ABSTRACT
Objective
To determine minimally clinically important differences (MCIDs) for Disability Rating Scale (DRS), Fugl-Meyer Upper Extremity Subscale (FM-UE), Fugl-Meyer Lower Extremity Subscale (FM-LE), and Fugl-Meyer Motor Scale (FMMS) in patients with chronic motor deficits secondary to traumatic brain injury (TBI).
Methods
Retrospective analysis from the 1-year, double-blind, randomized, surgical sham-controlled, Phase 2 STEMTRA trial (NCT02416492), in which patients with chronic motor deficits secondary to TBI (N = 61) underwent intracerebral stereotactic implantation of modified bone marrow-derived mesenchymal stromal (SB623) cells. MCIDs for DRS, FM-UE, FM-LE, and FMMS were triangulated with distribution-based, anchor-based, and Delphi panel estimates.
Results
Triangulated MCIDs were: 1) −1.5 points for the Disability Rating Scale; 2) 6.2 points for the Fugl-Meyer Upper Extremity Subscale; 3) 3.2 points for the Fugl-Meyer Lower Extremity Subscale; and 4) 8.4 points for the Fugl-Meyer Motor Scale.
Conclusions
For the first time in the setting of patients with chronic motor deficits secondary to TBI, this study reports triangulated MCIDs for: 1) DRS, a measure of global outcome; and 2) Fugl-Meyer Scales, measures of motor impairment. These findings guide the use of DRS and Fugl-Meyer Scales in the assessment of global disability outcome and motor impairment in future TBI clinical trials.
1. Introduction
Success in clinical trials in chronic neurological diseases is challenging to define and measure. Studies must show a statistically significant difference between treatment and control groups for the primary endpoint, and a defined clinical meaningfulness for the difference in primary endpoint values. Traditionally, the Glasgow Outcome Scale-Extended (GOS-E) has been the most commonly used measure of global outcome after acute Traumatic Brain Injury (TBI) and as the primary endpoint in TBI clinical trials [Citation1–3]. However, there are multiple limitations in using GOS-E in chronic TBI.
The GOS-E has been cited in more than two thirds of the over 190 randomized controlled trials for acute TBI, which were published between 1980 and 2016 [Citation4]. The GOS-E is broadly accepted, is a core data element selected by the National Institute of Neurological Disorders and Stroke and the Common Data Elements Workgroup for TBI research, and is the primary outcome measure accepted in TBI registrational studies by the US Food and Drug Administration [Citation2,Citation5,Citation6]. The GOS-E assesses global outcome by categorizing patients with acute TBI using an eight-point ordinal scale, based on the patient’s level of disability with an emphasis on functioning in daily life [Citation7]. Despite its wide acceptance, the GOS-E is reported to have a number of limitations in chronic TBI and stroke including lack of sensitivity to small but meaningful changes in function, lack of reliability, ceiling effects, dichotomization of outcomes which excludes consideration of life satisfaction, and poor associations with psychological, neurocognitive, and quality of life measures [Citation7,Citation8]. The reported insufficient sensitivity of the GOS-E may be a contributing factor to the failure to date of all interventional clinical trials in acute TBI where GOS-E was used as a primary outcome measure [Citation8].
The limitations of the GOS-E in elucidating incremental changes in chronic TBI has prompted efforts to consider other measurement instruments. Several investigations from the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study have highlighted the need for alternatives, including measures of motor impairment in chronic TBI for which certain global outcome measures maybe insensitive [Citation7,Citation9].
The Disability Rating Scale (DRS) is a 30-point global outcome scale for TBI which assesses eight areas of function, focusing on assessment of neurological function and restrictions of daily living [Citation8]. The DRS was developed to assess rehabilitative progress of patients with severe TBI from coma to reintegration into the community, and is the main alternative global outcome scale to the GOS-E [Citation8,Citation10] A critical difference between the DRS and the GOS-E is that self-care ratings (i.e., feeding, toileting, and grooming) on the DRS only consider cognitive ability. The DRS is more sensitive in detecting improvement in patients with TBI during in-hospital rehabilitation than the Glasgow Outcome Scale [Citation11]. The DRS has also been shown to be superior to other scales designed to assess global disability outcomes. For example, the DRS is more sensitive in detecting changes in TBI patients over a short-time period and more appropriate for detecting long-term deficits than the Functional Independence Measure, an alternative disability measure developed in the rehabilitation setting [Citation12]. A survey conducted in community-based patients with chronic TBI (average five years post-injury), showed that the DRS detected a range of deficits demonstrating its sensitivity to detect long-term functional outcomes in patients with chronic TBI [Citation13].
