. Both authors made contribution to the conception and design, and to the acquisition of data. E Axelsson conducted the statistical analyses and drafted the manuscript. Both authors reviewed the manuscript for intellectual content, contributed with revisions, and approved the final manuscript.
Data deposition
Data derived from the systematic review will be made freely available via the Open Science Framework upon acceptance (https://osf.io/pa89t/).
Supplemental Material
Download Zip (128.1 KB)Acknowledgments
This work used the BASS platform from the eHealth Core Facility at Karolinska Institutet. No writing assistance, based on artificial intelligence or otherwise, was utilised in the production of this manuscript.
Data availability statement
Data derived from the systematic review are reported in and will be made freely available via the Open Science Framework upon acceptance (https://osf.io/pa89t/). Individual participant data from the secondary original study fall under Swedish and European Union data protection and privacy legislation and can therefore not be share in their entirety. Reasonable requests may be directed to the corresponding author, and will be considered on a case-by-case basis.
Supplemental Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2250915.
Additional information
Funding
References
- Warwick HM, Salkovskis PM. Hypochondriasis. Behav Res Ther. 1990;28:105–117. doi: 10.1016/0005-7967(90)90023-C
- Axelsson E, Hedman-Lagerlöf E. Validity and clinical utility of distinguishing between DSM-5 somatic symptom disorder and illness anxiety disorder in pathological health anxiety: Should we close the chapter? J Psychosom Res. 2023;165:111133. doi: 10.1016/j.jpsychores.2022.111133
- Stein DJ, Kogan CS, Atmaca M, et al. The classification of obsessive-compulsive and related disorders in the ICD-11. J Affect Disord. 2016;190:663–674. doi: 10.1016/j.jad.2015.10.061
- Olatunji BO, Deacon BJ, Abramowitz JS. Is hypochondriasis an anxiety disorder? Br J Psychiatry. 2009;194(6):481–482. doi: 10.1192/bjp.bp.108.061085
- Abramowitz JS, Moore EL. An experimental analysis of hypochondriasis. Behav Res Ther. 2007;45:413–424. doi: 10.1016/j.brat.2006.04.005
- Leonidou C, Panayiotou G. How do illness-anxious individuals process health-threatening information? A systematic review of evidence for the cognitive-behavioral model. J Psychosom Res. 2018;111:100–115. doi: 10.1016/j.jpsychores.2018.06.001
- Weck F, Richtberg S, Neng J. Epidemiology of hypochondriasis and health anxiety: Comparison of different diagnostic criteria. Curr Psychiatry Rev. 2014;10(1):14–23. doi: 10.2174/1573400509666131119004444
- Olde Hartman TC, Borghuis MS, Lucassen PL, et al. Medically unexplained symptoms, somatisation disorder and hypochondriasis: course and prognosis. A systematic review. J Psychosom Res. 2009;66:363–377. doi: 10.1016/j.jpsychores.2008.09.018
- Sunderland M, Newby JM, Andrews G. Health anxiety in Australia: prevalence, comorbidity, disability and service use. Br J Psychiatry. 2013;202(1):56–61. doi: 10.1192/bjp.bp.111.103960
- Norbye AD, Abelsen B, Førde OH, et al. Health anxiety is an important driver of healthcare use. BMC Health Serv Res. 2022;22. doi: 10.1186/s12913-022-07529-x.
