ABSTRACT
Introduction
OnabotulinumtoxinA (OBT-A) and monoclonal antibodies (mAbs) targeting the calcitonin gene-related peptide (CGRP) pathway are two of the few treatments that ameliorate chronic migraine (CM) in randomized controlled trials and real-life studies. Separate clinical practice guidelines have been developed for the management of CM with OBT-A or CGRP-targeting mAbs.
Areas covered
Considering the concomitant availability of OBT-A and CGRP-targeting mAbs as therapeutic treatment options, Italian migraine experts reviewed the evidence supporting the efficacy of OBT-A and CGRP-targeting mAbs in CM in order to rationalize the management of CM patients treated with OBT-A. Experts addressed everyday practice needs to shape the optimal pharmacological management by balancing adherence to regulatory indications, ethical considerations, and clinical expertise. Considering the remarkable challenge of improving the health and quality of life of patients with CM, even partial improvements may be clinically meaningful, particularly for those who are resistant or intolerant to oral migraine treatments.
Expert opinion
In this collaborative effort, we propose a treatment algorithm that integrates the relevant aspects of managing patients with CM to provide ready-to-use practical guidance regarding the appropriate use of OBT-A.
1. Introduction
Migraine is the most common disabling brain disorder. Systematic analysis of the burden of neurological disorders by the GBD 2016 Neurology Collaborators for the Global Burden of Disease Study found that migraine is the second highest contributor to worldwide neurological disability-adjusted life-years (DALYs) [Citation1]. Furthermore, among people aged 15 to 49 years, the age group most commonly affected by migraine, migraine is the leading cause of disability [Citation2]. However, this condition is still underdiagnosed and undertreated, and there is poor global awareness of its burden [Citation3,Citation4].
Chronic migraine (CM), defined as the occurrence of at least 15 days with headache per month for at least 3 months, with headache having migraine characteristics for at least 8 days per month [Citation5,Citation6], affects 2–3% of the general population and is the most disabling form of migraine, representing a clinically distinct, more aggressive subtype of migraine [Citation6,Citation7,Citation8]. Both the American Migraine Prevalence and Prevention (AMPP) and the Chronic Migraine Epidemiology and Outcomes (CaMEO) longitudinal cohort studies found significantly more severe headache-related disability in those with chronic versus episodic migraine (EM) [Citation9]. Compared to EM, CM has a higher impact on physical, social, and occupational functioning and is characterized by a poorer health-related quality of life (HRQoL) [Citation10,Citation11,Citation12]. Patients with CM were reported to be twice as likely than those with EM to have psychiatric comorbidities, such as depression and anxiety [Citation13,Citation14]. Circulatory and endocrine conditions are also significantly more likely to be reported by those with CM [Citation15].
CM patients are problematic and difficult to treat, and only partial benefit is obtained from oral preventive medications [Citation3]. Managing CM is extremely challenging for several reasons. First, less than 50% of patients seek advice from a headache specialist, and a minority receive adequate acute and preventive treatment [Citation4,Citation16,Citation17]. Only few drugs have a clearly established level of evidence of efficacy [Citation16–18]; however, these medications are often poorly tolerated and their efficacy does not exceed, on average, 50% of cases [Citation19].
To date, onabotulinumtoxinA (OBT-A) is one of the few treatments that proved effective in CM in randomized controlled trials (RCTs) [Citation20,Citation21] and in real-life studies [Citation22–45]. OBT-A is specifically approved for CM prevention [Citation46] and is currently recommended for the treatment of patients who have not responded adequately or who are intolerant to a specified number (two, according to Italian regulations) of oral migraine treatments [Citation47,Citation48]. Recently, monoclonal antibodies (mAbs) targeting the calcitonin gene-related peptide (CGRP) pathway have been developed and investigated for EM and CM prevention, showing that the blockade of both the peptide and its receptor are effective mechanisms to reduce the frequency of migraine attacks [Citation18].
Separate guidelines are available for the management of CM with OBT-A or with CGRP-targeting mAbs [Citation48–50]. As a group of Italian migraine experts, considering the concomitant availability of these two different therapeutic strategies, we felt it important to rationalize the management of CM patients treated with OBT-A. This paper reports the decisional process and the treatment algorithm resulting from the discussion.
2. Methods
In September 2019, a panel of 7 Italian headache specialists (we, the authors) met in Rome to discuss the opportunity of reviewing the existing CM management algorithm for patients who start OBT-A according to the common practice of most of the more representative Italian Headache Centers. Such an opportunity was suggested by both the well-consolidated clinical experience with OBT-A and the advent of new treatments. To this purpose, we performed a complete review of the published RCTs and pooled analyses [Citation20,Citation21,Citation51–56] and real-world evidence data about OBT-A in CM [Citation22–45], and of the RCTs and a real-life studiey of the CGRP-targeting mAbs in CM patients [Citation57–66], together with an analysis of the current guidelines and recommendations for CM management [Citation48–50]. The overall data were reviewed and discussed, taking into account our personal clinical experience, with the aim of providing practical guidance for the optimal management of CM patients with OBT-A over a period of 3 years. A proposed algorithm was developed from the interactive discussion and is presented here.
