ABSTRACT
Introduction
Antibody-mediated rejection (ABMR) is a major cause of late kidney allograft failure, but its economic and humanistic impacts have not been well-characterized in the literature.
Areas covered
We reviewed available literature on economic burden (costs and healthcare resource use) and humanistic burden (health-related quality of life impacts [HRQOL] and utility estimates) in patients diagnosed with kidney transplant rejection; ABMR-specific studies were of particular interest. In total, 21 publications reporting economic and humanistic burden were included in the review; 9 of these reported ABMR-specific outcomes. The reviewed studies consistently showed a greater burden associated with ABMR-related transplant rejection than with non-ABMR transplant rejection.
Expert opinion
Evidence suggests greater economic burden and increased HRQOL impairment with ABMR-related kidney transplant rejection relative to non-ABMR, although small sample sizes and missing definitions for ABMR make meaningful comparisons between studies challenging. Because no International Classification of Diseases (ICD)-10 codes currently describe the etiologies of transplant rejection, it is difficult to characterize the burden of distinct types of transplant rejection. The paucity of high-quality data on the burden of ABMR in kidney transplant rejection demonstrates the need for more etiology-centric ICD-10 codes.
1. Introduction
Kidney transplant is the preferred treatment for end-stage renal disease, but an estimated 34% to 50% of transplant recipients will experience allograft failure within 10 years post-transplant [Citation1]. Kidney rejection is a major cause of allograft failure, and transplant recipients who experience allograft failure must resume dialysis or await re-transplantation. Thus, kidney transplant rejection may negatively impact transplant recipients’ health-related quality of life (HRQOL) and result in an economic burden for the healthcare system.
Kidney transplant rejection may be T-cell – mediated, antibody-mediated, or may have mixed etiology. T-cell – mediated rejection is generally clinically manageable; however, antibody-mediated rejection, which includes active, chronic active, and chronic inactive subtypes, is a significant driver of late allograft failures [Citation2,Citation3Citation4,Citation5]. In recent years, ABMR diagnoses have become more common with the availability of modern solid-phase assays to detect anti-HLA antibodies, as well as the development of more precise Banff diagnostic criteria for ABMR [Citation3]. Although it is recognized as a major cause of allograft loss, there are currently no approved therapies for ABMR; furthermore, existing treatment guidelines are based on low-level evidence, in part due to the limited number of prospective randomized trials conducted for the treatment of ABMR [Citation3]. Similarly, there are also limited data published on the humanistic and economic impacts of kidney transplant rejection due to ABMR.
Given the complex clinical course and lack of approved therapies for ABMR, we hypothesized that antibody-mediated kidney transplant rejection would be associated with considerable economic and humanistic burden. To better understand the impact of kidney transplant rejection and ABMR, we conducted a literature review evaluating economic burden (costs and healthcare resource use) and humanistic burden (HRQOL impacts and utility estimates) in individuals who experience kidney transplant rejection, with a particular focus on ABMR.
2. Materials and methods
We performed a literature review using a prespecified protocol to identify studies reporting medical costs, healthcare resource use, effects on employment, HRQOL, and utility estimates in patients diagnosed with kidney transplant rejection. Studies in patients with ABMR-related decline in kidney function or kidney failure were of special interest. Literature searches were conducted in Embase, PubMed, EconLit, and Cochrane databases for articles published from 1 January 2011 to 6 December 2021. The date limit was chosen because studies published more than 10 years ago are generally considered out-of-date (due to changes in practice patterns and cost). The literature search strategies were designed using Emtree terms, Medical Subject Headings (MeSH), and free-text terms (Tables A-1 to A-9, Supplementary Material). The geographical limits of the review were the United States (US), the United Kingdom (UK), Germany, Spain, France, and Italy. Our review was limited to these countries, as it was expected that the most relevant data could be found in these regions. The references of identified literature reviews and economic evaluations were searched to identify any primary studies not identified in the database searches. Additionally, the websites of relevant conferences and patient organizations were searched for relevant studies. The conference abstracts were searched from 2011 to 2021 to be consistent with the date range for the database search and to explore additional avenues for identifying ABMR-specific studies. The conferences indexed in Embase were ISPOR (The Professional Society for Health Economics and Outcomes Research), American Society of Nephrology Kidney Week, American Transplant Congress, American Society of Transplantation, and Transplantation Society Congress. Conferences and other websites not indexed in Embase included OpenGrey, American Kidney Foundation, International Transplant Nurses Society, National Kidney Foundation, and American Association of Kidney Patients; these sites were manually searched.