The Fugl-Meyer Motor Scale (FMMS) is designed to assess recovery from hemiplegia, and was developed because previous measures focused on global neurological outcomes and not specifically on the motor domain of neurological function [Citation14,Citation15]. The FMMS is a 100-point scale which consists of a 66-point Fugl-Meyer Upper Extremity Subscale (FM-UE), and a 34-point Fugl-Meyer Lower Extremity Subscale (FM-LE) [Citation14–16]. The FMMS is widely recognized as a clinically relevant measure of motor impairment in stroke, and its use is recommended in clinical trials that assess stroke rehabilitation [Citation15,Citation17]. A systematic review of robot assisted therapy for chronic stroke reported that the FMMS was used as the primary outcome measure in 60% of randomized controlled trials, while more recent randomized controlled trials for chronic stroke have also used the FMMS as the primary outcome measure [Citation18–22]. In addition to its successful use in chronic stroke, the FMMS is also used as the primary outcome measure for the assessment of motor impairment in two randomized controlled trials for chronic TBI [Citation23,Citation24].
Minimally clinically important difference (MCID) is defined by Malec et al. [Citation25] as ‘the smallest change on a measure that is reliably associated with a meaningful change in a patient’s clinical status, function, or quality of life.’ Comparing the proportion of patients in treatment and control arms of clinical trials who achieve the MCID threshold for a specific measure can be more useful than comparing a response difference between arms, as statistically significant differences in response may not necessarily be clinically meaningful to patients [Citation26]. Furthermore, MCID is also used in clinical practice to classify patients who achieve meaningful change in their status [Citation26]. Although there are no standard methods for determining MCID for any measure, an extensive literature search has identified three principal approaches: distribution-based, anchor-based, and Delphi panel, each of which has been used to estimate MCID for measures in a wide range of diseases [Citation25–35]. Because of strengths and limitations associated with distribution-based, anchor-based, and Delphi panel methods of estimating MCID, the procedure of triangulation (i.e., calculation of the arithmetic mean) is recommended to produce a final MCID for each measure [Citation26,Citation34,Citation36,Citation37].
The MCID for the FMMS may consider each extremity separately or in combination, as patients included in clinical trials have different levels of motor impairment in upper and lower extremities. In some instances the impairment is limited to one extremity only, or the impairment affects predominantly one extremity, and requiring the MCID to be related to the total FMMS may not reflect the clinical condition. Therefore, establishing separate MCIDs for FM-UE and FM-LE is at least as clinically relevant as doing so for the total FMMS. MCIDs have not been determined for DRS, FM-UE, FM-LE, or total FMMS in the setting of chronic TBI. Therefore this study defines triangulated minimally clinically important differences for the Disability Rating Scale, Fugl-Meyer Upper Extremity Subscale, Fugl-Meyer Lower Extremity Subscale, and Fugl-Meyer Motor Scale using distribution-based, anchor-based, and Delphi panel estimates for patients with chronic motor deficits secondary to TBI in a retrospective analysis of the randomized, controlled, Phase 2 ‘STEM cell therapy for TRAumatic brain injury’ STEMTRA trial.
2. Patients and methods
This study involved a retrospective analysis of the 1-year, double-blind, randomized, surgical sham-controlled, Phase 2 STEMTRA trial (clinicaltrials.gov: NCT02416492) which evaluated the safety and efficacy of intracerebral stereotactic implantation of modified bone marrow-derived mesenchymal stromal (SB623) cells in patients with chronic motor deficits secondary to TBI [Citation24]. Institutional review boards approved clinical study protocols, and patients provided written informed consent for participation in the parent study [Citation24].