3. Proposed new treatment algorithm for CM
OBT-A has been shown to be effective and safe in CM in RCTs () [Citation20,Citation21] and in real-world studies () [Citation22–45]. A recent review focused on long-term treatment data and on the optimal timing of prophylaxis with OBT-A concluded that OBT-A represents a therapeutic option that should be proposed to patients as early as possible [Citation47]. The effectiveness of OBT-A has also been demonstrated in both RCTs [Citation20,Citation21] and in real-life studies [Citation26,Citation28–30] in patients with medication-overuse headache (MOH), for whom our algorithm should also be considered. Four CGRP-targeting mAbs have recently been developed and evaluated in RCTs in patients with EM and CM [Citation18], one targeting the CGRP receptor (erenumab) and three targeting CGRP itself (eptinezumab, fremanezumab, and galcanezumab). Placebo-controlled trials of CGRP-targeting mAbs in CM have been shown to reduce migraine frequency () [Citation57–61]. A recent guideline on CGRP-targeting mAbs concluded that there is medium to high-quality evidence to recommend erenumab, fremanezumab, and galcanezumab in patients with CM [Citation49,Citation50].
Table 1. Summary of pivotal randomized clinical trials with onabotulinumtoxinA in patients with chronic migraine
Table 2. Summary of real-world studies with onabotulinumtoxinA in patients with chronic migraine
Table 3. Summary of randomized clinical trials with CGRP-targeting monoclonal antibodies in patients with chronic migraine
The new algorithm presented in is aimed at providing guidance for the management of OBT-A therapy in CM patients, taking into account that in several countries OBT-A is not approved as a 1st-line treatment, meaning that patients can be started on OBT-A only after having failed or not tolerated a specified number of previous oral medications. The decision on further patient management after the initial administration of OBT-A should be reassessed every 3 months during the first year of treatment to establish efficacy (). After the first year of treatment, timing to reevaluate the patient is dependent on the clinical response (), which should be evaluated using headache diaries or other outcome indicators () [Citation14,Citation22,Citation47,Citation48,Citation52,Citation53,Citation54,Citation55,Citation68,Citation69,Citation70,Citation72–77]. As additional efficacy/benefit indicators, we identified the following: headache intensity, intake of acute medications, headache-related disability, QoL, and patient-reported impression of effectiveness. The assessment of all levels of response should refer to the changes from baseline and should be performed using validated tools, e.g. a categorical, 4-level rating scale or 11-point numerical rating scale to rate the intensity of headache [Citation78], MIDAS or HIT-6 score for disability [Citation79,Citation80], MSQ for QoL [Citation81], PGCI for patient’s impression of efficacy [Citation82].
Figure 1. Proposed treatment algorithm for the preventive treatment of chronic migraine according to the clinical response. TP: Therapy; mAB: monoclonal antibodies; 75+ responder: ≥75% reduction in headache days and/or equivalent improvement in one of the efficacy/benefit indicators (see text); 50+ responder: ≥50% and <75% reduction in headache days and/or equivalent improvement in one of the efficacy/benefit indicators; 30+ responder: ≥30% and <50% reduction in headache days and/or equivalent improvement in one of the efficacy/benefit indicators; Nonresponder: <30% reduction in headache days and no improvements in any of the efficacy/benefit indicators. All patients should be assessed for response to treatment (changes from baseline) using a headache diary and validated tools for the detection of the other efficacy/benefit indicators
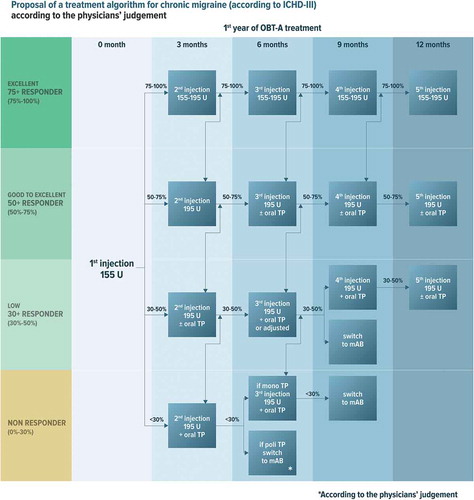
Figure 2. Proposed treatment algorithm for the use of onabotulinumtoxinA for preventive treatment of chronic migraine after the first year according to the clinical response
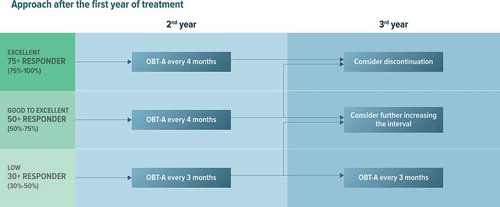
Table 4. Criteria of response to treatments in chronic migraine from the literature
For the proposed treatment algorithm, patients are classified as:
75+ responders when they experience a ≥ 75% reduction in headache days from baseline or a ≥ 75% improvement in one of the efficacy/benefit indicators listed above. These subjects are defined as excellent responders.
