ABSTRACT
Objective
To analyze the cost-effectiveness of weekly somatrogon compared to daily growth hormones (GH-d) in the pediatric population of Spain with growth hormone deficiency (GHD).
Methods
Markov model with two states (patients with or without GH-d or somatrogon treatment) in prepubertal children (3 to 11 years and 3 to 12 years in girls and boys, respectively) with GHD in isolation or as part of multiple pituitary hormone deficiency and without previous treatment, from the perspective of the National Health System. The simulation of the economic model ends at the age of 18. The costs of hormones and monitoring were obtained from Spanish sources. The utilities were obtained from the literature. Spanish clinical experts validated the assumptions of the model.
Results
In the deterministic analysis, somatrogon would be cost-effective, compared to GH-d, with a cost per QALY (quality-adjusted life year) gained of €19,259 and a clinically relevant QALY gain (0.336). This result was confirmed in deterministic sensitivity analyses. According to the probabilistic analysis, somatrogon would be the dominant treatment, with a 61% probability of a willingness to pay of €25,000 per QALY gained.
Conclusion
Compared to GH-d, somatrogon is cost-effective in the Spanish pediatric population with GHD.
1. Introduction
Short stature is defined by a height less than −2 standard deviations (SD) for age, sex and reference population. Only one in five children who meet this definition have underlying pathology, which rises to 50% if height falls below −3 SD [Citation1]. Height follows a normal distribution in the population according to sex and age [Citation2,Citation3] and, approximately 0.6% to 2.3% of healthy children are short [Citation3,Citation4]. The prevalence of GH deficiency (GHD) in childhood ranges between 1/3480, 1/4018 and 1/5600, depending on the series [Citation5–7]. The current annual cost of monitoring and treating a patient with GHD is estimated, based on a study published in 2012 [Citation8] at €10,146.
The main goal of growth hormone (GH, somatropin) treatment during childhood and puberty in patients with GHD is to achieve a normal height in accordance with genetic height [Citation9]. Daily injections of recombinant human growth hormone (somatropin) (GH-d) are the current standard treatment for GHD, which has been shown to be safe and effective [Citation10]. However, GH-d is associated with suboptimal adherence (prevalence of non-adherence from 5% to 82%) [Citation11,Citation12], one of the main reasons is patient exhaustion due to the need for long-term daily injections [Citation12,Citation13]. Suboptimal adherence is associated with decreased efficacy of GH-d therapy, leading to lower annual growth [Citation12,Citation14–16] and decreased long-term response [Citation12].
Somatrogon is a 47.5 kDa recombinant GH molecule formed by joining the 191 amino acid sequence of human growth hormone (hGH) with 3 copies of the C-terminal peptide (CTP) of human chorionic hormone (hCG). Glycosylated CTP determines the prolongation of the half-life of hGH by allowing its once-weekly subcutaneous administration, and has been shown to be clinically non-inferior to GH-d [Citation12,Citation17] and significantly less vital interference compared to daily somatropin [Citation2,Citation18]. Therefore, the weekly Somatrogon injections can increase patients’ adherence, decrease treatment burden and improve their quality of life, which would lead, in the long term, to an improvement in adult height versus long-term GH-d [Citation12].
The present study aims to analyze the cost-effectiveness/cost-utility of somatrogon compared to GH-d in Spain, using an economic model in GHD in the pediatric age group.
2. Methods
2.1. Design and structure
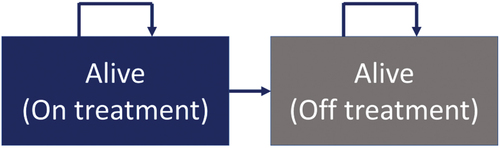
This Markov model was originally developed in Excel (Evidera) to simulate the evolution of patients aged 3 to 12 who start treatment with somatrogon or GH-d. The model consists of two health states (): (i) alive and on-treatment patients: patients receiving GH therapy (daily GH or weekly somatrogon). These patients will experience growth recovery due to GH therapy; and (ii) alive, untreated patients: patients who discontinue treatment. It is assumed in the model that these patients return to the standard deviations (SD) of the initial heights. Death status is not considered, as the modeled time horizon ends at 18 years of age, with reduced mortality. It is also considered that GHD is not associated with excess mortality compared to the general population [Citation12].