2.1. Study population
The STEMTRA trial enrolled patients who were at least one year post-injury with motor impairment that correlated with MRI-observed focal cerebral injury and had at least moderate functional disability (GOS-E: 3–6) at enrollment [Citation24]. Of 63 randomized patients, 61 underwent SB623 cell (N = 46) or sham-control (N = 15) treatment [Citation24]. The DRS and FMMS analyses were conducted on 61 patients, whereas FM-UE and FM-LE analyses were conducted on 55 patients exhibiting upper extremity deficits, and 56 patients exhibiting lower extremity deficits, respectively. The trial was conducted from June 2016 to March 2019 at 27 clinical sites in the United States (21 sites), Japan (5 sites), and Ukraine (1 site) [Citation24].
2.2. Statistical analysis
2.2.1. Distribution-based MCID estimates
In common with previous studies, distribution-based MCID estimates were calculated for each outcome scale using 0.5 x Standard Deviation (SD) of baseline values for the population of patients who completed 24 and 48 weeks of treatment in the STEMTRA trial [Citation26–34].
2.2.2. Anchor-based MCID estimates
This study used an anchor-based method for determining MCID described previously by Malec and Ketchum [Citation26]. The measure of interest used as an anchor in this study was the Global Rating of Perceived Change assessed by Clinician and Subject (Patient) (GRPC-C&S). GRPC-C&S values were calculated as the worse (i.e., lower) score from the GRPC-C and GRPC-S questionnaires. Therefore, it is a measure of perceived change in the patient’s motor function as assessed by both the clinician and patient using a seven-point Likert scale (1 = much worse; 2 = a little worse, meaningful; 3 = a little worse, not meaningful; 4 = about the same; 5 = a little better, not meaningful; 6 = a little better, meaningful; and 7 = much better) [Citation38]. Spearman correlations between the change from baseline for each outcome scale and GRPC-C&S at each time point were low (<0.25, data not shown); therefore, data for each outcome scale were divided between patients who achieved GRPC-C&S scores of 6 or 7 (indicating meaningful improvement) and patients who achieved scores of 1 to 5 (indicating no meaningful improvement). For each outcome scale, each time point (24 weeks or 48 weeks), and each possible integer cut off value, a variable was defined as ‘1’ if change from baseline value was greater than or equal to the cut off value and ‘0’ if the change from baseline value was less than the cut off value. Accuracy, sensitivity, specificity, and Youden’s Index (maximizing statistical sensitivity and specificity [sensitivity + specificity – 1]) were calculated using GRPC-C&S as truth and the cut off value for each outcome scale at each time point, and the cut off value that maximized Youden’s Index was selected as the MCID estimate. Receiver Operator Characteristic (ROC) curves using GRPC-C&S as the dependent variable and the outcome scales as independent variables at each time point were generated using SAS PROC LOGISTIC.
2.2.3. Delphi panel MCID estimates
Delphi panel MCID estimates for each outcome scale were determined using the RAND/UCLA modified Delphi method described by Mattke and colleagues [Citation35]. Briefly, the process involved a literature review, the recruitment of a multi-disciplinary independent expert panel (IEP) of 10 members, the development of TBI clinical vignettes, which were unrelated to the STEMTRA trial by independent physicians, and the rating of these clinical vignettes for meaningfulness of improvements and numeric changes by the IEP for each outcome scale using two separate individual rating rounds and a consensus building meeting [Citation35].
2.2.4. Triangulated MCID estimates
Because of the strengths and limitations associated with distribution-based, anchor-based, and Delphi panel MCID estimates, the triangulation method (i.e., calculation of the arithmetic mean) was used to calculate final MCIDs. Triangulation allows for a multifaceted approach to determining MCID, and has been used in multiple therapeutic areas [Citation26,Citation34,Citation36,Citation37]. Briefly, the arithmetic means of distribution-based, anchor-based, and Delphi panel MCID estimates for each outcome score at 24 and 48 weeks were calculated using the following equation: Distribution-Based MCID Estimate + Anchor-Based MCID Estimate + Delphi Panel MCID Estimate /3
Final MCIDs were defined as whichever estimate (24 weeks or 48 weeks) was higher.