Selection of studies was performed according to inclusion and exclusion criteria predefined in the study protocol. The screening was conducted in two stages: level 1, where titles and abstracts were screened for eligibility; and level 2, where full-text articles for studies included at level 1 were retrieved and their eligibility for inclusion in the review was confirmed. Screening was performed by one researcher, and a second independent researcher checked any queries. A third researcher was consulted to reach a consensus if there was uncertainty surrounding the eligibility of a study. After completion of level 2 screening, one researcher extracted data from the eligible studies into a predefined extraction table, and a second researcher performed a quality check of all extracted data against the original sources.
Included studies were required to be conducted in adults (aged ≥18 years) who had received a kidney transplant and were experiencing a decline in kidney function, as well as studies that specifically mentioned ABMR (as defined by individual study authors). Because the research question focused on humanistic and economic burden, studies also needed to report on direct medical costs, medical resource use, indirect costs, or humanistic burden (HRQOL and utility). Only studies published in English were reviewed. Studies conducted in children (aged <18 years), literature reviews, and studies published before 2011 were excluded from review.
3. Results
A total of 1,446 records (titles and abstracts) were selected for screening after elimination of duplicates (databases = 1,432; internet searches = 3; manual searches = 11). After the initial (level 1) screening of titles and abstracts, 140 publications (database searches = 131; internet searches = 0; manual searches = 9) progressed to level 2 screening of full-text articles. At the level 2 screening, 21 publications (database searches = 19; internet searches = 0; manual searches = 2) met the predefined inclusion criteria and were selected for data extraction and inclusion. The PRISMA flowchart presented in shows the number of studies included and excluded at each stage of screening.
Figure 1. PRISMA diagram.
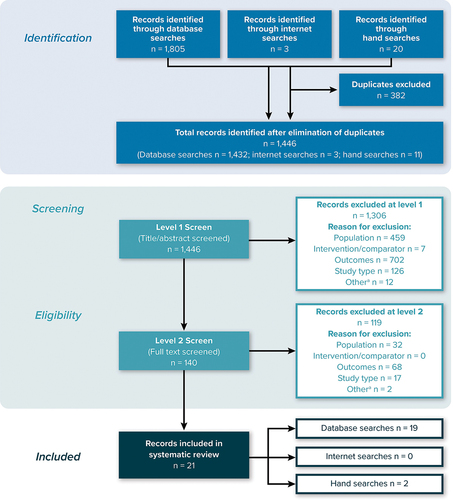
Of the 21 publications included in this review, 9 publications [Citation6–14] reported ABMR-specific burden, while the remaining 12 publications reported burden in the wider kidney transplant rejection population. Twelve publications reported medical costs and resource-use outcomes [Citation7,Citation11–19], 1 reported the effects of kidney allograft on employment [Citation20], and 9 reported HRQOL and utility outcomes [Citation6,Citation19,Citation21–27]. A summary of study outcomes is presented in , which summarizes the included articles. The studies in bold and italics represent those that reported burden related to ABMR-specific kidney transplant rejection.
Table 1. Summary of outcomes reported by study.