Patient growth was modeled using age- and sex-specific growth curves for each age range [Citation12]. Once the treatment completion age (18 years) is reached, patients in the ‘alive on-treatment’ health state remain in the same health state without accruing further costs or benefits from GH treatment.
2.2. Population
Prepubertal children (aged 3–10 years for girls and 3–11 years for boys) with isolated GHD or GHD as part of multiple pituitary hormone deficiency and no previous treatment (in line with the phase III clinical trial population comparing somatrogon and GH-d, reference NCT02968004 on ClinicalTrials.gov) [Citation17]. Patients whose etiology was an intracranial tumor were excluded.
2.3. Comparators
Somatrogon (weekly administration) is compared with GH-d marketed in Spain (Genotonorm®, Norditropin®, Saizen®, Humatrope®, Nutropin AQ®, Zomacton®, Omnitrope®).
2.4. Perspective and time horizon
The perspective of the study is that of the Spanish National Health System (NHS). Therefore, only direct health costs are considered. The simulation of the economic model ends at the age of 18. An annual cost and utility discount of 3% was applied.
2.5. Model assumptions and clinical validation
The main assumptions of the model are presented in and the supplementary tables (Tables S1 to S5) [Citation9,Citation15,Citation16,Citation19–25]. All model assumptions were clinically validated by a panel of three Spanish clinical experts with extensive experience managing GHD, co-authors of the present paper (MAAC, LCF, JILA). Among the different assumptions of the model, the following are worth highlighting, corroborated by clinical expert opinion: (i) It was assumed that adherence would be 5% higher with weekly than with daily injections of growth hormone, according to a study published in 2021 [Citation19]; (ii) that this increase in adherence associated with weekly versus daily GH would not decrease over time; and (iii) treatment adherence rates over 10 years, according to studies by Maggio et al. [Citation15] and Rodriguez-Arnao et al.[Citation16]
Table 1. Main assumptions of the economic model.
2.6. Efficacy data
The model uses efficacy data from the Phase 3 clinical trial [Citation17], whose protocol and results are available on the clinicaltrials.gov clinical trials database [Citation26]. The main results are presented in . Somatrogon, injected once a week, has been shown to be clinically non-inferior to somatropin [Citation12,Citation18].
Table 2. Efficacy result of clinical trial with somatrogon [Citation26].
2.7. Estimating resources and costs
Two costs have been considered in the economic model: the pharmacological cost of GH-d and somatrogon, and the cost of disease monitoring. The acquisition cost of GH-d was calculated, considering that the price per mg of GH-d is €17.50 per mg, according to the Royal Decree on reference prices of medicines in the Spanish National Health System [Citation21]. This average price was weighted according to the market shares in Spain of the GH-d. The parity price of somatrogon compared to GH-d was calculated, excluding in the base case of the analysis the 4% deduction for orphan drugs, established in articles 8, 9 and 10 of Royal Decree-Law 8/2010, as amended by Royal Decree-Law 9/2011 (). The monitoring cost included outpatient endocrinology consultations, primary care physician consultations, blood tests, hand X-rays and growth hormone stimulation tests. In this case, it was assumed the pituitary function test would include the following tests: prolactin, LH, FSH, TSH, free T4, ACTH, cortisol, GH and IGF-1, according to clinical expert opinion [MAAC, LCF JILA] (Table S4) [Citation22]. Although the Pharmacy Department is involved in the storage and collection of the treatment, it was not accounted for in the model.
2.8. Utilities
The model considered utilities by height (Table S5) and the additional utilities of weekly versus daily injection [Citation23] (). These utilities were used to calculate the quality-adjusted life years (QALYs) gained in a patient treated with somatrogon and GH-d.
