ABSTRACT
Introduction
In patients with cervical dystonia (CD), pain is a major contributor to disability and social isolation and is often the main reason patients seek treatment. Surveys evaluating patient perceptions of their CD symptoms consistently highlight pain as a troublesome and disabling feature of their condition with significant impact on daily life and work.
Areas covered
In this article, the authors review the epidemiology, assessment, possible mechanisms and treatment of pain in CD, including a meta-analysis of randomized controlled trial data with abobotulinumtoxinA.
Expert opinion
Mechanisms of pain in CD may be muscle-based and non-muscle based. Accumulating evidence suggests that non-muscle-based mechanisms (such as abnormal transmission and processing of nociceptive stimuli, dysfunction of descending pain inhibitory pathways as well as structural and network changes in the basal ganglia, cortex and other areas) may also contribute to pain in CD alongside prolonged muscle contraction. Chemodenervation with botulinum toxin is considered the first-line treatment for CD. Treatment with botulinum toxin is usually effective, but optimization of the injection parameters should include consideration of pain as a core symptom in addition to the motor problems
1. Introduction
Cervical dystonia (CD) is a is focal dystonia and is clinically characterized by sustained or intermittent cervical muscle contractions causing abnormal, often repetitive movements, twisted neck postures, or both. Dystonic movements are typically patterned, twisting and may be tremulous [Citation1]; the motor disorder is accompanied by pain in most patients [Citation2]. It is one of the most common forms of focal dystonia with a recent estimated incidence of about 1.18 per 100,000 person-years [Citation3].
As opposed to other movement disorders, such as Parkinson’s disease [Citation4], CD has only recently been recognized as possessing nonmotor features. Evidence now supports the presence of nonmotor symptoms such as sensory deficits, mood and sleep disorders, and pain [Citation5–7], and the interplay between these non-motor symptoms and how these impact on the quality of life and disability in CD, cannot be overemphasized [Citation8]. When present, pain is a major contributor to disability [Citation9,Citation10] and social isolation [Citation11], and is often the main reason patients seek treatment for CD [Citation12,Citation13]. Surveys evaluating patient perceptions of their CD symptoms consistently highlight pain as a troublesome and disabling feature of their condition with significant impact on daily life and work [Citation14,Citation15]. However, while pain has long been recognized as a core feature of CD, its phenomenology is not well understood.
In this article, we sought to review the literature of the epidemiology, assessment, possible mechanisms and treatment of pain in CD. Treatment with botulinum toxin (BoNT) is considered first-line treatment for the management of pain in CD [Citation2,Citation16]. We also performed a meta-analysis of patient-level data from randomized, controlled trial data of abobotulinumtoxinA on pain in CD.
2. Pain in CD
Among all types of dystonia, CD has the highest prevalence of pain [Citation13,Citation17]. Affecting between 55% and 90% of treated patients [Citation10,Citation13,Citation18], pain in CD is typically described as a diffuse, sharp shooting or sometimes burning pain over the neck and shoulders that often (but not always) irradiates to the side of head deviation and may extend to the ipsilateral arm [Citation19]. About a third of patients recognize a sensation of pulling in the neck [Citation18] and 10–20% of patients with CD also report headache [Citation20].
Early studies suggested that up to 30% of patients eventually diagnosed with CD had pain in the neck muscles before the appearance of dystonic movements [Citation21,Citation22]. More recently, it has been suggested that (nonspecialist) physician focus on pain management rather than the underlying cause of pain may be one reason why up to 50% of patients experience a delay receiving a diagnosis of CD [Citation3]. Recently, Marciniec and colleagues [Citation23] investigated how painful the different head and neck postures are according to the Col-Cap classification system. They reported that the risk of cervical pain was 3.78 fold higher in patients with a pure-caput (involving muscles around the atlanto-occipital joint) presentation than in those with a pure -collis type (involving muscles around the cervical spine) [Citation23]. Similarly, the latero presentation was reported as more painful than other types.