3.1. Costs and resource use associated with ABMR
Six studies reported total costs associated with ABMR-related kidney transplant rejection, although there were varying definitions of total costs, and in some cases no exact definitions of total costs were reported. Banga et al. [Citation7] and Kulkarni and Hall. [Citation10] reported total costs of ABMR transplant rejection versus non-ABMR transplant rejection in the US. The studies are not directly comparable, as the components of the total costs are not clearly reported by either study, and the cost-year was only reported by Banga et al. [Citation7]; however, ABMR kidney transplant rejection was associated with higher total costs than non-ABMR transplant rejection in both studies (). Hart et al. [Citation8] reported mean total costs per patient 60 days prior to an ABMR event and for up to 2 years after an ABMR event for ABMR patients compared with matched non-ABMR controls in the US (). Similar to results in Banga et al. [Citation7] and Kulkarni and Hall. [Citation10], costs reported by Hart et al. [Citation8] were considerably higher for the ABMR patients than for non-ABMR patients, but p values were not provided to assess if the differences were statistically significant. Of note, in the absence of International Classification of Diseases, 10th Revision (ICD-10) or Current Procedural Terminology (CPT) codes for ABMR, the authors developed an algorithm to identify AMBR cases for the cost analyses; thus, ABMR cases were selected by exposure to a treatment pattern typical of ABMR.
Figure 2. Total costs per patient, ABMR versus non-ABMR.
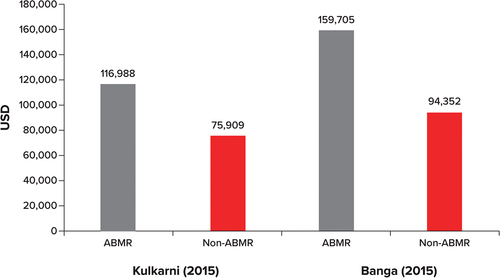
Table 2. Mean total costs.
Other key results from the ABMR articles include medication costs and resource utilization for ABMR patients. Banga et al. [Citation7] estimated mean medication/pharmacy costs in the year post-transplant to be $17,511 for ABMR patients versus $7,997 for non-ABMR patients. Muduma et al. [Citation12,Citation13] developed a cost-effectiveness model that estimated the prices for treatment regimens as patients transitioned into ABMR onset; costs ranged between £6,818.46 and £7,014.21, depending on the treatment regimen, although a comparison with treatment cost for non-ABMR was not estimated. Kim et al. [Citation17] also provided an overview of the prices for exploratory ABMR therapies (e.g. rituximab, bortezomib, and eculizumab) ranging from $1,200 to $28,000 per dose, although no comparison with non-ABMR patients was provided.
In terms of resource utilization for ABMR patients versus non-ABMR patients, including intensive care unit stays and hospital inpatient stays, Irish et al. [Citation9] reported 3.3 versus 1.69 days for intensive care unit stays and 20 versus 11 days for inpatient stays, respectively.
3.2. General costs of kidney failure
McDermott et al. [Citation11] estimated an annual cost of dialysis, from a cohort of 64 ABMR patients, of $88,000 (US dollars) per kidney transplant patient. Although the estimate for dialysis was assumed to be the same for both ABMR and non-ABMR patients, the cost – benefit analysis conducted by McDermott et al. [Citation11] illustrated the benefit of preventing graft failure. The cost – benefit ratio of ABMR treatment was estimated to be $6.59, meaning that for every dollar spent on ABMR treatment, $6.59 was projected to be saved on dialysis for the ABMR patient. The authors suggested that this resulted in $22,357,786 saved on dialysis for the cohort, equating to a median dialysis cost avoided of $387,682 per ABMR patient. It must be noted, however, that the timeframe for this saving is not clear, and the cost-savings calculation assumed that all patients with ABMR would have required dialysis. Similarly, while not specific to ABMR, Vo et al. [Citation14] estimated annual dialysis costs to be $84,639 (2011 US dollars) per kidney transplant patient, consistent with McDermott and colleagues’ [Citation11] estimated annual cost of dialysis. No studies identified outcomes relating to the impact of returning to the transplant waiting list or undergoing nephrectomy. The availability of data by cost and resource-use outcomes of interest in each publication is presented in .
Table 3. Summary of cost and resource use outcomes.
We found that the economic burden for ABMR patients was consistently higher than for non-ABMR patients across both costs and resource use. Cost and/or resource use associated with return to the kidney transplant waiting list, return to dialysis, re-transplantation, and nephrectomy for ABMR patients were not evaluated in any of the studies identified. Additionally, some of the reviewed studies did not include clear definitions for methodologies and outcomes used in the analyses, and some did not report the year in which the costs were incurred, preventing comparison between these studies. Additional details of the resource-use and cost studies are presented in Table A-10 (Supplementary Material).