3. Results
The majority of patients in the study were white (68.9%) and male (70.5%). Patients had a mean age of 34.4 years (standard deviation: 11.8 years), had at least moderate functional disability (GOS-E median score: 4, interquartile range: 1) and motor impairments after TBI, and were 1.4–28.4 years post-injury ().
Table 1. Patient baseline demographics
3.1. Triangulated MCIDs for DRS
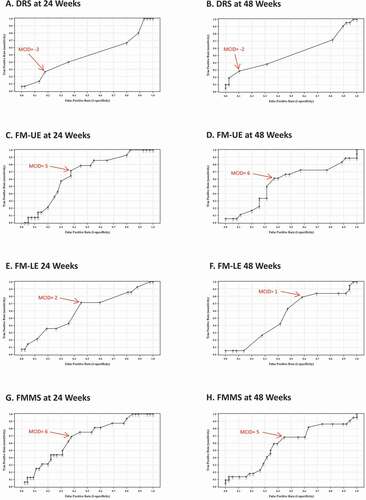
Distribution-based MCID estimates for DRS were −1.4 points at both 24 and 48 weeks (). DRS anchor-based MCID estimates were −2 points at both 24 and 48 weeks (, and ), and DRS Delphi panel MCID estimates were −1 point at both 24 and 48 weeks (). Triangulated MCIDs for DRS were −1.5 points at both 24 and 48 weeks (), therefore the final MCID for DRS was −1.5 points.
Table 2. MCIDs for DRS, FM-UE, FM-LE, and FMMS
3.2. Triangulated MCIDs for FM-UE
Distribution-based MCID estimates for FM-UE were 6.5 points at both 24 and 48 weeks (). FM-UE anchor-based MCID estimates were 5 points at 24 weeks and 6 points at 48 weeks (, and ), and FM-UE Delphi panel MCID estimates were 6 points at both 24 and 48 weeks (). Triangulated MCIDs for FM-UE were 5.8 points at 24 weeks and 6.2 points at 48 weeks (), therefore the final MCID for FM-UE was 6.2 points.
3.3. Triangulated MCIDs for FM-LE
Distribution-based MCID estimates for FM-LE were 2.7 points at both 24 and 48 weeks (). FM-LE anchor-based MCID estimates were 2 points at 24 weeks and 1 point at 48 weeks (, and ), and FM-LE Delphi panel MCID estimates were 5 points at both 24 and 48 weeks (). Triangulated MCIDs for FM-LE were 3.2 points at 24 weeks and 2.9 points at 48 weeks (), therefore the final MCID for FM-LE was 3.2 points.
3.4. Triangulated MCIDs for FMMS
Distribution-based MCID estimates for FMMS were 9.1 points at 24 weeks and 9.2 points at 48 weeks (). FMMS anchor-based MCID estimates were 6 points at 24 weeks and 5 points at 48 weeks (, and ), and FMMS Delphi panel MCID estimates were 10 points at both 24 and 48 weeks (). Triangulated MCIDs for FMMS were 8.4 points at 24 weeks and 8.1 points at 48 weeks (), therefore the final MCID for FMMS was 8.4 points.
4. Discussion
This study is the first to establish triangulated MCIDs for the DRS and Fugl-Meyer Scales in patients with chronic motor deficits secondary to TBI. This was a retrospective analysis of data from the 1-year, double-blind, randomized, surgical sham-controlled, Phase 2 STEMTRA trial, which enrolled patients with chronic motor deficits secondary to TBI [Citation24].
Motor deficits are a significant long-term sequelae experienced by approximately 43% of surviving hospitalized patients with TBI in the US [Citation39], with over 30% of patients with severe TBI experiencing at least one motor impairment two years after inpatient rehabilitation [Citation40]. Overall an estimated 5.3 million individuals live with long-term motor deficits secondary to TBI in the US [Citation41], underscoring the need to establish MCIDs for measurement scales that are sensitive to changes in long-term functional outcomes and motor impairment for both clinical research and clinical practice.