Like parkinsonian pain [Citation24], CD pain does not seem to fully correlate with motor symptomatology or changes in posture. For example, a proportion of patients with CD are pain-free, even with dystonic movements of similar severity and duration to those in patients with pain [Citation10,Citation13,Citation18,Citation19], and patients with similar degrees of dystonia often report different severities of pain [Citation25]. In some patients, treatment with BoNT can improve dystonic contractions and postures without relieving pain, while in others BoNT can relieve pain without much impact on the abnormal postures [Citation26–29]. Indeed, studies of BoNT for CD have found that some patients will often request re-injection when pain returns but before the return of their postural symptoms, and others report pain relief disproportionate to motor benefit [Citation30]. Studies of deep brain stimulation (DBS) for CD also show temporal dissociation of improvement in posture severity and pain [Citation31,Citation32]. Finally, pain may be on the same or opposite side of the dystonic muscle posturing, suggesting an origin from either dystonic muscle contraction or from non-dystonic muscles (e.g. due to passive stretching or active compensatory contraction) [Citation33].
3. Evaluation of pain in CD
As a construct, pain is difficult to assess. There is no unit for measurement as it is a subjective experience and each person’s interpretation of pain will differ based on past experiences and expectations. Indeed, a meta-analysis of large international observational studies found some geographic differences in the perception of CD pain. Patients treated in the US reported higher levels of CD-related pain than patients from Europe or rest of world (mean scores of 8.4 vs. 6.0 and 6.9, respectively) as assessed by the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) pain subscale. Of note, there were no apparent geographical differences in TWSTRS ratings of disability, suggesting that the difference may be related to the perception and reporting of pain rather than its impact on daily life [Citation34].
However, several different rating scales have been used to evaluate pain in CD (). The TWSTRS is a composite scale which covers different features of CD, including pain [Citation35]. The TWSTRS pain subscale (range 0 = no pain to 20 = highest possible) comprises a severity score for the patient’s usual, worst, and best pain in the last week (0–10 points), a duration of pain component (0–5 points), as well as an assessment of disability due to pain (0–5 points). It does not however, consider the location of the pain. These three components have been retained in the updated TWSTRS-2 scale, but severity is now rated separately for best pain, worst pain and usual pain (each rated on a scale of 0–10 summing to a severity scale of 0–30) and so the total TWSTRS-2 pain scale now ranges from 0 to 40 [Citation36].
Table 1. Rating scales for pain in CD
Patient-reported outcomes are particularly relevant when it comes to the evaluation of pain. The CDIP-58 questionnaire also includes a pain and discomfort subscale consisting of five items (aching shoulders, shoulder pain, tired neck and shoulders, tightness in neck and tightness in shoulders) [Citation37,Citation38]. Patients self-rate each item for the prior 2-week timeframe on a scale of 1 = not at all bothersome to 5 = extremely bothersome. The CDIP-58 has been shown to be more sensitive in detecting statistical and clinical change than comparable subscales of the TWSTRS and SF-36 [Citation38], perhaps reflecting the specific attention to the location of the pain in CD.
Pain is also a significant contributor to poor quality of life (QoL), and as such is assessed both in disease-specific QoL instruments such as the CDQ-24 [Citation39] and generic scales such as the EQ5D [Citation40]. Whereas the CDIP-58 asks patients about ‘aching,’ ‘tiredness’ or ‘tightness’ in the neck and shoulders, the CDQ-24 mentions ‘burning’ and ‘pulling’ sensations and also includes the face and head [Citation39]. Specifically, the CDQ-24 asks the patient to rate how frequently they experience pain/burning sensation in the face, head, or neck region or have been prevented from falling asleep by pain or a pulling sensation, as well as whether they have felt hindered by pain/burning sensation in the face, head or neck region [Citation39].
Thus, no single scale captures the full range of painful sensations a patient with CD might have, and studies often combine such scales with the Pain Numeric Rating Scale (PNRS) which is a validated, single-item question on the current level of pain (range 0–10) as experienced by the patient with established cut-points of 0–3 for mild, 4–6 for moderate and 7–10 for severe [Citation41].