3.3. Productivity impairment associated with kidney transplant
We did not find any productivity loss costs associated with ABMR in patients with kidney allograft. However, Julián-Mauro et al. [Citation20] reported indirect effects related to kidney transplantation in general. The study analyzed the mean cost of lost labor productivity for patients in eight Spanish hospitals using Human Capital Theory. The estimated mean (95% confidence interval) cost of lost labor productivity was €5,079.69 (€4,127.90–€6,030.50) for kidney transplant and €6,546.30 (€5,727.10–€7,366.10) for hemodialysis using an annual timeframe for the costs and a cost-year of 2009 euros. In the published data or gray literature we did not find any indirect effects associated with ABMR in kidney transplant recipients, such as caregiver burden, transport costs to treatment, and other out-of-pocket expenses. There is often a lack of indirect cost data in the published literature due to the general difficulty in quantifying indirect costs regardless of condition. Additionally, many studies are performed from a payor perspective, so these do not consider the societal impact of a condition.
3.4. HRQOL impairment associated with ABMR
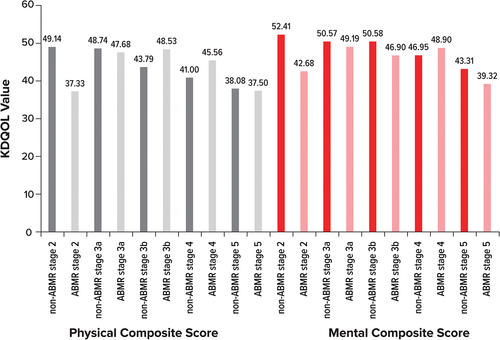
We identified 1 study that reported HRQOL for patients with ABMR-related decline in kidney function. Augustine et al. [Citation6] evaluated scores on the Kidney Disease Quality of Life Instrument (KDQOL)-36 effects of the kidney disease (EKD) domain for biopsy-proven ABMR and non-ABMR patients by chronic kidney disease (CKD) stage. Mean (standard deviation) KDQOL-36 EKD score for ABMR patients decreased from 78.91 (18.82) to 51.14 (21.47) when progressing from CKD stage 2 to stage 5 (higher KDQOL-36 EKD scores indicate better HRQOL). Non-ABMR controls decreased from 88.23 (18.31) to 59.62 (17.93) during the same stages of progression. These results demonstrated considerable impairments in HRQOL for both ABMR patients and non-ABMR controls as patients progressed through CKD stages. Augustine et al. [Citation6] also evaluated physical and mental component scores of the KDQOL-36 by CKD stage for ABMR and non-ABMR patients. Non-ABMR patients generally had numerically higher KDQOL-36 physical and mental component scores than ABMR patients, except for ABMR patients with stage 3b CKD (who had higher physical component scores than non-ABMR patients) and ABMR patients with stage 4 CKD (who had higher physical and mental component scores than non-ABMR patients) ().
Figure 3. KDQOL-36 values for physical and mental composite scores by ABMR Stage.
3.5. Utility estimates associated with decline in kidney function
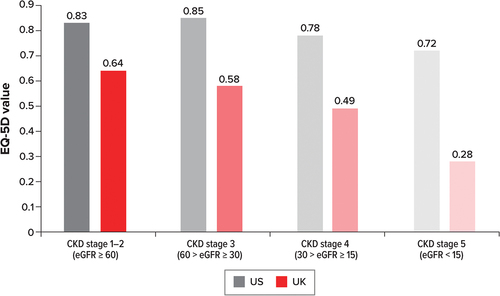
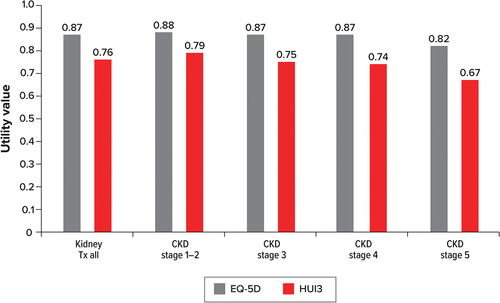
The review identified two further humanistic burden studies: Neri et al. [Citation25] and Neri et al. [Citation26]. While these studies did not report ABMR-specific utility data, these articles reported utility values (EQ-5D [Citation25,Citation26] and Health Utilities Index version 3 [HUI3] [Citation26]) by CKD stage, which could be extrapolated to the ABMR population as graft function declines. As with KDQOL-36, a higher score for the EQ-5D and HUI3 indicates a higher quality of life. EQ-5D scores declined with each CKD stage in the UK and the US, with the exception being an increase of 0.02 between stage 1–2 and stage 3 in the US (). The EQ-5D value was also considerably lower for the UK than for the US across all CKD stages; however, no p value was provided.