The DRS is recognized as a measure of global outcomes in TBI [Citation8]; however no triangulated MCID has been established to date. In this study, the triangulated MCID for the DRS in patients with chronic motor deficits secondary to TBI was −1.5 points. The triangulated values reflect the near-identical distribution-based (−1.4 points), anchor-based (−2 points), and Delphi panel (−1 point) MCID estimates at 24 and 48 weeks in the STEMTRA trial. Although anchor-based MCID estimates were the same at both 24 and 48 weeks, AUC and sensitivity values were low, suggesting limited association between the DRS and the GRPC-C&S anchor measure of interest.
MCIDs for the FM-UE Subscale have been reported in several clinical trials for chronic stroke. In the Everest trial, in which patients with chronic stroke were treated with electrical epidural motor cortex stimulation, an FM-UE score of 4.5 points was considered to be clinically meaningful [Citation42]. A change of 3.4 points was clinically meaningful in a Brain-Machine interface trial, in which patients with upper limb impairments were able to lift and stretch the arm, turn the forearm, and extend the wrist and fingers from a baseline of no function [Citation43]. The design of a robotic therapy clinical trial for patients with chronic stroke considered 3 points to be clinically meaningful based on data from the Kansas City Stroke Study, in which a 3-point change in FM-UE was equivalent to a meaningful change of 20 points in the Stroke Impact Scale [Citation19]. In addition, a mean change of 5.25 points in FM-UE was determined to be a clinically important difference using the Global Rating of Change Scale as an anchor in a study of patients with chronic stroke [Citation44]. In the current study, distribution-based, anchor-based, and Delphi panel MCID estimates were similar, between 5 and 6.5 points. The triangulated MCID for FM-UE was 6.2 points in the STEMTRA trial, which compared to a clinically meaningful range of 3 to 5.25 points in chronic stroke. Although clinically meaningful values from individual clinical trials are important, and data from stroke trials are pertinent, the triangulated MCIDs for FM-UE presented in this TBI study are the product of three TBI-based MCID estimates.
An anchor-based MCID for the FM-LE Subscale of 6 points was established in a small prospective observational study of patients with chronic stroke having hemiparesis who were treated with conventional motor therapy, using the Global Rating of Perceived Change-Patient and the Functional Ambulation Classification as anchors [Citation45]. In the current study, distribution-based and anchor-based MCID estimates were similar, between 1 and 2.7 points, while Delphi panel MCID estimates were higher at 5 points [Citation35]. The triangulated MCID for FM-LE was 3.2 points in the STEMTRA trial, which was consistent with the MCID being 10% of the total range of the scale (3.4 points) suggested by van der Lee and colleagues for the FM-UE and the Action Research Arm Test [Citation46]. The current anchor-based MCID estimates in TBI were 1 and 2 points, lower than the anchor-based value of 6 points previously reported in stroke [Citation45], possibly representing differences in patient populations or disease pathophysiology.
The FMMS is recognized as a clinically relevant measure of motor impairment in chronic stroke, and is widely used in clinical trials that assess stroke rehabilitation [Citation15,Citation17]. Clinical trials in chronic stroke which employed forced use, constraint-induced, and implanted stem cell treatments used an MCID for the total FMMS of ≥10 points, although this was not adjusted for baseline FMMS and does not reflect the relative degree of improvement [Citation46–48]. In the current study, the triangulated MCIDs for the FMMS in patients with chronic motor deficits secondary to TBI were 8.1 and 8.4 points, the narrow range reflecting small differences of distribution-based and anchor-based MCID estimates at 24 and 48 weeks in the STEMTRA trial. Distribution-based MCID estimates were 9.1 and 9.2 points, which was caused by slight differences in the population of patients who reached 24 weeks (N = 61) and 48 weeks (N = 60), however these estimates closely match the MCID for chronic stroke. Anchor-based MCID estimates were 6 and 5 points, these lower values possibly resulting from the limited association between the GRPC-C&S anchor and the FMMS, reflected by relatively low AUC, sensitivity, and specificity values. Although the Delphi panel felt that individual FM-UE and FM-LE MCID estimates were more useful than the FMMS, an MCID of 10 points was recommended which closely matched both the distribution-based MCID estimates and the established MCID for chronic stroke [Citation35]. The final triangulated MCID for the FMMS was 8.4 points.