4. Mechanisms of pain in CD (muscle based and non-muscle based)
In their comprehensive assessment of patients with CD, Chan and colleagues [Citation13] found that pain was strongly associated with constant head turning (p < 0.05), greater severity of head turning (p < 0.01) and presence of spasms (p < 0.01), and suggested that the high incidence of pain in CD could be related to the relatively large size of muscles in spasm (particularly the trapezius and sternocleidomastoid versus the smaller muscles affected in blepharospasm which is generally less painful), the strong forces generated by the twisted posturing of the neck, or possibly the high density of deep pain receptors in the neck muscles. In addition, preclinical evidence suggests that sustained muscle contraction may trigger muscle cell ischemia inducing excess ATP and H+ release alongside substance P resulting in excitability of A delta and C fibers [Citation42,Citation43]. However, it is increasingly clear that prolonged muscle contraction is not the only cause of cervical pain. As will be described below, research suggests that abnormal transmission and processing of nociceptive stimuli, as well as dysfunction of descending pain inhibitory pathways, may also contribute to the development of ‘non-muscle-based’ pain in CD [Citation25,Citation44,Citation45].
In one of the first studies to evaluate pain perception in patients with CD, Lobbezoo and colleagues [Citation46] found that the threshold of pain perception in the sternocleidomastoid and upper trapezius muscles was decreased in patients with CD versus age-matched control subjects. Interestingly, they also showed that patients whose pain is on the same side as their posture deviation had lower self-reported ratings for the intensity and unpleasantness of their pain compared with those patients whose pain was on the opposite side to their deviation [Citation46]. Another study showed that patients with dystonia had two-times lower pain thresholds in both the dystonic or nonaffected muscles compared to age and sex-matched controls [Citation7]. This fits with our clinical observation that there is often little correspondence between the location of dystonia and the location of pain.
Using Quantitative Sensory Testing (QST), Paracka and colleagues [Citation47] showed impaired C-fiber transmission (as evidenced by impaired cold pain thresholds) as well as impaired Aδ and Aβ fibers (as evidenced by impaired hot detection thresholds and increased dynamic mechanical allodynia, respectively). In contrast to the findings by Lobbezoo, Tinazzi and colleagues [Citation48] have shown that patients with CD show normal ascending nociceptive pathway function, but have an impaired conditioned pain modulation response indicating that the descending pain inhibitory control system is primarily defective in CD [Citation49]. This is consistent with the growing evidence-base which supports the concept that chronic pain is associated with a dysregulation in descending pain modulation. Disruption of the balance of descending modulatory circuits to favor facilitation may promote and maintain chronic pain [Citation50].
While sometimes conflicting, these studies add to the accumulating evidence-base for sensory dysfunction as a core feature of CD. In particular, numerous imaging and electrophysiological studies have demonstrated abnormalities in sensorimotor network processing in dystonia [Citation51], and the ability of some patients to use alleviating maneuvers (‘geste antagoniste’ such as a light touch to certain areas of the head or face) also supports a role of the sensory system in clinical manifestation of CD [Citation52,Citation53]. While the effects of geste antagoniste on pain have not been systematically studied, patients often report that this alleviating maneuver can temporarily reduce the discomfort from abnormal positioning. The neuroanatomical basis of dystonia remains unclear, but basal ganglia involvement has long been assumed due to observations of dystonia following basal ganglia lesions and neurodegenerative diseases affecting these nuclei [Citation53]. More recently, the cortico-cerebellar circuit has also been implicated in dystonia [Citation54,Citation55] and imaging studies have shown structural and network changes in the basal ganglia, thalamus, motor cortex, premotor cortex, frontal, temporal and parietal cortices, cerebellum and brainstem of the patients with CD [Citation56,Citation57]. Several potential central mechanisms such as decreased inhibition, altered plasticity, and dysfunction of oscillatory activity appear to be involved and it has been hypothesized that people with dystonia have deficient subcortical and intracortical inhibition as well as abnormal sensorimotor integration and reorganization [Citation53,Citation57,Citation58]. These same cortical and subcortical centers communicate directly with descending pain modulatory circuits providing a mechanistic basis to explain how exogenous factors can influence the expression of chronic pain in a susceptible individual [Citation59].
Ultimately, the pain experienced by patients is likely to be multifactorial, with the severity of dystonic postures, chronicity of the disease, as well as the presence of comorbid medical conditions (e.g. arthritis) or painful orthopedic complications of CD (e.g. premature degeneration of the spine, disk herniation and radiculopathy [Citation60]) all playing a contributory role.