The utilities for the different heights were calculated according to the estimates made by NICE in the report ‘Human growth hormone (somatropin) for the treatment of growth failure in children Technology appraisal guidance [TA188],’ published on 26 May 2010 [Citation23]. In a study carried out in England, in a general population of 14,416 adults, the inter-relationship between quality of life and height SDS was estimated using EuroQoL (EQ-5D) using linear regression, controlling for age, body weight, sex, social class and long-standing illness. ‘The study identified a positive correlation between an increase in height and a participant’s EQ-5D score. An increase in height SDS of 1.0 was associated with an increase in EQ-5D score in the shortest group of 0.061, an increase of 0.010 in the middle group, and an increase of 0.002 in the group with average or above average height’ [Citation23] (Table S5).
2.9. Analyses performed
A deterministic base case was performed according to the assumptions shown in . Univariate deterministic sensitivity analyses were also carried out, modifying the values of 15 variables (with the intervals indicated in ). Three additional sensitivity analyzes were performed. A first analysis including the 4% discount on the price of somatrogon, as it is an orphan drug.
According to the article by Deal et al [Citation17], the estimated levels of mean IGF-I, according to the PK/PD models, remain within normality and only 1.9% of the samples presented levels above 2 SDS (this corresponded to three patients). Somatrogon PK/PD levels indicate that sample collection 96 hours after somatrogon administration represents the mean IGF-I level over the weekly dosing interval. For this reason, the control is very similar to that done with daily GH and it is recommended to obtain the sample 96 hours after the administration of the somatrogon dose. Obviously, if the IGF-I level is elevated, it should be repeated. In the base case analysis, it was assumed that somatrogon monitoring will be very similar to that of daily GH. However, we performed a second sensitivity analysis, considering that the cost of monitoring was 20% higher with somatrogon than with GH-d. Finally, a sensitivity analysis was performed, considering a 2% increase in adherence with somatrogon vs GH-d.
A probabilistic analysis was also performed using second-order Monte Carlo simulations [Citation27,Citation28] to calculate the probability that somatrogon is cost-effective versus GH-d.
3. Results
3.1. Deterministic base case
In the base case, weekly somatrogon would be cost-effective versus GH-d, as the cost of gaining one QALY would be €19,259, below a willingness to pay of €25,000 to €60,000 per QALY gained [Citation29] (). For each patient treated with weekly somatrogon, there would be, compared to GH-d, an additional expenditure of €6,471 and a gain of 0.336 QALYs (a clinically relevant difference) [Citation30–33]. Furthermore, according to the model results, there would be a 4.11 cm gain in height compared to GH-d (170.28 cm and 166.17 cm, respectively).
Table 3. Result of the deterministic base case.
3.2. Univariate sensitivity analysis
In the tornado analysis, including all sensitivity analyses, all net monetary benefit results were positive, meaning that somatrogon was the cost-effective treatment versus GH-d for a willingness to pay of €25,000 per QALY gained (the threshold below which the most effective treatment is also considered cost-effective), with only one exception: when the upper limit of somatrogon acquisition cost was considered (). When the 4% price discount for somatrogon was included, the cost per QALY gained was reduced to €3,899, compared to GH-d. Considering that the cost of monitoring was 20% higher with somatrogon than with GH-d, the cost of gaining a QALY with somatrogon amounted to €23,042. Finally, a sensitivity analysis was performed, considering a 2% increase in adherence with somatrogon vs GH-d. The result was also cost-effective: €10,605 per QALY gained.
Table 4. Univariate sensitivity analysis.
3.3. Probabilistic analysis
The result of the probabilistic analysis is presented in and . The mean cost of gaining one QALY with somatrogon compared to GH-d would be €19,796, with a 95% CI of €19,126 to €20,438 (). The probability that, compared to GH-d, weekly somatrogon would be the cost-effective treatment would be 61% (for a willingness to pay of €25,000 per QALY gained), with somatrogon being the dominant (most effective, lowest cost) treatment in 12.4% of simulations (; ).