Figure 4. Adjusted mean EQ-5D values by CKD stage for the US and UK.
Both EQ-5D and HUI3 scores declined as US patients progressed through CKD stages (). Again, p values were not provided, so conclusions regarding the statistical significance cannot be drawn. In contrast to has a larger sample size and is based on data that are unadjusted, which may account for the lower rate of decline in HRQOL than that seen in . Both studies showed that quality of life declines as CKD progresses, and they imply a causal connection between the two. Further details of the HRQOL and utility studies are presented in Table A-11 (Supplementary Material).
Figure 5. EQ-5D and HUI3 values by CKD stage.
4. Discussion
In this review, we found that patients with ABMR-related kidney transplant rejection experience greater economic burden than kidney transplant patients without ABMR, driven primarily by consistently higher direct costs and consistently greater resource use [Citation7–10]. Furthermore, while HRQOL declines as patients experience a decline in kidney function due to transplant rejection regardless of etiology, patients with ABMR experience even greater HRQOL impairments than non-ABMR patients as they progress through CKD stages 2 through 5 [Citation6]. However, it is worth noting that these findings are based on a small number of studies and may not comprehensively represent the burden of ABMR.
Owing to a known paucity of data in ABMR-related decline in kidney function or transplant rejection, our review was designed pragmatically. Namely, we expanded the conference abstract search outside the typical limit of 2 years for reviews, acknowledging that older abstracts and posters would be key sources of relevant studies for the review. In addition, while the original review protocol was limited to US studies, only minimal data were identified; we then formally amended the protocol to expand the searches to key European markets (the UK, Germany, Spain, France, and Italy), thus identifying more studies on kidney transplant rejection in ABMR patients for inclusion and broadening the findings of the review. However, there were still considerable gaps in the data, especially on the effect of ABMR-related decline in kidney function or transplant rejection on employment and quality of life. High-quality data, including estimates of statistical significance in particular, were lacking. Of the studies on ABMR-related transplant rejection, some included information on cost and resource-use burden, but none included data on nephrectomy, return to dialysis, or re-transplantation. A recognition of the lack of approved therapies for ABMR was noted in Kim et al. [Citation17], along with the price per dose of exploratory ABMR therapies developed in different disciplines and adopted by the transplant community because of their effect on the humoral immune system. The impact of experimental therapies was not well reflected in our research and may further add to the economic burden of ABMR. It must be noted that a number of studies on the economic burden of ABMR were available only in abstract or poster form and thus included limited information on cost years, cost calculations, or outcome definitions. The evidence on economic burden was also subject to heterogeneity in terms of geographies, healthcare settings, populations studied, and study design. Thus, we found it challenging to make comparisons across studies on cost and resource-use burden. None of the identified studies distinguished between or specified if their populations had active ABMR or chronic active ABMR, which represented an additional gap in the literature. Further research to understand the difference in both economic and humanistic burden for active ABMR and chronic active ABMR is warranted. While our review primarily focused on ABMR-specific transplant rejection, we allowed the inclusion of studies on the wider kidney allograft population (noting that ABMR is rarely diagnosed) so that reported data could be used as a proxy in the absence of published and publicly available ABMR-specific data. Acknowledging the limitations of the evidence, this review summarizes the available literature on the topic of the economic and humanistic burden of ABMR and kidney graft failure, and contributes to the research on this topic. While the results of the review highlight the paucity of evidence, the research contributes to an understanding of the AMBR literature and the key gaps in the literature.