5. Pain management in CD
5.1. Management with analgesics and muscle relaxants
Studies conducted before treatment with BoNT became an established first-line treatment reported that at least two-thirds of patients use analgesics during the course of the disease [Citation61], and more recently, the ANCHOR-CD registry study reported that around 45% of patients presenting for treatment with abobotulinumtoxinA (aboBoNT-A) were also taking analgesic medications (predominantly non-steroidal anti-inflammatory drugs) for their CD [Citation62]. Opioid medications should generally be avoided. A recent study conducted at expert dystonia sites found that up to 11% of patients with CD met criteria for substance abuse and that opiate use was significantly higher in CD patients with substance abuse than without (p = 0.006) [Citation63]. Risk factors for substance abuse in CD patients were younger age (<55 years), male sex with mood disorders. Pain ratings were higher in patients who used opiates versus those who didn’t [Citation63]. Caution should be exercised when prescribing drugs with potential for abuse in these patients.
Other medications such as the muscle relaxants, baclofen and trihexyphenidyl are also used in routine practice [Citation62] although studies have shown that the pain relief afforded by treatment with BoNT-A is better than with trihexyphenidyl [Citation64].
5.2. Management with botulinum toxin, including a meta-analysis of controlled clinical trial data for abobotulinumtoxinA in the management of pain CD
The efficacy of BoNT in reducing pain in CD has long been established in several studies, although many of the earlier randomized controlled studies were limited because they used the Tsui scale for CD evaluation which misses pain [Citation65]. A recent review and meta-analysis by Rodrigues and colleagues (2020) estimated a mean difference in TWSTRS pain subscore of 2.11 [95% CI: 1.38, 2.83] points for patients treated with BoNT-A (any brand) versus placebo at Week 4 [Citation66].
However, the Rodrigues review was mostly limited to published data at peak effect (Week 4) and provides limited information about the efficacy over a longer time frame (e.g. Week 12) and with repeated injections. Recent analyses of pivotal trial data for abobotulinumtoxinA (aboBoNT-A) in CD have suggested that 72.6–81.5% of aboBoNTA-treated patients did not require retreatment before 16 weeks [Citation67]. To assess the impact of aboBoNT-A on pain in CD over a longer timeframe, we searched PubMed, PubMed Central, EMBASE and Google Scholar for clinical studies of aboBoNT-A in CD patients which evaluated TWSTRS post-injection (published in English between 2000 and 2021). Search terms used were abobotulinumtoxinA or Dysport, cervical dystonia or spasmodic torticollis, and pain. Four studies and their open label extensions were identified [Citation68–73] and we obtained the patient level data from the study sponsors associated datasets. Changes from baseline to Week 4 and Week 12 in TWSTRS pain scores were assessed using an ANCOVA with treatment as fixed effect, baseline values as covariates, and subject as random effect. In the randomized controlled studies, baseline TWSTRS pain scores were 10.5 ± 4.0 in the pooled aboBoNT-A group (n = 340) and 10.8 ± 4.3 in the placebo group (n = 203). Treatment with AboBoNT-A significantly reduced Week 4 pain scores by a mean ± SE of −3.2 ± 0.2 points in the AboBoNT-A group vs −1.0 ± 0.3 points in the placebo group (treatment difference: 2.2 ± 0.4 points; P < 0.0001). Statistical significance versus placebo was maintained at Week 12 (treatment difference: −1.3 ± 0.4 points vs placebo; P = 0.0006) ()). Moreover, in the open-label studies, reductions in Week 4 and Week 12 pain scores were maintained with repeat treatment with AboBoNT-A, ensuring relatively consistent pain control for patients across multiple injection cycles ()).
Figure 1. Meta-analysis of patient level data for patients treated with abobotulinumtoxinA in (a) 4 randomized controlled studies and (b) their open-label extensions
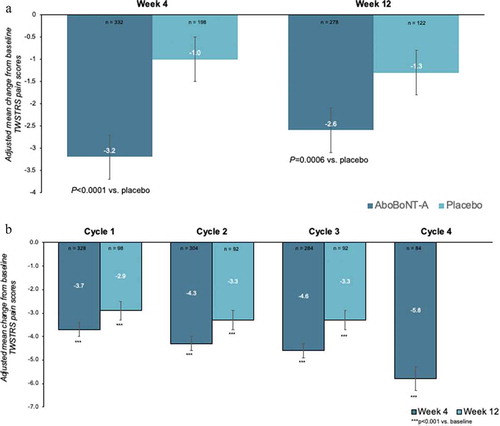
Similar findings have also been reported in a meta-analysis of aboBoNT-A as used in routine practice although baseline pain levels were lower than in the RCTs which typically include more severely affected patients. The MetaCD study analyzed TWSTRS data from three observational studies and showed that pain was significantly reduced at Week 4 by a mean [95%CI] of −2.4 [−2.8, −2.0] (p < 0.0001) points vs. baseline, equating to >30% improvement [Citation74].