Although the importance of MCID for FMMS in assessing upper and lower extremity motor impairment in chronic stroke is well recognized, use of an MCID that is focused on FMMS in patients with impairment in only one extremity will inaccurately reflect the clinical condition for patients with stroke or TBI. This limitation demonstrates that MCIDs for FM-UE and FM-LE are at least as clinically relevant as the MCID for FMMS in chronic stroke or TBI populations.
Although MCIDs were established for DRS and Fugl-Meyer Scales using patients with the following mean baseline values: DRS = 4.6, FM-UE = 31.7, FM-LE = 20.5, FMMS = 52.2 (), the meaningfulness of MCIDs for patients with greater or lesser disability should also be considered. For example, in a patient with a DRS score of 2, the effect of a-1.5 point change in DRS (the MCID) may reflect the transition from noncompetitive to competitive employment, and thereby represents a major change in functional status. In contrast, in a patient with a DRS score of 27, a -1.5 point decrease may reflect a change in wakefulness only (e.g., eye-opening stimulated by pain to spontaneous eye-opening), which, in isolation, is of little consequence to functional outcome.
In further examples of clinical change, the effect of a 6.2 point change (the MCID) in FM-UE could reflect the transition from partial to full volitional movement (i.e., shoulder abduction and flexion, pronation/supination), representing a major improvement of upper extremity motor function in a patient with a baseline FM-UE score of 32. Similarly, the effect of a 3.2 point change (the MCID) in FM-LE could reflect the transition from partial to full volitional movement (i.e., knee flexion and ankle dorsiflexion) and reflex activity (i.e., knee flexors, Achilles, patellar), representing a major improvement in lower extremity motor function in a patient with a baseline FM-LE score of 20. The effect of an 8.4 point change (the MCID) in FMMS could reflect transition from partial to full volitional movement and reflex activity, representing a meaningful improvement in upper and lower motor function in a patient with a baseline FMMS score of 52.
4.1. Study strengths and limitations
The findings of this study should be interpreted in light of the fact that this is a retrospective analysis using a small patient population from the STEMTRA clinical trial. In addition, although MCIDs were triangulated, the strengths (e.g., longitudinal approach, statistical significance, sample variability, and measurement precision) and limitations (e.g., limited association between the GRPC-C&S anchor and DRS and Fugl-Meyer Scales, multiplicity of MCID estimates, loss of patient perspective, and relationship between baseline and post-treatment score changes) associated with each method of calculating MCIDs should be considered [Citation27,Citation49,Citation50].
4.2. Conclusions
This study has established MCIDs for the DRS and Fugl-Meyer Scales in patients with TBI who had motor impairment and at least moderate functional disability at enrollment. The establishing MCIDs for the DRS and Fugl-Meyer Scales provides improved precision for assessing long-term functional outcomes and motor impairment, respectively, as compared to the widely used GOS-E Scale, which is most appropriate for use in acute TBI. The findings of this study support the use of DRS and Fugl-Meyer Scales in the evaluation of clinical outcomes, and defines the amplitude of clinically meaningful improvement for future chronic TBI clinical trials.
Article highlights
This study was a retrospective analysis from the Phase 2 STEMTRA trial (NCT02416492) in which patients with chronic motor deficits secondary to TBI received SB623 cells.
For the first time MCIDs for DRS and Fugl-Meyer Scales were established in patients with chronic motor deficits secondary to TBI.
MCIDs were triangulated using distribution-based, anchor-based, and Delphi-panel estimates.
Final triangulated MCIDs: 1) DRS was −1.5 points; 2) FM-UE was 6.2 points; 3) FM-LE was 3.2 points; and 4) FMMS was 8.4 points.