- Axelsson E, Hedman-Lagerlöf E. Cognitive behavior therapy for health anxiety: systematic review and meta-analysis of clinical efficacy and health economic outcomes. Expert Rev Pharmacoecon Outcomes Res. 2019;19(6):663–676. doi: 10.1080/14737167.2019.1703182
- Vincent C. Understanding and responding to adverse events. N Engl J Med. 2003;348(11):1051–1056. doi: 10.1056/NEJMhpr020760
- Rozental A, Kottorp A, Boettcher J, et al. Negative effects of psychological treatments: An Exploratory Factor analysis of the negative effects questionnaire for Monitoring and reporting adverse and unwanted events. PLoS One. 2016;11(6):e0157503. doi: 10.1371/journal.pone.0157503
- Moritz S, Fieker M, Hottenrott B, et al. No pain, no gain? Adverse effects of psychotherapy in obsessive–compulsive disorder and its relationship to treatment gains. J Obsessive Compuls Relat Disord. 2015;5:61–66. doi: 10.1016/j.jocrd.2015.02.002
- Rozental A, Magnusson K, Boettcher J, et al. For better or worse: An individual patient data meta-analysis of deterioration among participants receiving Internet-based cognitive behavior therapy. J Consult Clin Psychol. 2017;85(2):160–177. doi: 10.1037/ccp0000158
- Swift JK, Greenberg RP. Premature discontinuation in adult psychotherapy: a meta-analysis. J Consult Clin Psychol. 2012;80(4):547–559. doi: 10.1037/a0028226
- Fernandez-Arias I, Garcia-Fernandez G, Bernaldo-de-Quiros M, et al. Premature termination of psychological treatment for anxiety disorders in a clinical setting. Psicothema. 2016;28(3):241–246. doi: 10.7334/psicothema2015.201
- Santana L, Fontenelle LF. A review of studies concerning treatment adherence of patients with anxiety disorders. Patient Prefer Adherence. 2011;5:427–439. doi: 10.2147/PPA.S23439
- Gersh E, Hallford DJ, Rice SM, et al. Systematic review and meta-analysis of dropout rates in individual psychotherapy for generalized anxiety disorder. J Anxiety Disord. 2017;52:25–33. doi: 10.1016/j.janxdis.2017.10.001
- Swift JK, Greenberg RP. A treatment by disorder meta-analysis of dropout from psychotherapy. J Psychother Integr. 2014;24(3):193–207. doi: 10.1037/a0037512
- Bentley KH, Cohen ZD, Kim T, et al. The Nature, Timing, and symptom Trajectories of dropout from Transdiagnostic and Single-diagnosis cognitive-behavioral therapy for anxiety disorders. Behav Ther. 2021;52(6):1364–1376. doi: 10.1016/j.beth.2021.03.007
- Fernandez D, Vigo D, Sampson NA, et al. Patterns of care and dropout rates from outpatient mental healthcare in low-, middle- and high-income countries from the World health Organization’s World Mental health Survey Initiative. Psychol Med. 2021;51(12):2104–2116. doi: 10.1017/S0033291720000884
- Johnco C, McGuire JF, Roper T, et al. A meta-analysis of dropout rates from exposure with response prevention and pharmacological treatment for youth with obsessive compulsive disorder. Depress Anxiety. 2020;37(5):407–417. doi: 10.1002/da.22978
- Ong CW, Clyde JW, Bluett EJ, et al. Dropout rates in exposure with response prevention for obsessive-compulsive disorder: What do the data really say? J Anxiety Disord. 2016;40:8–17. doi: 10.1016/j.janxdis.2016.03.006
- Bisby MA, Karin E, Hathway T, et al. A meta-analytic review of randomized clinical trials of online treatments for anxiety: Inclusion/exclusion criteria, uptake, adherence, dropout, and clinical outcomes. J Anxiety Disord. 2022;92:102638. doi: 10.1016/j.janxdis.2022.102638
- Aderka IM, Anholt GE, van Balkom AJ, et al. Differences between early and late drop-outs from treatment for obsessive-compulsive disorder. J Anxiety Disord. 2011;25:918–923. doi: 10.1016/j.janxdis.2011.05.004
- Bennemann B, Schwartz B, Giesemann J, et al. Predicting patients who will drop out of out-patient psychotherapy using machine learning algorithms. Br J Psychiatry. 2022;220:1–10. doi: 10.1192/bjp.2022.17