There is clearly a difference in the symptom relief achieved at peak BoNT-A effect (assessed at Week 4) versus across the injection cycle and clinicians are typically more interested in how well symptoms, including pain, are covered in between dosing sessions. In a recent survey of CD patients treated with BoNT-A (all formulations), pain was often the first symptom to reappear between doses and the mean time to the patients noticing waning of BoNT-A effects was 10.5 weeks suggesting that many patients have to live with significant symptom reemergence for at least a few weeks before they are reinjected [Citation14]. Residual pain has also been identified as a predictor of non-satisfaction with long-term BoNT-A [Citation75]. Of note, most patients treated with aboBoNT-A in the MetaCD studies did not fully return to baseline before their next injection and their improvement in TWSTRS scores, including pain, remained statistically significant versus baseline even at the end of the treatment cycle (mean of 16 weeks) [Citation74]. This persistence of benefit not only suggests that treatment with aboBoNT-A afforded good symptom coverage across the cycle, but that patients and physicians do not wait for the full waning of effect before the next injection.
The question of BoNT-A dosing for pain remains a source of debate [Citation25]. In a large observational study of onabotulinumtoxinA (onaBoNT-A) for CD, Charles and colleagues [Citation10] showed that patients with moderate or severe pain had higher TWSTRS scores, required higher doses of onaBoNT-A and were injected in more muscles, demonstrating the critical importance of optimizing the BoNT injection paradigm in the management of pain in CD patients. However, other studies have found no such correlation with the dose of aboBoNT-A and pain relief [Citation76,Citation77], with some authors suggesting that the toxin dose for pain relief may not be as high as the doses required to relieve the motor disorder [Citation77].
5.3. Mechanisms of BoNT-A on pain
The best known mechanism of BoNT-A action is the inhibition of acetylcholine release from the pre-synaptic terminal at the neuromuscular junction with consequent local reduction of muscle fiber activity [Citation78]. It has been suggested that the relaxation of hyperactive muscles may, at least in part, contribute to pain relief through the decompression of the nerve fibers due to the reduction in muscle tone or volume, decreasing afferent activity of spindles and reducing excitability of motoneurons [Citation79–81]. In addition, a reduction of muscle hypertonicity should provide relief of ischemia, reduction in lactate production, and reduced traction-related and positional pain [Citation81,Citation82].
However, the inhibition of acetylcholine release at the neuromuscular junction is not the only mechanism of BoNT action and a growing evidence base suggests that the mechanism behind the antinociceptive effects of BoNT in the management of CD is more complex than simple muscle relief. First and foremost, as described earlier, pain relief has been reported in CD patients who did not show considerable improvements in hyperactive muscle contraction [Citation26–29]. Based on experimental and clinical evidence in neuropathic pain conditions [Citation83], it is also thought that BoNT may inhibit neurogenic inflammation and peripheral sensitization by also inhibiting the release of local neuropeptides from sensory nerves that are involved in pain transmission such as substance P, calcitonin gene-related peptide, glutamate and transient receptor potential vanilloid 1 [Citation84–86]. There is also accumulating evidence that changes to the afferent input caused by BoNT-A may also result in short- and long-term plastic changes in the networks associated with pain in CD [Citation87], and this reorganization of the brain may have an additional therapeutic effect [Citation82]. Whether this is a direct effect in the brain or explained by cortical plasticity following an altered cortical representation of the affected muscles (or both) is yet another source of debate. However, there is clear evidence that BoNT has clear effect on muscle spindles (which act as sensory proprioceptors) and as such a modification of the central motor programming may occur in CD, with BoNT acting either as a ‘sensory trick’ or as a form of ‘short‐term plasticity’ [Citation88]. This parallel chemodenervation of intrafusal muscles by BoNT-A derives significance in the clinics, wherein deep cervical muscle injections may be considered when relief from usual large superficial muscle injections is not optimally achieved. Feng and colleagues [Citation89] documented overactivity of deep-seated cervical muscles in CD, and showed that the deeper overactive muscles have high densities of muscle spindles, compared to the superficial muscles.