Figure 2. Cost-effectiveness plane of the probabilistic analysis. Weekly somatrogon vs. GH-d.
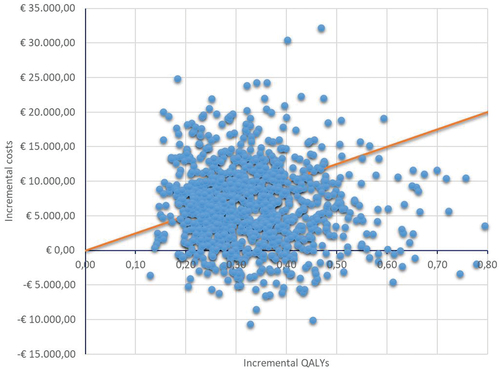
Figure 3. Acceptability curves of the probabilistic analysis. Weekly somatrogon vs. GH-d.
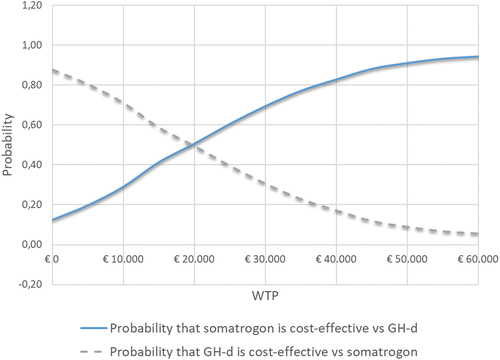
Table 5. Probabilistic analysis result.
3.4. Price threshold
The threshold price of somatrogon (for a willingness to pay of €25,000/QALY) is €5.75/mg. That is, above that price, the cost of gaining 1 QALY with somatrogon compared to GH-d would exceed €25,000.
4. Discussion
Clinical studies of somatrogon have shown that it is not inferior to somatropin. However, the effects of long-term growth hormone deficiency treatment in clinical practice need to be investigated, particularly the implications of weekly versus daily dosing on treatment adherence and consequent effectiveness.
According to the present analysis, weekly somatrogon is a cost-effective treatment compared to GH-d, with a cost per QALY gained of €19,796 (95%CI €19,126; €20,438) and a clinically relevant QALY gain (0.33) per treated patient. On this last aspect, it should be noted that a difference of 0.33 QALY in favor of somatrogon compared to GH-d is clinically relevant, as it is generally considered that the minimum difference in utility that the patient can detect between two interventions (measured by the EQ-5D, HUI2, HUI3 and SF-6D instruments) would be, depending on the instrument used, 0.074, 0.030, 0.030 and 0.033 QALYs, respectively [Citation30–33].
The economic model simulated, lato sensu, the evolution of a cohort of patients with short stature due to GHD treated with somatrogon or GH-d. To properly evaluate the results of the study, both the possible limitations of the study and its consistencies must be taken into account. The study’s main strength lies in the stability of the economic model (the direction of the base case result was maintained in the sensitivity analyses), which was confirmed in most deterministic analyses and the probabilistic analysis. According to the latter, compared to GH-d, somatrogon treatment of GHD patients would be cost-effective in 61% of patients for a willingness to pay of €25,000 per QALY gained, with a 95% confidence interval of €19,126 to €20,438. However, a limitation of the study is that it is a theoretical model and therefore a simplified simulation of reality. Therefore, several assumptions about using medical resources in the GHD had to be made. On the contrary, it should be considered a strength of the study that all assumptions made in the economic model were clinically validated by a panel composed of three Spanish clinical experts with extensive experience in the management of GHD, co-authors of the present paper (MAAC, LCF, JILA). In this regard, it is important to point out that most of the assumptions of the economic model were made by the panel of experts. The justification of the assumptions is explained in , according to the opinion of the panel of experts.
In this study, only direct healthcare costs were considered, since it was carried out exclusively from the perspective of the Spanish National Health System.