50+ responders, when they experience a reduction in headache days from baseline ranging from ≥50% to <75% and/or experience an improvement ranging from ≥50% to <75% in one of the efficacy/benefit indicators listed above. These subjects are defined as good responders.
30+ responders, when they experience a reduction in headache days from baseline ranging from ≥30% to <50% and this is associated to a clinically meaningful improvement in at least one of the efficacy/benefit indicators indicated above. These subjects are defined low responders.
Non– responders when they experience <30% reduction in headache days from baseline and no improvement in the other outcome indicators.
3.1. Excellent responders
After the first injection of OBT-A 155 U, the dose can be kept steady or escalated to 195 UI to consolidate the clinical response (). We suggest administering 155–195 U injection every 3 months for 1 year. During the second year, if the excellent response persists, we suggest increasing the inter-injection interval to 4 months (). After the second year, discontinuation may be considered for patients showing stable clinical improvement also with the increased (4-month) inter-injection period ().
3.2. Good responders
After the first injection of OBT-A 155 U, we suggest increasing the dose to 195 U at the second cycle to try to improve the clinical response (). Thereafter, patients who further improve will follow the algorithm for excellent responders; for those who remain good-responders, we suggest considering the addition of a second drug starting from the third injection cycle onward in order to further improve clinical outcomes. In more detail, if the number of monthly headache days is consistently <8 with OBT-A 195 U, OBT-A can be continued as monotherapy; if the monthly headache days are ≥8, we suggest the addition of a second preventative therapy (). The second preventative should be selected considering the clinical and pharmacological history and comorbidities of the patient. The second preventive drug can theoretically be represented by a CGRP-targeting mAB, though evidence in favor of the association is currently lacking and local regulations may not allow the combination.
In patients who have benefited from this treatment approach, OBT-A should be administered every 3 months also during the second year of treatment (). During the third year of treatment, the inter-injection interval of OBT-A 195 U administrations can be tentatively increased to 4 months.
3.3. Low responders
In patients qualifying in this group after the first injection of OBT-A 155 U, we suggest increasing the dose to 195 U during the second injection and considering to add an oral preventive () or modifying the dosage of the oral therapy in the first course. For patients who achieve a good response with this approach, we suggest following the algorithm for good responders. For those who continue to be low responders, there are two possible options: 1) to continue with OBT-A plus oral preventative or 2) to switch to an approved CGRP-targeting mAb.
In patients who show partial improvement with the former approach, we suggest maintaining the combined OBT-A+ oral prevention approach for at least 1 year if patients are good or near-good responders (). During the second year of treatment, we suggest trying to taper off the oral drug, while continuing OBT-A. During the third year of treatment, only in cases of good-to-excellent response, it will be possible to increase the inter-injection interval of OBT-A 195 U to 4 months (). Conversely, if there is a fluctuation between good and low response, we suggest continuing OBT-A at 3-month intervals.
For patients who remain low responders after 2 cycles of OBT-A in association with an oral treatment, we suggest stopping OBT-A and switching to a CGRP-targeting mAb ().
3.4. Non-responders
For non-responders after one injection of OBT-A 155 U, we suggest increasing the OBT-A dose to 195 U at the second injection and adding an oral preventative (). In patients who are still non-responders after 2 cycles of OBT-A with the combination of oral treatment for at least 3 months, we suggest switching to a CGRP-targeting mAb. If there is some improvement at 6 months, but the response is still low, we suggest following the algorithm for low responders, and thus to go ahead with a further OBT-A injection, then consider maintaining or withholding treatment, taking into consideration the clinical response at 9 months.
4. Discussion
The primary objective of treating CM is to reduce the frequency and duration of headache attacks and migraine-related disability, to decrease the burden of the disease and its impact on patients’ lives [Citation83,Citation84]. OBT-A is the first treatment specifically approved for the prevention of CM in the European Union and guidelines recommended preventive medication for CM [Citation48,Citation67,Citation68]. However, further significant progress is currently being made in the preventive treatment of migraine, prompting clinicians to rethink the treatment algorithm for CM in the light of the new preventive therapies.