- Zimmermann D, Rubel J, Page AC, et al. Therapist effects on and predictors of non-Consensual dropout in psychotherapy. Clin Psychol Psychother. 2017;24:312–321. doi: 10.1002/cpp.2022
- Hedman E, Axelsson E, Andersson E, et al. Exposure-based cognitive-behavioural therapy via the internet and as bibliotherapy for somatic symptom disorder and illness anxiety disorder: randomised controlled trial. Br J Psychiatry. 2016;209:407–413. doi: 10.1192/bjp.bp.116.181396
- Axelsson E, Andersson E, Ljótsson B, et al. Effect of Internet vs face-to-face cognitive behavior therapy for health anxiety: A randomized Noninferiority clinical trial. JAMA Psychiatry. 2020;77(9):915–924. doi: 10.1001/jamapsychiatry.2020.0940
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71
- Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. doi: 10.3163/1536-5050.104.3.014
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4
- Dimsdale JE, Creed F, Escobar J, et al. Somatic symptom disorder: an important change in DSM. J Psychosom Res. 2013;75(3):223–228. doi: 10.1016/j.jpsychores.2013.06.033
- R core Team. R: a Language and Environment for statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x
- Junqueira DR, Zorzela L, Golder S, et al. CONSORT harms 2022 statement, explanation, and elaboration: updated guideline for the reporting of harms in randomised trials. BMJ. 2023;381.
- Axelsson E, Andersson E, Ljótsson B, et al. The health preoccupation diagnostic interview: inter-rater reliability of a structured interview for diagnostic assessment of DSM-5 somatic symptom disorder and illness anxiety disorder. Cogn Behav Ther. 2016;45:259–269. doi: 10.1080/16506073.2016.1161663
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33.
- Hedman-Lagerlöf E, Axelsson E. Cognitive behavioral therapy for health anxiety. In: Hedman-Lagerlöf E, editor. The Clinician's Guide to Treating Health Anxiety: diagnosis, Mechanisms, and Effective Treatment. Cambridge, MA: Academic Press; 2019. 9780128118061.
- Furer P, Walker JR. Treatment of hypochondriasis with exposure. J Contemp Psychother. 2005;35(3):251–267. doi: 10.1007/s10879-005-4319-y
- Salkovskis PM, Rimes K, Warwick H, et al. The health anxiety Inventory: development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychol Med. 2002;32(5):843–853. doi: 10.1017/S0033291702005822
- Ljótsson B, Hesser H, Andersson E, et al. Provoking symptoms to relieve symptoms: a randomized controlled dismantling study of exposure therapy in irritable bowel syndrome. Behav Res Ther. 2014;55:27–39. doi: 10.1016/j.brat.2014.01.007
- Svanborg P, Åsberg M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating scale. Acta Psychiatr Scand. 1994;89(1):21–28. doi: 10.1111/j.1600-0447.1994.tb01480.x
- Leon AC, Olfson M, Portera L, et al. Assessing psychiatric impairment in primary care with the Sheehan Disability scale. Int J Psychiatry Med. 1997;27(2):93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3(4):257–260. doi: 10.1016/0005-7916(72)90045-6
- Boettcher J, Rozental A, Andersson G, et al. Side effects in Internet-based interventions for social anxiety disorder. Internet Interv. 2014;1(1):3–11. doi: 10.1016/j.invent.2014.02.002
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037/0022-006X.59.1.12
- Olatunji BO, Etzel EN, Tomarken AJ, et al. The effects of safety behaviors on health anxiety: an experimental investigation. Behav Res Ther. 2011;49:719–728. doi: 10.1016/j.brat.2011.07.008
- Fallon BA, Basaraba C, Pavlicova M, et al. Differential treatment response between hypochondriasis with and without Prominent somatic symptoms. Front Psychiatry. 2021;12:691703. doi: 10.3389/fpsyt.2021.691703
- Österman S, Axelsson E, Lindefors N, et al. The 14-item short health anxiety inventory (SHAI-14) used as a screening tool: appropriate interpretation and diagnostic accuracy of the Swedish version. BMC Psychiatry. 2022;22. doi: 10.1186/s12888-022-04367-3.