5.4. Surgery
Selective peripheral denervation remains a surgical option in the treatment of CD for treatment-refractory patients and is generally effective against pain in these hard-to-treat patients [Citation90–92]. However, while the majority of patients experience a significant relief of symptoms, there is a substantial risk of reinnervation (particularly in the splenius) and/or change in the pattern of the CD, which may impact on pain relief. DBS of the internal pallidum or the subthalamic nucleus is another option for severe and drug‐refractory CD, and pain scores have been reported to improve by up to 92% [Citation93–95]. However, a number of sham-controlled DBS studies showed no impact on pain and quality of life [Citation96]. As noted earlier, pain improvement following DBS can be temporally dissociated from motor and postural improvement, supporting the idea that pain relief is not always secondary to the improvement of motor postures.
5.5. Non-pharmacologic approaches
Non-pharmacologic approaches may also be of benefit. The benefits of physiotherapy for pain are increasingly accepted with increasing evidence that physiotherapy consistently reduces self-reported pain in adults suffering from a variety of painful conditions [Citation97]. In view of the presence of non-motor manifestations in CD, a multi-modal, holistic neurorehabilitation program has recently been advocated [Citation97]. However, physiotherapy for CD is an emerging field, and the approaches to therapy (EMG biofeedback, electrotherapy, relaxation training, massage therapy, muscle stretching techniques, kinesiotaping mobilization and manipulation, vestibular stimulation and cognitive behavioral therapy) are diverse and difficult to standardize [Citation98]. A personalized intensive physiotherapy program has been applied in CD that involves realignment of the muscles using mirrors, reducing tone and stretching dystonic muscles, and reinforcing corrective muscles promoting the activation of the muscles groups opposing the dystonic posture [Citation99].
In general, the evidence for physiotherapy in CD is lacking (mainly due to poor methodologic quality of the clinical trials), but there is evidence that a multimodal physiotherapy program consisting of active exercises, stretching and relaxation in addition to BoNT-A treatment induces beneficial effects on pain alleviation and disability in CD patients [Citation98,Citation100,Citation101]. Only a few CD rehabilitation studies have considered pain as a key outcome. In one study, Tassorelli and colleagues [Citation102] evaluated whether the association of a rehabilitative program improves the clinical efficacy of BoNT-A treatment in a single-center (n = 40), randomized cross-over study of BoNT-A plus physiotherapy versus BoNT-A alone. Compared with those treated with BoNT-A alone, patients who also received physiotherapy showed a more marked reduction in subjective pain (−13.35 vs. 6.95 points) scores. In another study, Hu and colleagues [Citation103] evaluated the benefits of a home exercise program (stretching, range-of-motion and isometric exercises) following BoNT-A injection and showed that patients who followed the home program significantly reduced pain versus BoNT-A alone.
Finally, while also limited by poor quality evidence, the application of noninvasive neuromodulation techniques for CD pain is another growing field that is based on the concept of dystonia as a network-based disorder [Citation55]. Recent studies have shown that in CD, the Cortical Silent Period, Short Interval Cortical Inhibition, and Afferent-induced inhibition are reduced compared to controls [Citation104]. Case studies using transcranial stimulation (alternating and direct current approaches) applied to the motor cortex have reported a 55–75% reduction in pain [Citation105,Citation106], while the effects of repetitive transcranial magnetic stimulation on CD symptoms, including pain are now starting to be systematically evaluated [Citation107].
6. Conclusions
Pain in CD is common and a source of considerable disability for patients with CD. BoNT-A remains the first-line treatment for CD. Treatment with BoNT is effective for most patients, but optimization of the injection parameters should include a holistic approach to management including consideration of pain as a core symptom. Many scales are available to assess pain in CD, but none assess pain in the same way, and it may be useful to combine instruments for detailed assessments. Pain is a subjective construct and, as such, patient reported outcomes such as the PNRS may provide the simplest insights into a patient’s pain history for routine practice.