The first issue under discussion is the definition of responders, given that, as previously pointed out, several different definitions are available in the literature, and there is not a generally accepted one. This appears also to be confirmed in clinical practice. The previously mentioned 2017 Italian survey [Citation84] revealed that nearly 60% of participants, i.e., representing 46 third-level headache centers experienced in the use of OBT-A in CM according to the PREEMPT protocol, defined response to treatment with OBT-A as a ≥ 50% reduction from baseline in the number of headache days (50+ responders), while one-quarter of them defined it as a ≥ 30% reduction (30+ responders). The remaining 15% also considered as response a reduction in headache days lower than 30% (<30-responders) if it was associated with the improvement of at least another efficacy variable, such as patient satisfaction with treatment, intensity of headache, use of medications for symptom relief, and duration of headache attacks [Citation84]. The patients’ perspective also emerged from the survey. In the opinion of their physicians, patients placed more value on an improvement in their QoL rather than in a simple reduction in headache days. While not hard clinical outcomes, the relevance of such patient-reported outcomes should not be underestimated, especially since they are important for patient and may also reflect isolated improvement in pain.
Another question still being debated is whether it is appropriate to administer further courses of treatment in case of low or non-response to the first course of OBT-A. Analyzing the pooled data of the PREEMPT program, approximately 10% of non-responders to the first OBT-A cycle responded to each of the two subsequent cycles [Citation74]. Real-life studies have also shown progressive improvements with continuous OBT-A treatment [Citation26,Citation29]. As a general rule, the expert group agreed on the administration of at least one additional course of OBT-A at an increased dosage (195 U) in all patients who receive a first administration of a 155-U dose. It is further recognized that patient-reported outcomes such as MIDAS and HIT-6, as well as general quality of life indicators, are heterogeneous measures that are quantitatively different. It is also possible that the different scales do not give equal weight to headache intensity and frequency. Despite this possible shortcoming, different outcome measurements nonetheless provide valuable information on the efficacy of different treatments and help to categorize responses to various therapies. While it is possible that a decrease in headache intensity alone might be a good outcome measure for efficacy, this has been poorly tested to date.
Regarding the optimal treatment duration in responders, some evidence from real-world studies suggests that discontinuing treatment in responding patients may lead to worsening of the disease [Citation24,Citation26]. The Italian survey found that the most frequent duration of OBT-A therapy in clinical practice is 1 year, with a tendency to enlarge the interval between cycles when prolonging treatment beyond 1 year [Citation84]. When considering that CM is an aggressive type of migraine that tends to be treatment-resistant and to relapse over time, it is reasonable and ethical to consider prolonging OBT-A treatment into the second year in excellent responders, and into the third year in less brilliant responders, while putting in place corrective measures, such as distancing of cycles in patients showing a satisfactory improvement, or adding additional drugs in those with limited improvement.
5. Conclusions
Oral migraine preventive medications are the first-line treatment for patients with frequent debilitating migraines, but most evidence on the efficacy of these medications in CM is extrapolated from studies in patients with high-frequency EM [Citation85,Citation86]. Of note, in patients with CM, poor adherence to therapy is mostly due to insufficient effectiveness [Citation87,Citation88]. However, combining treatments for migraine prevention when a patient has an inadequate response to a single therapy, although supported by limited evidence, is a common practice in the clinical setting [Citation45]. We considered that approved oral medications could be a potentially useful add-on therapy in CM patients partially responding to OBT-A, if not tried before and not contraindicated. Erenumab, fremanezumab, and galcanezumab ameliorate CM, reducing the number of headache/migraine days and the days of intake of acute medications, and improving disability, with a favorable safety profile [Citation57–60,Citation62,Citation63].
Until now, clinical data on the association of CGRP-targeting mAbs and OBT-A are lacking. Initial real-life data suggest that patients who are OBT-A non-responders may benefit from the combined therapy with CGRP-targeting mAbs [Citation64]. However, there is still a proportion of patients who are non-responders to CGRP-targeting mAbs or OBT-A alone. Patients with refractory migraine are a noticeably difficult-to-treat subgroup [Citation89]. In those patients, further studies are needed to understand the possible benefits of combining the two treatments. Even in the current absence of clinical evidence, we think that the combination of OBT-A with monoclonal antibodies targeting CGRP may provide clinical benefit to a subset of treatment-refractory CM patients and may be ethically indicated when all other options have proved ineffective. The association of OBT-A with anti-CGRP treatments should be assessed, in our opinion, on solid efficacy outcomes, such as decrease in headache days and use of acute medications. Assessing the combined efficacy of two potent anti-migraine injectables is problematic if it is based solely upon patient-reported outcomes. Demonstrating this will require well-designed observational studies based on solid outcome assessments, given that OBT-A and anti-CGRP are both very effective treatments.