- Shabahang R, Aruguete MS, McCutcheon L. Video-based cognitive-behavioral intervention for COVID-19 anxiety: a randomized controlled trial. Trends Psychiatry Psychother. 2021;43:141–150. doi: 10.47626/2237-6089-2020-0056
- Bourgault-Fagnou MD, Hadjistavropoulos HD. A randomized trial of two forms of cognitive behaviour therapy for an older adult population with subclinical health anxiety. Cogn Behav Ther. 2013;42:31–44. doi: 10.1080/16506073.2012.717302
- Warwick HM, Clark DM, Cobb AM, et al. A controlled trial of cognitive-behavioural treatment of hypochondriasis. Br J Psychiatry. 1996;169:189–195. doi: 10.1192/bjp.169.2.189
- Clark DM, Salkovskis PM, Hackmann A, et al. Two psychological treatments for hypochondriasis: A randomised controlled trial. Br J Psychiatry. 1998;173(3):218–225. doi: 10.1192/bjp.173.3.218
- Visser S, Bouman TK. The treatment of hypochondriasis: Exposure plus response prevention vs cognitive therapy. Behav Res Ther. 2001;39:423–442. doi: 10.1016/S0005-7967(00)00022-X
- Barsky AJ, Ahern DK. Cognitive behavior therapy for hypochondriasis: a randomized controlled trial. JAMA. 2004;291(12):1464–1470. doi: 10.1001/jama.291.12.1464
- Buwalda FM, Bouman TK, van Duijn MA. Psychoeducation for hypochondriasis: a comparison of a cognitive-behavioural approach and a problem-solving approach. Behav Res Ther. 2007;45:887–899. doi: 10.1016/j.brat.2006.08.004
- Greeven A, van Balkom AJ, Visser S, et al. Cognitive behavior therapy and paroxetine in the treatment of hypochondriasis: a randomized controlled trial. Am J Psychiatry. 2007;164:91–99. doi: 10.1176/ajp.2007.164.1.91
- Seivewright H, Green J, Salkovskis P, et al. Cognitive-behavioural therapy for health anxiety in a genitourinary medicine clinic: randomised controlled trial. Br J Psychiatry. 2008;193:332–337. doi: 10.1192/bjp.bp.108.052936
- Hedman E, Andersson G, Andersson E, et al. Internet-based cognitive-behavioural therapy for severe health anxiety: randomised controlled trial. Br J Psychiatry. 2011;198:230–236. doi: 10.1192/bjp.bp.110.086843
- Sørensen P, Birket-Smith M, Wattar U, et al. A randomized clinical trial of cognitive behavioural therapy versus short-term psychodynamic psychotherapy versus no intervention for patients with hypochondriasis. Psychol Med. 2011;41(2):431–441. doi: 10.1017/S0033291710000292
- Bovell CV. Randomized controlled Feasibility trial of a self-Help book for health anxiety. Regina, Canada: University of Regina; 2012.
- Hedman E, Axelsson E, Görling A, et al. Internet-delivered exposure-based cognitive-behavioural therapy and behavioural stress management for severe health anxiety: randomised controlled trial. Br J Psychiatry. 2014;205:307–314. doi: 10.1192/bjp.bp.113.140913
- Tyrer P, Cooper S, Salkovskis P, et al. Clinical and cost-effectiveness of cognitive behaviour therapy for health anxiety in medical patients: a multicentre randomised controlled trial. Lancet. 2014;383(9913):219–225. doi: 10.1016/S0140-6736(13)61905-4
- Weck F, Neng JM, Richtberg S, et al. Cognitive therapy versus exposure therapy for hypochondriasis (health anxiety): A randomized controlled trial. J Consult Clin Psychol. 2015;83:665–676. doi: 10.1037/ccp0000013
- de Meza Skjernov M. Group cognitive behaviour therapy for severe health anxiety: a randomised controlled trial. Copenhagen, Denmark: University of Copenhagen; 2017.