7. Expert opinion
While the jury is out on the importance of BoNT-A dosing to the management of pain in CD, clinical experience shows that the location of the pain reported may not always coincide with the dystonic pattern. Current injection practice is to be guided by the dystonic posturing, although evidence from observational studies suggests more muscles, including deeper muscles, may need to be injected [Citation10]. While little is known about the direct relevance of guidance techniques to the pain outcomes in CD, the use of ultrasound or other guidance techniques is increasingly accepted as important to verify precise needle positioning in the proper muscle (particularly in deeper muscles), thereby optimizing motor and safety outcomes in general [Citation108,Citation109]. Pain is a key driver for patients to seek treatment, and it is also often the first symptom to reemerge when the effects of BoNT-A start to wane [Citation12,Citation14]. It is therefore important to consider how long each BoNT-A injection lasts – accumulating evidence suggests that the duration of symptom coverage may differ between the BoNT-A products [Citation110,Citation111].
In cases of suboptimal response, it is useful to consider the caput versus collis presentations (although most patients will have a mix of both). For example, expert injectors have reported a benefit of injecting into the obliquus capitus inferior for patients with the painful rotational caput presentation [Citation112,Citation113]. Previously this (and other) muscles were ignored largely because it is less involved in collis presentations, but also because it is deeper and requires ultrasound guidance for accurate targeting. Indeed, over the last few years the improvements in guidance techniques have allowed us to inject with more accuracy and have improved how we treat CD [Citation113]. In clinical practice, it may also be useful to combine BoNT-A treatment with physical therapy to seek beneficial synergies, in analogy to physical therapy and BoNT-A in the management of spasticity [Citation114]. Finally, clinicians should consider other sources of pain, including pain from comorbidities and complications of CD that treatment with BoNT can’t treat (e.g. accelerated degenerative arthritis or spinal stenosis). For these patients, a referral to a pain clinic is often helpful.
On the whole, the evidence base supports the concept that the etiology of pain in CD is multifactorial and extends beyond simple muscle pain. The relevance of sensory brain networks remains to be fully elucidated but offers several opportunities for enhancement of clinical practice. For example, it is theoretically possible that earlier treatment with BoNT-A may help to avoid the centralization of pain. The role of noninvasive neuromodulation techniques, with or without BoNT-A treatment, is another exciting area for future work.
Looking to the future, in the short (immediate) term, the management of pain in CD will remain reliant on the effective management of the movement disorder with BoNT-A products. Clinicians should remain vigilant in tracking their patients’ symptoms, including pain, over the course of the injection cycle and look for ways to optimize the therapy plan to avoid the ‘roller-coaster’ ride of symptom relief and then symptom reemergence before the next injection that patients often describe [Citation14]. New BoNT-A formulations such as daxibotulinumtoxinA [Citation115] and prabotulinumtoxinA [Citation116,Citation117] are also in development and may reach the market within the next few years.
In the medium term, new treatments that take advantage of the diverse BoNT family of proteins are under development [Citation118,Citation119]. SNARE proteins are a family of proteins involved in regulating exocytosis, which in neurons mediate neurotransmitter release. Each BoNT serotype (types A-G) specifically cleaves one SNARE protein (SNAP25 and synaptobrevin, except for BoNT-C which can cleave both), thus inhibiting synaptic vesicle exocytosis and neurotransmission at the neuromuscular junction in different ways [Citation118,Citation120]. This natural diversity offers the potential to modify clinical properties of BoNT proteins to meet the needs of patients living with a variety of movement disorders, including CD, and it is conceivable that some products will have greater (or lesser) effects on pain mechanisms than others [Citation120]. Moreover, protein engineering offers a huge potential for new BoNTs with enhanced clinical properties. For example, it appears possible to improve the neuronal binding of Type A [Citation121,Citation122], enhance the catalytic activity of Type B [Citation123] and enhance the syntaxin-specific catalytic activity of Type C [Citation124]. Looking even further forward, with BoNTs as their scaffold, targeted secretion inhibitors are a new class of biopharmaceuticals that can inhibit cellular secretion for very prolonged periods of time [Citation125,Citation126] and therefore may also offer new potential for patients living with painful chronic conditions.
Article highlights
When present, pain is a major contributor to disability in cervical dystonia.
Pain in CD does not always fully correlate with motor symptomatology or changes in posture.
No single scale captures the full range of painful sensations a patient with CD might experience.