6. Expert opinion
The treatment of CM is a dynamic and rapidly evolving area of research, and significant advances have been made in the preventive treatment of CM. Clinicians managing patients with migraine now have multiple choices ranging from oral medications to OBT-A and CGRP-targeting mAbs. Strong evidence supports the efficacy of OBT-A and CGRP-targeting mAbs, while the level of evidence in favor of oral treatments is limited. The benefit of OBT-A in CM has been established in real-life studies, as well as RCTs, but it is important to establish the optimal treatment duration and to determine whether patients are responding to OBT-A, as different durations of treatment or higher doses may be required to allow the full benefits of treatment to emerge, and there may be issues of worsening rebound following treatment interruption. To optimize patient outcomes, it is crucial that treatment is continued at an appropriate dosage for an adequate period of time, and it is also important to know when to continue or discontinue treatment when a patient appears as not responding, or whether there is value in adding another therapy to OBT-A.
Besides scientific evidence, the choice of drugs in everyday practice needs to be shaped by adherence to regulatory indications, ethical considerations, and the expertise of clinicians. The availability of an individualized treatment algorithm that acknowledges all of these aspects and incorporates them into a ready-to-use, practical guidance for physicians who undertake the difficult challenge of improving the health and the life of CM patients is extremely useful, as it is our belief that even partial improvements in these patients are extremely important and clinically meaningful.
The treatment algorithm presented here is the result of the collaborative efforts of an expert panel of Italian headache specialists with extensive expertise in the management of patients with migraine. The algorithm also has relevance for patients with medication-overuse headache. It represents an evidence- and experience-based synthesis of clinical information that provides a practical tool for clinicians to guide and individualize their approach to the management of this difficult-to-treat patient group. Many of our suggestions, including the classification of responders, some of the suggested outcomes to evaluate, and the possibility of prolonging the time intervals between the doses of OBT-A are based upon common clinical practice and not entirely evidence-based. Nevertheless, our suggestions might serve as a basis for commonly accepted guidelines, subject to field testing and further improvement.
Although the algorithm enhances the decision-making process for determining OBT-A dosage and injection interval based on clearly defined responder profiles and provides options for the addition of other therapies, including CGRP-targeting mAbs, the management of CM remains challenging. However, our understanding of the pathophysiology of migraine is rapidly advancing, and ongoing research will provide new insights into the genetic causes, anatomical and physiological aspects, and disease mechanisms of CM. Identification of additional therapeutic targets and and a better understanding of how the different pharmacological interventions can be best combined will possibly allow more efficacious and individualized treatments for this disabling condition.
Article highlights
Chronic migraine, a highly disabling neurological disease, is burdened by a high negative impact on the quality of life of patients and is a significant contributor to worldwide neurological disability.
The condition remains underdiagnosed and undertreated.
The availability of medications with proven efficacy is limited and the management of chronic migraine is challenging.
OnabotulinumtoxinA (OBT-A) has been shown to be safe and effective for the prevention of chronic migraine in randomized controlled trials and in real-life studies and is a valid therapeutic option that should be proposed to patients as early as possible.
OBT-A is also effective in patients with medication-overuse headache.
Monoclonal antibodies (mAbs) targeting the calcitonin gene-related peptide (CGRP) pathway have been developed and investigated for the prevention of episodic and chronic migraine. They ameliorate migraine in clinical trials.
Although separate guidelines are available for the management of chronic migraine with OBT-A or with CGRP-targeting mAbs, guidance on the integration of these two different therapeutic strategies to optimize the management of chronic migraine is lacking.
This proposed new algorithm aims to provide guidance for the management of OBT-A therapy taking into account that patients who start OBT-A have already failed or not tolerated at least two previous oral preventive medications.
Decisions on further patient management after the initial administration of OBT-A should be reassessed every 3 months for the first year of treatment to establish efficacy. Subsequently, the timing to re-evaluate the patient will be guided by the response to treatment.
Once criteria for the definition of responder petients are established, OBT-A dose and dosing interval, the continuation of OBT-A or switching to another therapy, or initiating complementary strategies with CGRP-targeting mAbs can be considered to optimize patient outcomes.