This study supports the use of DRS and Fugl-Meyer Scales in the evaluation of long-term functional outcomes and motor impairment in future clinical trials of patients with chronic motor deficits secondary to TBI.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
SC Cramer is a consultant for Abbvie, Constant Therapeutics, MicroTransponder, Neurolutions, SanBio, Fujifilm Toyama Chemical Co., NeuExcell, Elevian, Medtronic, and TRCare. S Mattke serves on the board of directors of Senscio Systems, Inc., and the scientific advisory board of AiCure Technologies, and Boston Millennia Partners. S Mattke has also received consulting fees from AARP, Biotronik, Bristol-Myers Squibb, Eisai, and Defined Health. S Paadre was an employee of Biostatistical Consulting Inc. D Bates was an employee and is currently a paid consultant of SanBio, Inc. B Nejadnik is an employee of SanBio, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Author contributions
MA McCrea, SC Cramer, DO Okonkwo, S Mattke, B Nejadnik, and JT Giacino contributed to the analysis and interpretation of the data, critical revision of the manuscript for intellectual content, and final approval of the manuscript for publication. S Paadre contributed to the conception and design of the study, critical revision of the manuscript for intellectual content, and final approval of the manuscript for publication. D Bates contributed to the conception and design of the study, analysis and interpretation of the data, critical revision of the manuscript for intellectual content, and final approval of the manuscript for publication. All authors agree to be accountable for all aspects of the work.
Data availability
De-identified patient data from the STEMTRA trial are available to qualified external researchers two years after completion of the trial. Researchers may submit a data sharing request to the Chief Medical Officer of SanBio, Inc., who will assess the scientific appropriateness of the request.
Additional information
Funding
References
- Jennett B, Snoek J, Bond MR, et al. Disability after severe head injury: observations on the use of the glasgow outcome scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285–293.
- Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil. 2010;91(11):1650–1660.e17.
- Sharma B, Lawrence DW. Top-cited articles in traumatic brain injury. Front Hum Neurosci. 2014;8:879.
- Bragge P, Synnot A, Maas AI, et al. A state-of-the-science overview of randomized controlled trials evaluating acute management of moderate-to-severe traumatic brain injury. J Neurotrauma. 2016;33(16):1461–1478.
- Thurmond VA, Hicks R, Gleason T, et al. Advancing integrated research in psychological health and traumatic brain injury: common data elements. Arch Phys Med Rehabil. 2010;91(11):1633–1636.
- Yeatts SD, Palesch YY, Temkin N. Biostatistical issues in TBI clinical trials. In: Skolnick BE, Alves WM, editors. Handbook of neuroemergency clinical trials. San Diego (CA): Academic Press; 2017. p. 167–183.
- Ranson J, Magnus BE, Temkin N, et al. TRACK-TBI investigators. Diagnosing the GOSE: structural and psychometric properties using item response theory, a TRACK-TBI pilot study. J Neurotrauma. 2019;36(17):2493–2505.
- McMillan T, Wilson L, Ponsford J, et al. The glasgow outcome scale - 40 years of application and refinement. Nat Rev Neurol. 2016;12(8):477–485.
- Bodien YG, McCrea M, Dikmen S, et al. Optimizing outcome assessment in multicenter TBI trials: perspectives from TRACK-TBI and the TBI endpoints development initiative. J Head Trauma Rehabil. 2018;33(3):147–157.
- Rappaport M, Hall KM, Hopkins K, et al. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63(3):118–123.
- Hall K, Cope DN, Rappaport M. Glasgow outcome scale and disability rating scale: comparative usefulness in following recovery in traumatic head injury. Arch Phys Med Rehabil. 1985;66(1):35–37.
- Hammond FM, Grattan KD, Sasser H, et al. Long-term recovery course after traumatic brain injury: a comparison of the functional independence measure and disability rating scale. J Head Trauma Rehabil. 2001;16(4):318–329.
- Hall KM, Bushnik T, Lakisic-Kazazic B, et al. Assessing traumatic brain injury outcome measures for long-term follow-up of community-based individuals. Arch Phys Med Rehabil. 2001;82(3):367–374.
- Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31.
- Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240.
- Lin JH, Hsu MJ, Sheu CF, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840–850.
- See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27(8):732–741.
- Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22(2):111–121.