The results of several cost-effectiveness analyses of somatrogon compared to GH-d have been published. According to a Markov model with three health states and a 7-year time horizon, the cost of gaining one QALY with somatrogon compared to GH-d would be €190,430 in Germany and £51,957 in the UK [Citation34]. These results are not comparable to those obtained in our study, given that it is a different model in terms of structure and assumptions, and refers to health systems other than the Spanish NHS. Preliminary results of the present model were recently published in five countries (Canada, Spain, United States, Ireland and Sweden) [Citation12]. This concluded that somatrogon treatment led to 1.71 to 4.11 cm height gain and 0.19 to 0.43 QALYs compared with d-HGs. Somatrogon was generally cost-effective versus GH-d, with GH-d dosing, loss of utilities associated with injection frequency, unit costs of GH-d, and adherence to somatrogon being the key cost-effectiveness factors [Citation12]. The full results for Ireland have recently been published [Citation35]. This study concluded that somatrogon weekly injections were estimated to result in higher near adult height, higher QALYs, lower overall cost and lower cost per cm gained than GH-d, in pediatric growth hormone deficiency.
5. Conclusions
In summary, and according to the analyses performed, it can be concluded that somatrogon weekly is a cost-effective treatment compared to GH-d, with a mean cost per QALY gained of €19,796 (95%CI €19,126; €20,438), below a willingness to pay of €25,000 per QALY gained, and with a clinically relevant QALY gain (0.33) per treated patient.
Article highlights
Clinical studies of somatrogon have shown that it is not inferior to somatropin. However, the effects of long-term growth hormone deficiency treatment in clinical practice need to be investigated, particularly the implications of weekly (somatrogon) versus daily somatropin (GH-d) dosing on treatment adherence and consequent effectiveness.
According to the Markov model carried out, somatrogon would be cost-effective, compared to GH-d, with a cost per QALY (quality-adjusted life year) gained of €19,259 and a clinically relevant QALY gain (0.336).
This result was confirmed in the probabilistic analysis; somatrogon would be the dominant treatment, with a 61% probability of a willingness to pay of €25,000 per QALY gained.
Author’s contribution
C. Rubio-Terres and D. Rubio-Rodríguez have developed the adaptation to the Spanish National Health System of this economic model. M.A. Andreu Crespo, L. Castro-Feijóo, J.I. Labarta-Aizpún, C. Peral and J.A. Barrueta contributed to study conceptualization, design and revision of model. All authors had accessed to data, contributed to study conceptualization, methodology development and manuscript preparation. All authors listed made substantial contributions to the study in conceptualization and/or design of study, analysis and/or interpretation of data and manuscript preparation and/or review. All authors read, edited and approved the final manuscript. C. Rubio-Terres is the guarantor of the overall content of this manuscript.
Declaration of interest
C. Peral and J.A. Barrueta are employees of Pfizer SLU, Madrid, Spain. M.A. Andreu Crespo, L. Castro-Feijóo and J.I. Labarta-Aizpún has received speaker honoraria from Pfizer (Spain) and have participated in Pfizer advisory boards. D. Rubio-Rodríguez and C. Rubio-Terrés are employees of Health Value who received an honorarium from Pfizer (Spain) in connection with the development of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are an investigator in clinical trials of somatrogon for OPKO Health, a consultant for Pfizer and receive research funding from Pfizer. They are also a consultant for Ascendis which also makes a once weekly growth hormone approved in the US and elsewhere. They are a consultant for GenSci which also makes a once weekly growth hormone available in China. They are a consultant for and receive research funding from Novo Nordisk which also makes a once weekly growth hormone available in the US and elsewhere. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download (24.5 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2256473
Additional information
Funding
References
- Cheetham T, Davies JH. Investigation and management of short stature. Arch Dis Child. 2014;99(8):767–771. doi: 10.1136/archdischild-2013-304829
- López JP, Ariza AB. Talla baja de etiología no determinada y cada vez menos idiopática. Rev Esp Endocrinol Pediatr. 2021;12:21–34.