Reviewer disclosures
A peer reviewer on this manuscript has received grants for research (no personal compensation) from: Alder, Allergan, Amgen, Dr. Reddy’s, ElectroCore, Eli Lilly, eNeura, Neurolief, Novartis, Satsuma, Scion Neurostim, Teva and Zosano. They have also acted as a consultant and/or on advisory boards (honoraria) for: Acorda, Aeon, Alexsa, Align Strategies, Allergan, Alphasights, Amgen, Aperture Venture Partners, Aralez Pharmaceuticals Canada, Axsome Therapeutics, Becker Pharmaceutical Consulting, BioDelivery Sciences International, Biohaven, Charleston Labs, CRG, Currax, Decision Resources, DeepBench, Eli Lilly, eNeura, Equinox, ExpertConnect, GLG, GSK, Guidepoint Global, Healthcare Consultancy Group, Health Science Communications, Impel, Lundbeck, M3 Global Research, Magellan Rx Management, Marcia Berenson Connected Research and Consulting, Medicxi, Navigant Consulting, Neurolief, Nordic BioTech, Novartis, Pulmatrix, Reckner Healthcare, Relevale, Revance, SAI MedPartners, Satsuma, Scion Neurostim, Slingshot Insights, Sorrento, Spherix Global Insights, Sudler and Hennessey, Synapse Medical Communications, Teva, Theranica, Thought Leader Select, Trinity Partners, XOC, Zosano. They receive a salary from Dartmouth-Hitchcock Medical Center and the American Headache Society. This reviewer also holds stock options in Nocira and Percept and has received CME honoraria from: American Academy of Neurology, American Headache Society, Cleveland Clinic Foundation, Diamond Headache Clinic, Elsevier, Forefront Collaborative, Hamilton General Hospital, Ontario, Canada, Headache Cooperative of New England, Henry Ford Hospital, Detroit, Inova, Medical Learning Institute Peerview, Medical Education Speakers Network, Miller Medical Communications, North American Center for CME, Physicians’ Education Resource, Rockpointe, ScientiaCME and WebMD/Medscape. Another reviewer on this manuscript has consulted for Allergan/Abbvie, Aeon, Supernus and Revance all of whom manufacture/sell neurotoxins. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Declaration of interest
S Sacco has received fees as speaker or for participation to advisory boards from Abbott, Allergan, Eli-Lilly, Medscape, Novartis, Teva. C Tassorelli has participated in advisory boards or lectured at symposia sponsored by Allergan, Eli Lilly, Novartis and Teva; she has lectured at symposia sponsored by Allergan, Eli Lilly, Novartis and Teva; she is principal investigator or collaborator in clinical trials sponsored by Alder, Eli-Lilly, Novartis and Teva. She has received research grants from the European Commission, the Italian Ministry of Health and the Italian Ministry of University. P Geppetti has received personal fees and non-financial support from Allergan, Electrocore, Eli Lilly, IBSA, Novartis, Pfizer, Sanofi,TEVA; non-financial support from Amgen, Chiesi; Scientific Advisory Board, Endosome Therapeutics; FloNext srl, shareholder; Associate Editor Physiological Reviews, Pain, Pain Therapeutics; The Journal of Headache and Pain. A Negro has participated in advisory boards or lectured at symposia sponsored by Allergan, Eli Lilly, Novartis and Teva; he is collaborator in clinical trials sponsored by Allergan, Novartis and Teva; he is in the Editorial Board of Neurology and Therapy and The Journal of Headache and Pain. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Acknowledgments
Medical writing support was provided by Renata Perego on behalf of Health Publishing & Services srl. The authors thank Ray Hill, an independent medical writer, who provided English-language editing and journal styling prior to submission on behalf of Health Publishing & Services Srl.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–480.
- GBD Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17:954–976.
- Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55:103–122.
- Lipton RB, Munjal S, Alam A, et al. Migraine in America Symptoms and Treatment (MAST) Study: baseline study methods, treatment patterns, and gender differences. Headache. 2018;58:1408–1426.
- Medrea I, Christi S. Chronic migraine - evolution of the concept and clinical implications. Headache. 2018;58:1495–1500.
- Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808.
- Adams AM, Serrano D, Buse DC, et al. The impact of chronic migraine: the Chronic Migraine Epidemiology and Outcomes (CaMEO) study methods and baseline results. Cephalalgia. 2015;35:563–578.
- Lipton RB, Fanning KM, Buse DC, et al. Identifying natural subgroups of migraine based on comorbidity and concomitant condition profiles: results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache. 2018;58:933–947.
- Lipton RB, Manack Adams A, Buse DC, et al. A Comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study and American Migraine Prevalence and Prevention (AMPP) Study: demographics and headache-related disability. Headache. 2016;56:1280–1289.
- Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301–315.
- Buse D, Manack A, Serrano D, et al. Headache impact of chronic and episodic migraine: results from the American migraine prevalence and prevention study. Headache. 2012;52:3–17.
- Leonardi M, Raggi A. A narrative review on the burden of migraine: when the burden is the impact on people’s life. J Headache Pain. 2019;20:41.
- Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al. Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry. 2016;87:741–749.
- Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache. 2016;56:821–834.
- D’Amico D, Sansone E, Grazzi L, et al. Multimorbidity in patients with chronic migraine and medication overuse headache. Acta Neurol Scand. 2018;138:515–522.
- Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49:1153–1162.
- Escher CM, Paracka L, Dressler D, et al. Botulinum toxin in the management of chronic migraine: clinical evidence and experience. Ther Adv Neurol Disord. 2017;10:127–135.
- Israel H, Neeb L, Reuter U. CGRP monoclonal antibodies for the preventative treatment of migraine. Curr Pain Headache Rep. 2018;22:38.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53:644–655.
- Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803.
- Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814.
- Khalil M, Zafar HW, Quarshie V, et al. Prospective analysis of the use of OnabotulinumtoxinA (BOTOX) in the treatment of chronic migraine; real-life data in 254 patients from Hull, U.K. J Headache Pain. 2014;15:54.