- Lo AC, Guarino P, Krebs HI, et al. Multicenter randomized trial of robot-assisted rehabilitation for chronic stroke: methods and entry characteristics for VA ROBOTICS. Neurorehabil Neural Repair. 2009;23(8):775–783.
- Conroy SS, Whitall J, Dipietro L, et al. Effect of gravity on robot-assisted motor training after chronic stroke: a randomized trial. Arch Phys Med Rehabil. 2011;92(11):1754–1761.
- Wu C-Y, Yang C-L, Chuang -L-L, et al. Effect of therapist-based versus robot-assisted bilateral arm training on motor control, functional performance, and quality of life after chronic stroke: a clinical trial. Phys Ther. 2012;92(8):1006–1016.
- Cramer SC, Dodakian L, Le V, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol. 2019;76(9):1079–1087.
- Wang S, Cheng H, Dai G, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84.
- Kawabori M, Weintraub AH, Imai H, et al. Cell therapy for chronic TBI: interim analysis of the randomized controlled STEMTRA trial. 2021;96:e1202–e1214.
- Malec JF, Kean J, Monahan PO. The minimal clinically important difference for the Mayo-Portland adaptability inventory. J Head Trauma Rehabil. 2017;32(4):E47–E54.
- Malec JF, Ketchum JM. A standard method for determining the minimal clinically important difference for rehabilitation measures. Arch Phys Med Rehabil. 2020;101(6):1090–1094.
- Revicki D, Hays RD, Cella D, et al., Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 61(2): 102–109. 2008.
- Turner D, Schünemann HJ, Griffith LE, et al. The minimal detectable change cannot reliably replace the minimal important difference. J Clin Epidemiol. 2010;63(1):28–36.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582e92.
- Brigden A, Parslow RM, Gaunt D, et al. Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: triangulation using three different methods. Health Qual Life Outcomes. 2018;16(1):202.
- Hung M, Saltzman CL, Kendall R, et al. What are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res. 2018;476(10):2027–2036.
- Chatham CH, Taylor KI, Charman T, et al. Adaptive behavior in autism: minimal clinically-important differences on the Vineland-II. Autism Res. 2018;11(2):270–283.
- Smid DE, Franssen FM, Houben-Wilke S, et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc. 2017;18(1):53–58.
- Lemay KR, Tulloch HE, Pipe AL, et al. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev. 2019;39(6):E6–E11.
- Mattke S, Cramer SC, Wang M, et al. Estimating minimal clinically important differences for two scales in patients with chronic traumatic brain Injury. Curr Med Res Opin. 2020;36(12):1999–2007.
- Leidy NK, Wyrwich KW. Bridging the gap: using triangulation methodology to estimate minimal clinically important differences (MCIDs). COPD. 2005;2(1):157–165.
- Myles PS, Myles DB, Galagher W, et al. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125(1):39–45.
- Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–170.
- Selassie AW, Zaloshnja E, Langlois JA, et al. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008;23(2):123–131.
- Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J Rehabil Res Dev. 2007;44(7):975–982.
- Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14(6):602–615.
- Levy RM, Harvey RL, Kissela BM, et al. Epidural electrical stimulation for stroke rehabilitation: results of the prospective, multicenter, randomized, single-blinded Everest trial. Neurorehabil Neural Repair. 2016;30(2):107–119.
- Ramos-Murguialday A, Broetz D, Rea M, et al. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74(1):100–108.
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92(6):791–798.
- Pandian S, Arya KN, Kumar D. Minimal clinically important difference of the lower-extremity Fugl-Meyer assessment in chronic-stroke. Top Stroke Rehabil. 2016;23(4):233–239.
- Van Der Lee JH, Beckerman H, Lankhorst GJ, et al. The responsiveness of the action research arm test and the Fugl-Meyer assessment scale in chronic stroke patients. J Rehabil Med. 2001;33(3):110–113.
- Page SJ, Levine P, Khoury JC. Modified constraint-induced therapy combined with mental practice: thinking through better motor outcomes. Stroke. 2009;40(2):551–554.
- Steinberg GK, Kondziolka D, Wechsler LR, et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg. 2018;23:1–11.
- Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407.
- Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546.