- Cohen LE. Idiopathic short stature: a clinical review. JAMA. 2014;311(17):1787–1796. doi: 10.1001/jama.2014.3970
- Murray PG, Clayton PE, Chernausek SD. A genetic approach to evaluation of short stature of undetermined cause. Lancet Diabetes Endocrinol. 2018;6(7):564–574. doi: 10.1016/S2213-8587(18)30034-2
- Lindsay R, Feldkamp M, Harris D, et al. Utah growth study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125(1):29–35. doi: 10.1016/S0022-3476(94)70117-2
- Vimpani GV, Vimpani AF, Lidgard GP, et al. Prevalence of severe growth hormone deficiency. Br Med J. 1977;2(6084):427–430. doi: 10.1136/bmj.2.6084.427
- Thomas M, Massa G, Craen M, et al. Prevalence and demographic features of childhood growth hormone deficiency in Belgium during the period 1986-2001. Eur J Endocrinol. 2004;151:67–72. doi: 10.1530/eje.0.1510067
- Donoso MA, Díaz S, Oyagüez I, et al. Impacto presupuestario de la utilización de hormona de crecimiento de la edad pediátrica a la adulta. Farm Hosp. 2012;36:3–10. doi: 10.1016/j.farma.2010.11.004
- Luzuriaga Tomás C, Oyarzabal Irigoyen M, Caveda Cepas E, et al. el grupo de investigadores españoles del estudio GeNeSIS. Seguridad y efectividad del tratamiento con hormona de crecimiento: estudio GeNeSIS en España. An Pediatr (Barc). 2016;84:139–147. doi: 10.1016/j.anpedi.2015.05.002
- Grimberg A, DiVall SA, Polychronakos C, et al. Drug and therapeutics committee and ethics committee of the pediatric endocrine society. guidelines for growth hormone and insulin-like growth factor-i treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. doi: 10.1159/000452150
- Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79(4):189–196. doi: 10.1159/000350251
- Rivolo S, Loftus J, Peter B, et al. Cost-effectiveness and cost-utility analysis of somatrogon once-weekly Injectable vs. Daily growth hormones for treating pediatric growth hormone deficiency (EE27). ISPOR Annual Meeting; Vienna, Austria: p. 6–9 Nov 2022. Available at: https://www.ispor.org/docs/default-source/euro2022/eva-27336-10-ispor-eu-a0-poster18oct22print-pdf.pdf?sfvrsn=79291a96_0 (access: 21 Nov 2022).
- Mohseni S, Heydari Z, Qorbani M, et al. Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab. 2018;31(1):13–20. doi: 10.1515/jpem-2017-0157
- Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148. doi: 10.1136/adc.2006.114249
- Maggio MC, Vergara B, Porcelli P, et al. Improvement of treatment adherence with growth hormone by easypod™ device: experience of an Italian centre. Ital J Pediatr. 2018; 44(1):113. doi: 10.1186/s13052-018-0548-z
- Rodríguez Arnao MD, Rodríguez Sánchez A, Díez López I, et al. Adherence and long-term outcomes of growth hormone therapy with easypod™ in pediatric subjects: Spanish ECOS study. Endocr Connect. 2019;8:1240–1249.
- Deal CL, Steelman J, Vlachopapadopoulou E, et al. Efficacy and safety of weekly somatrogon vs daily somatropin in children with growth hormone deficiency: a Phase 3 study. J Clin Endocrinol Metab. 2022;107(7):e2717–28. doi: 10.1210/clinem/dgac220
- Maniatis AK, Carakushansky M, Galcheva S, et al. Treatment Burden of weekly somatrogon vs daily somatropin in children with growth hormone deficiency: a randomized study. J Endocr Soc. 2022;6(10):bvac117. doi: 10.1210/jendso/bvac117
- Loftus J, Chen Y, Alvir JMJ, et al. Suboptimal adherence to daily growth hormone in a US real-world study: an unmet need in the treatment of pediatric growth hormone deficiency. Curr Med Res Opin. 2021;37(12):2141–2150. doi: 10.1080/03007995.2021.1982682
- Ngenla 24 y 60 mg solución inyectable en pluma precargada. Ficha técnica o Resumen de características del producto. Available at: https://www.ema.europa.eu/en/documents/product-information/ngenla-epar-product-information_es.pdf (access: 14 Apr 2023).