- Boudreau GP, Grosberg BM, McAllister PJ, et al. Prophylactic onabotulinumtoxinA in patients with chronic migraine and comorbid depression: an open-label, multicenter, pilot study of efficacy, safety and effect on headache-related disability, depression, and anxiety. Int J Gen Med. 2015;8:79–86.
- Cernuda-Morollon E, Ramon C, Larrosa D, et al. Long-term experience with onabotulinumtoxinA in the treatment of chronic migraine: what happens after one year? Cephalalgia. 2015;35:864–868.
- Grazzi L, Usai S. Onabotulinum toxin A (Botox) for chronic migraine treatment: an Italian experience. Neurol Sci. 2015;36(Suppl 1):33–35.
- Guerzoni S, Pellesi L, Baraldi C, et al. Increased efficacy of regularly repeated cycles with onabotulinumtoxinA in MOH patients beyond the first year of treatment. J Headache Pain. 2015;17:48.
- Pedraza MI, de la Cruz C, Ruiz M, et al. OnabotulinumtoxinA treatment for chronic migraine: experience in 52 patients treated with the PREEMPT paradigm. Springerplus. 2015;4:176.
- Negro A, Curto M, Lionetto L, et al. OnabotulinumtoxinA 155 U in medication overuse headache: a two years prospective study. Springerplus. 2015;4:826.
- Negro A, Curto M, Lionetto L, et al. A two years open-label prospective study of onabotulinumtoxinA 195 U in medication overuse headache: a real-world experience. J Headache Pain. 2015;17:1.
- Aicua-Rapun I, Martinez-Velasco E, Rojo A, et al. Real-life data in 115 chronic migraine patients treated with onabotulinumtoxin A during more than one year. J Headache Pain. 2016;17:112.
- Butera C, Colombo B, Bianchi F, et al. Refractory chronic migraine: is drug withdrawal necessary before starting a therapy with onabotulinum toxin type A? Neurol Sci. 2016;37:1701–1706.
- Demiryurek BE, Ertem DH, Tekin A, et al. Effects of onabotulinumtoxinA treatment on efficacy, depression, anxiety, and disability in Turkish patients with chronic migraine. Neurol Sci. 2016;37:1779–1784.
- Kollewe K, Escher CM, Wulff DU, et al. Long-term treatment of chronic migraine with onabotulinumtoxinA: efficacy, quality of life and tolerability in a real-life setting. J Neural Transm (Vienna). 2016;123:533–540.
- Russo M, Manzoni GC, Taga A, et al. The use of onabotulinum toxin A (Botox((R))) in the treatment of chronic migraine at the parma headache centre: a prospective observational study. Neurol Sci. 2016;37:1127–1131.
- Grazzi L. Onabotulinumtoxin A for chronic migraine with medication overuse: clinical results of a long-term treatment. Neurol Sci. 2017;38:141–143.
- Matharu M, Pascual J, Nilsson Remahl I, et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia. 2017;37:1384–1397.
- Andreou AP, Trimboli M, Al-Kaisy A, et al. Prospective real-world analysis of OnabotulinumtoxinA in chronic migraine post-national institute for health and care excellence UK technology appraisal. Eur J Neurol. 2018;25:1069–e83.
- Dikmen PY, Kosak S, Aydinlar EI, et al. A single-center retrospective study of onabotulinumtoxinA for treatment of 245 chronic migraine patients: survey results of a real-world experience. Acta Neurol Belg. 2018;118:475–484.
- Stark C, Stark R, Limberg N, et al. Real-world effectiveness of onabotulinumtoxinA treatment for the prevention of headaches in adults with chronic migraine in Australia: a retrospective study. J Headache Pain. 2019;20:81.
- Ahmed F, Gaul C, Garcia-Monco JC, et al. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. 2019;20:26.
- Rothrock JF, Adams AM, Lipton RB, et al. FORWARD Study: evaluating the comparative effectiveness of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache. 2019;59:1700–1713.
- Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13.
- Young WB, Ivan Lopez J, Rothrock JF, et al. Effects of onabotulinumtoxinA treatment in chronic migraine patients with and without daily headache at baseline: results from the COMPEL study. J Headache Pain. 2019;20:12.
- Young WB, Ivan Lopez J, Rothrock JF, et al. Effects of onabotulinumtoxinA treatment in patients with and without allodynia: results of the COMPEL study. J Headache Pain. 2019;20:10.
- Ornello R, Guerzoni S, Baraldi C, et al. Sustained response to onabotulinumtoxin A in patients with chronic migraine: real-life data. J Headache Pain. 2020;21:40.
- Allergan Inc. BOTOX (onabotulinumtoxinA) full prescribing information; 2017 [cited Mar 2020].