- Fallon BA, Ahern DK, Pavlicova M, et al. A randomized controlled trial of medication and cognitive-behavioral therapy for hypochondriasis. Am J Psychiatry. 2017;174(8):756–764. doi: 10.1176/appi.ajp.2017.16020189
- Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86(1):89–98. doi: 10.1037/ccp0000248
- Morriss R, Patel S, Malins S, et al. Clinical and economic outcomes of remotely delivered cognitive behaviour therapy versus treatment as usual for repeat unscheduled care users with severe health anxiety: a multicentre randomised controlled trial. BMC Med. 2019;17(1):16. doi: 10.1186/s12916-019-1253-5
- Hoffmann D, Rask CU, Hedman-Lagerlöf E, et al. Efficacy of internet-delivered acceptance and commitment therapy for severe health anxiety: results from a randomized, controlled trial. Psychol Med. 2020;51:1–11. doi: 10.1017/S0033291720001312
- Hedman E. Therapist guided internet delivered cognitive behavioural therapy. BMJ. 2014;348(mar10 2):g1977. doi: 10.1136/bmj.g1977
- Almeida L, Kashdan TB, Nunes T, et al. Who volunteers for phase I clinical trials? Influences of anxiety, social anxiety and depressive symptoms on self-selection and the reporting of adverse events. Eur J Clin Pharmacol. 2008;64(6):575–582. doi: 10.1007/s00228-008-0468-8
- Newby JM, Haskelberg H, Hobbs MJ, et al. The effectiveness of internet-delivered cognitive behavioural therapy for health anxiety in routine care. J Affect Disord. 2020;264:535–542. doi: 10.1016/j.jad.2019.11.087
- Olatunji BO, Deacon BJ, Abramowitz JS. The Cruelest Cure? Ethical Issues in the Implementation of exposure-based treatments. Cogn Behav Pract. 2009;16:172–180. doi: 10.1016/j.cbpra.2008.07.003
- Richard DCS, Gloster AT. Chapter 18 - exposure therapy has a public relations problem: a dearth of litigation amid a wealth of concern. In: Richard D Lauterbach D, editors. Handbook of exposure therapies. Academic Press; 2007. p. 409–425. doi: 10.1016/B978-012587421-2/50019-3
- Farrell NR, Deacon BJ, Kemp JJ, et al. Do negative beliefs about exposure therapy cause its suboptimal delivery? An experimental investigation. J Anxiety Disord. 2013;27(8):763–771. doi: 10.1016/j.janxdis.2013.03.007
- Trivasse H, Webb TL, Waller G. A meta-analysis of the effects of training clinicians in exposure therapy on knowledge, attitudes, intentions, and behavior. Clin Psychol Rev. 2020;80:101887. doi: 10.1016/j.cpr.2020.101887
- Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav. 2019;97:111–121. doi: 10.1016/j.addbeh.2018.08.027
- Danborg PB, Valdersdorf M, Gotzsche PC. Long-term harms from previous use of selective serotonin reuptake inhibitors: a systematic review. Int J Risk Saf Med. 2019;30:59–71. doi: 10.3233/JRS-180046
- Kelly K, Posternak M, Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci. 2008;10:409–418. doi: 10.31887/DCNS.2008.10.4/kkelly
- Rozental A, Kottorp A, Forsstrom D, et al. The negative effects questionnaire: psychometric properties of an instrument for assessing negative effects in psychological treatments. Behav Cogn Psychother. 2019;47:559–572. doi: 10.1017/S1352465819000018
- Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012