- Orden SCB/953/2019, de 13 de septiembre, por la que se procede a la actualización en 2019 del sistema de precios de referencia de medicamentos en el Sistema Nacional de Salud. BOE Nº. 2019 de;225(19):101906.
- Acuerdo de 18 de diciembre de 2020, del consejo de administración del ente público Osakidetza, por el que se aprueban las tarifas por prestación de servicios sanitarios y docentes a terceros obligados al pago durante el ejercicio 2021. Available at: https://www.euskadi.eus/contenidos/informacion/osk_servic_para_empresas/es_def/adjuntos/LIBRO_DE_TARIFAS_2021_CAS_Firmado.pdf (access: 7 Dec 2021).
- NICE. Human growth hormone (somatropin) for the treatment of growth failure in children technology appraisal guidance [TA188]. Published: 26 May 2010. Available at: https://www.nice.org.uk/guidance/ta188 (access: 07 Dec 2021).
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi: 10.1007/s10198-010-0224-8
- Ranke MB, Lindberg A. KIGS International Board. Height at start, first-year growth response and cause of shortness at birth are major determinants of adult height outcomes of short children born small for gestational age and Silver-Russell syndrome treated with growth hormone: analysis of data from KIGS. Horm Res Paediatr. 2010;74(4):259–266. doi: 10.1159/000289570
- Safety and Efficacy Phase 3 Study of Long-acting hGH (MOD-4023) in Growth Hormone Deficient Children. ClinicalTrials.Gov identifier: NCT02968004. Available at: https://clinicaltrials.gov/ct2/show/NCT02968004?term=NCT02968004&rank=1 (access: 27 Apr 2022).
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford (UK): Oxford University Press; 2006.
- Rubio-Terrés C, Rubio-Rodríguez D. Probabilistic analysis: sensitivity analysis or main result? (Editorial). Pharmacoecon Open Acc. 2016;1:2. doi: 10.4172/pe.1000e102
- Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? Gac Sanit. 2020;34:189–193. doi: 10.1016/j.gaceta.2019.06.007
- Brazier J, Roberts J, Tsuchiya A, et al. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13(9):873–884. doi: 10.1002/hec.866
- Horsman J, Furlong W, Feeny D, et al. The Health utilities index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1(1):54. doi: 10.1186/1477-7525-1-54
- Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes. 2003;1(1):4. doi: 10.1186/1477-7525-1-4
- Wee H-L, Machin D, Loke W-C, et al. Assessing differences in utility scores: a comparison of four widely used preference-based instruments. Value Health. 2007;10(4):256–265. doi: 10.1111/j.1524-4733.2007.00174.x
- Mehl A, Goh A, Gupta S Cost-effectiveness of once-daily somatropin from Sandoz versus once-weekly somatrogon for the treatment of growth hormone deficiency in children and adolescents (EE589). ISPOR Annual Meeting; Vienna, Austria: p. 6–9 Nov 2022. Available at: https://www.ispor.org/docs/default-source/euro2022/ee589ispor-eu-2022posterandrea-mehl-pdf.pdf?sfvrsn=cf0ba714_0 (access: 21 Nov 2022).
- Rivolo S, Loftus J, Peter B, et al. Cost-effectiveness and cost-utility analysis of somatrogon once-weekly injections vs. daily growth hormone injection for treating paediatric growth hormone deficiency in Ireland. J Med Econ. 2023;26(1):963–972. doi: 10.1080/13696998.2023.2228167