One limitation of this review is that double screening was not performed. Although screening was performed by one researcher, any uncertainties regarding inclusion or exclusion were discussed with a second researcher (and with the wider research team, where applicable) to reach consensus. In addition, the search strategy was targeted to identify data published in English within the past 10 years. Restricting the timeframe of the searches meant that older articles may have been missed, but we felt it was important to capture the most recent economic data reflecting current practice patterns and treatment guidelines and aligning with the most recent Banff criteria. Restricting the search strategy to English-language data had little impact on the number of hits retrieved by the searches (the number of hits retrieved was reduced by 2% when restricted); however, there is still a small risk that relevant studies published in another language may not have been identified. Despite this limitation, the review was conducted according to a predefined protocol and eligibility criteria, and the screening process was thoroughly documented using PRISMA reporting methods.
5. Conclusion
To the best of our knowledge, this is the first review of the economic and humanistic burden of kidney transplant rejection. While ABMR-specific data were not available for every outcome of interest, the available data demonstrated that patients with kidney transplant rejection associated with ABMR consistently experienced greater economic and humanistic burden than patients who did not experience ABMR or those who experienced transplant rejection due to other etiologies.
6. Expert opinion
Although ABMR transplant rejection was described in 2003, as part of an update to the 1997 International Banff Classification of kidney allograft rejection, and subclassifications of ABMR were established in the 2017 update to the Banff criteria [Citation2,Citation3,Citation28], the implications of the subclassifications are not well-described or well-documented in the literature. Indeed, while the relative rarity of kidney transplant patients diagnosed with ABMR could explain the gap in high-quality data, another contributing factor may be the lack of International Classification of Diseases, 10th Revision (ICD-10) codes for the different etiologies of kidney transplant failure, such as ABMR, T-cell – mediated rejection, and BK virus – induced nephropathy. While evidence from our review suggests additional economic and humanistic burden for kidney transplant patients with ABMR compared to those without ABMR, it is challenging to conduct analyses of the impact of different etiologies of kidney transplant rejection without being able to distinguish between them in a dataset. Only one database analysis was identified in our review: Hart et al. [Citation15], which established an algorithm for identifying ABMR patients.
Our research suggests the need for collaborative research to establish ICD-10 codes for the different types of kidney transplant rejection. Establishing separate ICD-10-CM codes for the etiology of kidney transplant rejection would enable greater precision in evaluating outcomes, subsequently improving the generation of real-world evidence in this disease area. Higher quality and quantity of research, in turn, should help to establish better treatment for the different types of kidney transplant rejection, including ABMR. New codes have been added in other disease areas [Citation29], which allows greater precision when recording insurance claims in databases. Similarly, familial hypercholesterolemia (FH) [Citation30] and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy are other examples where etiology-inclusive ICD-10 codes have been established. At the time new codes were established for FH, it was believed that greater precision in the ICD-10 codes for FH would help improve treatment for patients and generate better research [Citation30]. The introduction of new ICD-10 codes that discriminate between different etiologies of kidney transplant rejection will not have immediate impact, as time is needed for the new codes to be utilized in clinical practice and for sufficient data to be collected in order to generate real-world evidence. However, long-term improvement in evidence generation should be realized with the help of new ICD-10 codes. The design and implementation of prospective research focused on the impact of ABMR on patients’ quality of life, including those with active ABMR and those with chronic active ABMR, would also be beneficial. The lack of available ICD-10 codes for the different etiologies of kidney transplant rejection is a significant enough issue in the conduct of medical research that it may warrant a reevaluation of the existing ICD-10 code taxonomy for transplantation rejection. While this review primarily focused on ABMR-related kidney transplant rejection, it demonstrates the difficulty of identifying high-quality data based on specific etiologies of transplant rejection. Finally, one direction for future research is whether the treatment of ABMR is a cost-effective strategy to prolong graft survival and to prevent graft loss. Precise diagnosis of ABMR is essential for understanding the costs and outcomes associated with ABMR-related graft loss and the potential value of new therapies.