- Tassorelli C, Tedeschi G, Sarchielli P, et al. Optimizing the long-term management of chronic migraine with onabotulinumtoxinA in real life. Expert Rev Neurother. 2018;18:167–176.
- Bendtsen L, Sacco S, Ashina M, et al. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European headache federation. J Headache Pain. 2018 Sep 26;19:91.
- Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20:6.
- Sacco S, Bendtsen L, Ashina M, et al. Correction to: european headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20:58.
- Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921–936.
- Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373.
- Lipton RB, Rosen NL, Ailani J, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: pooled results from the PREEMPT randomized clinical trial program. Cephalalgia. 2016;36:899–908.
- Mathew NT, Jaffri SF. A double-blind comparison of onabotulinumtoxinA (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study. Headache. 2009;49:1466–1478.
- Magalhães E, Menezes C, Cardeal M, et al. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin Neurol Neurosurg. 2010;112:463–466.
- Blumenfeld AM, Schim JD, Chippendale TJ. Botulinum toxin type A and divalproex sodium for prophylactic treatment of episodic or chronic migraine. Headache. 2008;48:210–220.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–434.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113–2122.
- Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394:1030–1040.
- Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91:e2211–21.
- Dodick DW, Lipton RB, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia. 2019;39:1075–1085.
- Ashina M, Tepper S, Brandes JL, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38:1611–1621.
- Tepper SJ, Diener HC, Ashina M, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology. 2019;92:e2309–20.
- Ornello R, Casalena A, Frattale I, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21:32.
- Russo A, Silvestro M, Scotto Di Clemente F, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain. 2020;21:69.
- National Institute for Health and Clinical Excellence. Management of migraine (with or without aura); [ cited 2020 Mar]. Available from: https://pathways.nice.org.uk/pathways/headaches/management-of-migraine-with-or-without-aura#content=view-node%3Anodes-prophylactic-treatment
- American Academy of Neurology. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache; [ cited 2020 Mar]. Available from: https://www.aan.com/Guidelines/home/GetGuidelineContent/737
- National Institute for Health and Clinical Excellence. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine; [ cited 2020 Mar]. Available from: https://www.nice.org.uk/guidance/ta260
- National Institute for Health and Clinical Excellence. Erenumab for preventing migraine [ID1188]; [ cited 2020 Mar]. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10302
- Kouremenos E, Arvaniti C, Constantinidis TS, et al. Hellenic headache society. Consensus of the hellenic headache society on the diagnosis and treatment of migraine. J Headache Pain. 2019;20:113.
- Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20:92.
- Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484–495.
- Dodick DW, Turkel CC, DeGryse RE, et al. Assessing clinically meaningful treatment effects in controlled trials: chronic migraine as an example. J Pain. 2015;16:164–175.
- Silberstein SD, Dodick DW, Aurora SK, et al. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996–1001.
- Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56:S20–8.
- Haywood KL, Mars TS, Potter R, et al. Assessing the impact of headaches and the outcomes of treatment: A systematic review of patient-reported outcome measures (PROMs). Cephalalgia. 2018;38:1374–1386.
- Ford JH, Foster SA, Stauffer VL, et al. Patient satisfaction, health care resource utilization, and acute headache medication use with galcanezumab: results from a 12-month open-label study in patients with migraine. Patient Prefer Adherence. 2018 Nov 13;12:2413–2424.
- Skovlund E, Flaten O. Response measures in the acute treatment of migraine. Cephalalgia. 1995;15:519–522. discussion 450–511.
- Bagley CL, Rendas-Baum R, Maglinte GA, et al. Validating migraine-specific quality of life questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52:409–421.
- Stewart WF, Lipton RB, Dowson AJ, et al. Developmentand testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56:S20–8.
- Cole JC, Lin P, Rupnow MF. Minimal important differences in the Migraine-Specific Quality of Life Questionnaire (MSQ) version. Cephalalgia. 2009;29:1180–1187.
- Fischer D, Stewart AL, Bloch DA, et al. Capturing the patient’s view of change as a clinical outcome measure. JAMA. 1999;282:1157–1162.
- Schwedt TJ. Chronic migraine. BMJ. 2014;348:g1416.
- Tassorelli C, Aguggia M, De Tommaso M, et al. Onabotulinumtoxin A for the management of chronic migraine in current clinical practice: results of a survey of sixty-three Italian headache centers. J Headache Pain. 2017;18:66.
- Sun-Edelstein C, Rapoport AM. Update on the pharmacological treatment of chronic migraine. Curr Pain Headache Rep. 2016;20:6.
- Sarchielli P, Granella F, Prudenzano MP, et al. Italian guidelines for primary headaches: 2012 revised version. J Headache Pain. 2012;13:S31–70.
- Ford JH, Jackson J, Milligan G, et al. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache. 2017;57:1532–1544.
- Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20:22–33.
- Sacco S, Braschinsky M, Ducros A, et al. European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain. 2020;21:76.