Article highlights
We conducted a literature review to evaluate economic burden and humanistic burden in individuals who experience kidney transplant rejection, with a particular focus on ABMR.
Evidence from the 9 specific studies reviewed suggests greater economic burden and increased health-related quality-of-life impairment among individuals with ABMR-related kidney transplant rejection relative to individuals without ABMR.
Because no International Classification of Diseases (ICD)-10 codes currently describe the etiologies of kidney transplant rejection, it is difficult to characterize the burden of distinct types of transplant rejection. Establishing etiology-specific ICD-10 codes for kidney transplant rejection would enable greater precision in evaluating outcomes, subsequently improving the generation of real-world evidence in this disease area.
Declaration of interest
J Lee, D Reichenbach, S Yan, and K Thiruvillakkat report employment with CSL Behring. E Moss and S Mitchell are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which was retained by CSL Behring to conduct the research which is the subject of this manuscript. Their compensation is unconnected to the studies on which they work. A Burrell reports consulting fees from CSL Behring, Neumentum, and Healx.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
All authors were involved in the conception and design of the research, in the interpretation of the data, the drafting of the paper and final approval. The authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (156.8 KB)Acknowledgments
Writing assistance was provided by Kate Lothman, BA, and Brian Samsell, PhD, of RTI Health Solutions. Editorial support was provided by Synergy Medical Communications and funded by CSL Behring in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2024.2305140
Additional information
Funding
References
- Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 annual data report: kidney. Am J Transplant. 2019 Feb;19 Suppl 2:19–123.
- Loupy A, Haas M, Roufosse C, et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20(9):2318–2331. doi: 10.1111/ajt.15898
- Schinstock CA, Mannon RB, Budde K, et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 expert consensus from the transplantation society working group. Transplantation. 2020;104(5):911–922. doi: 10.1097/TP.0000000000003095
- Ho J, Okoli GN, Rabbani R, et al. Effectiveness of T cell-mediated rejection therapy: a systematic review and meta-analysis. Am J Transplant. 2022;22(3):772–785. doi: 10.1111/ajt.16907
- Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9(11):2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x
- Augustine T, Gill J, Budde K, et al. International, matched-cohort checkerboard study of quality of life and disease burden in kidney transplant (TX) patients with antibody-mediated rejection (AMR). Transplant Int. 2019;32:310.
- Banga R, Schinstock C, Boscoe A, et al. Burden of early antibody-mediated rejection (AMR): complications, resource utilization and cost-differential in treatment of AMR. Am J Transplant. 2015;15(Suppl 3):A296.
- Hart A, Zaun D, Itzler R, et al. Cost, healthcare utilization, and outcomes of antibody-mediated rejection in kidney transplant recipients in the US. J Med Econ. 2021;24(1):1011–1017. doi: 10.1080/13696998.2021.1964267
- Irish W, Boscoe A, Ryan M, et al. Economic burden of antibody mediated rejection following kidney transplantation: comparative analysis using the Premier hospital database. Value Health. 2015;18(3):A187. doi: 10.1016/j.jval.2015.03.1082
- Kulkarni S, Hall I. Association of de novo antibody-mediated rejection and increased hospital costs following kidney transplant in immunologically low-risk patients. Am J Transplant. 2015;15(Suppl 3): A127.
- McDermott C, Carsky K, Ferrin P, et al. Analysis of cost effectiveness of treatment for acute antibody-mediated rejection. Am J Transplant. 2018;18:734.
- Muduma G, Odeyemi I, Pollock RF. Evaluating the economic implications of non-adherence and antibody-mediated rejection in renal transplant recipients: the role of once-daily tacrolimus in the UK. J Med Econ. 2015;18(12):1050–1059. doi: 10.3111/13696998.2015.1074584
- Muduma G, Odeyemi IA, Pollock RF. Evaluating the economic implications of nonadherence and antibody-mediated rejection in renal transplant recipients: the role of once-daily, prolonged-release tacrolimus in the UK setting. Value Health. 2015;18(3):A187. doi: 10.1016/j.jval.2015.03.1081
- Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation. 2013;95(6):852–858. doi: 10.1097/TP.0b013e3182802f88
- Hart A, Singh D, Brown SJ, et al. Incidence, risk factors, treatment, and consequences of antibody-mediated kidney transplant rejection: a systematic review. Clin Transplant. 2021;35(7):e14320. doi: 10.1111/ctr.14320
- Gheorghian A, MA S, DA A, et al. The implications of acute rejection and reduced allograft function on health care expenditures in contemporary US kidney transplantation. Transplantation. 2012;94(3):241–249. doi: 10.1097/TP.0b013e318255f839
- Kim M, Martin ST, Townsend KR, et al. Antibody-mediated rejection in kidney transplantation: a review of pathophysiology, diagnosis, and treatment options. Pharmacotherapy. 2014;34(7):733–744. doi: 10.1002/phar.1426
- Schnitzler MA, Johnston K, Axelrod D, et al. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91(12):1347–1356. doi: 10.1097/TP.0b013e31821ab993
- Sussell J, Silverstein AR, Goutam P, et al. The economic burden of kidney graft failure in the United States. Am J Transplant. 2020;20(5):1323–1333. doi: 10.1111/ajt.15750
- Julián-Mauro JC, Cuervo J, Rebollo P, et al. Employment status and indirect costs in patients with renal failure: differences between different modalities of renal replacement therapy. Nefrologia. 2013;33(3):333–341. doi: 10.3265/Nefrologia.pre2012.Dec.11767
- Calia R, Lai C, Aceto P, et al. Effects of switching from twice-daily to once-daily tacrolimus formulation on quality of life, anxiety, and transplant benefit perception after kidney transplantation. Transplant Proc. 2011;43(4):1020–1023. doi: 10.1016/j.transproceed.2011.03.048
- Gibbons A, Bayfield J, Cinnirella M, et al. Changes in quality of life (QoL) and other patient-reported outcome measures (PROMs) in living-donor and deceased-donor kidney transplant recipients and those awaiting transplantation in the UK ATTOM programme: a longitudinal cohort questionnaire survey with additional qualitative interviews. BMJ Open. 2021;11(4):e047263. doi: 10.1136/bmjopen-2020-047263
- Gibbons A, Bradley C. The effects of kidney alone or simultaneous pancreas and kidney transplant on quality of life and health status: findings from ATTOM programme cohorts with baseline data. Nephrol Dial Transplant. 2016;31(suppl_1):i450. doi: 10.1093/ndt/gfw189.33
- Kochar GS, Rega SA, Feurer ID, et al. Association of kidney function with patient reported outcomes after kidney transplantation. Am J Transplant. 2021;21(Suppl 4):667.
- Neri L, McEwan P, Sennfält K, et al. Characterizing the relationship between health utility and renal function after kidney transplantation in UK and US: a cross-sectional study. Health Qual Life Outcomes. 2012;10(1):139. doi: 10.1186/1477-7525-10-139
- Neri L, Dukes J, Brennan DC, et al. Impaired renal function is associated with worse self-reported outcomes after kidney transplantation. Qual Life Res. 2011;20(10):1689–1698. doi: 10.1007/s11136-011-9905-8
- Perl J, Zhang J, Gillespie B, et al. Reduced survival and quality of life following return to dialysis after transplant failure: the dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2012;27(12):4464–4472. doi: 10.1093/ndt/gfs386
- Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation. 2018;102(11):1795–1814. doi: 10.1097/TP.0000000000002366
- Moore K. New diagnosis codes effective Oct. 1. Here are some family physicians should know. American Academy of Family Physicians: FPM journal [Internet]. 2021 Aug 1 [cited 12 Aug 2022]; Getting Paid, A Blog from FPM Journal. Available from: https://www.aafp.org/journals/fpm/blogs/gettingpaid/entry/new_diagnosis_codes.html
- Family Heart Foundation. The ICD-10 codes for familial hypercholesterolemia are approved! Family heart foundation [Internet]. 2016 Jun 30 [cited 14 Apr 2022]; ICD-10 Code Approved. Available from: https://thefhfoundation.org/the-icd-10-codes-for-familial-hypercholesterolemia-are-approved