Mechanisms of pain in CD may be muscle-based and non-muscle based. Accumulating evidence suggests that non-muscle-based mechanisms (such as abnormal transmission and processing of nociceptive stimuli, dysfunction of descending pain inhibitory pathways as well as structural and network changes in the basal ganglia, cortex and other areas) may also contribute to pain in CD alongside prolonged muscle contraction.
As a leading complaint in most patients with CD, dystonic pain can almost always be markedly reduced by BoNT-A therapy. Meta-analysis of patient level data from abobotulinumtoxinA studies showed that significant pain reductions at peak effect (Week 4), that were still apparent at Week 12, and which were maintained with repeat treatment.
Declaration of interests
RL Rosales reports being a clinical trialist for Ipsen on BoNT-A applications for Cervical Dystonia and Post-Stroke Spasticity. In the past, he was engaged in an Allergan BoNT-A Cervical Dystonia Trial. He has received travel honoraria for lectures on BoNT-A for Ipsen, Allergan and Merz. L Cuffe is employed by Ipsen. Benjamin Regnault provides statistical consultancy to Ipsen. R Trosch has received personal fees from Ipsen and Supernus. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this paper has received funding from Ipsen, manufacturer of Dysport. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
The authors thank Anita Chadha-Patel, PhD, of ACP Clinical Communications Ltd (Hertfordshire, UK) for providing medical writing support, which was funded by Ipsen (Paris, France) in accordance with Good Publication Practice guidelines.
Additional information
Funding
References
- Grütz K, Klein C. Dystonia updates: definition, nomenclature, clinical classification, and etiology. J Neural Transm. 2021;128(4):395–404.
- Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18(1):5–18.
- LaHue SC, Albers K, Goldman S, et al. Cervical dystonia incidence and diagnostic delay in a multiethnic population. Mov Disord. 2020;35(3):450–456.
- Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–450.
- Stamelou M, Edwards MJ, Hallett M, et al. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2011;135(6):1668–1681.
- Avanzino L, Cherif A, Crisafulli O, et al. Tactile and proprioceptive dysfunction differentiates cervical dystonia with and without tremor. Neurology. 2020;94(6):e639–e50.
- Torres J, Rosales RL. Nonmotor symptoms in dystonia. Int Rev Neurobiol. 2017;134:1335–1371.
- Smit M, Kuiper A, Han V, et al. Psychiatric co-morbidity is highly prevalent in idiopathic cervical dystonia and significantly influences health-related quality of life: results of a controlled study. Parkinsonism Relat Disord. 2016;30:7–12.
- van den Dool J, Tijssen MA, Koelman JH, et al. Determinants of disability in cervical dystonia. Parkinsonism Relat Disord. 2016;32:48–53.
- Charles PD, Adler CH, Stacy M, et al. Cervical dystonia and pain: characteristics and treatment patterns from CD PROBE (cervical dystonia patient registry for observation of onabotulinumtoxinA efficacy). J Neurol. 2014;261(7):1309–1319.
- Muller J, Kemmler G, Wissel J, et al. The impact of blepharospasm and cervical dystonia on health-related quality of life and depression. J Neurol. 2002;249(7):842–846.
- Novak I, Campbell L, Boyce M, et al. Botulinum toxin assessment, intervention and aftercare for cervical dystonia and other causes of hypertonia of the neck: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):94–108.
- Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6(2):119–126.
- Comella C, Ferreira JJ, Pain E, et al. Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia. J Neurol. 2021;268(3):903–912.
- Comella C, Bhatia K. An international survey of patients with cervical dystonia. J Neurol. 2015;262(4)837-848.
- Simpson DM, Hallett M, Ashman EJ. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache. Neurology. 2016;86:1–9.
- Williams L, McGovern E, Kimmich O, et al. Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur J Neurol. 2017;24(1):73–81.
- Jankovic J, Leder S, Warner D, et al. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41(7):1088–1091.
- Kutvonen O, Dastidar P, Nurmikko T. Pain in spasmodic torticollis. Pain. 1997;69(3):279–286.
- Barbanti P, Fabbrini G, Pauletti C, et al. Headache in cranial and cervical dystonia. Neurology. 2005;64(7):1308–1309.
- Sheehy MP, Marsden CD. Trauma and pain in spasmodic torticollis. Lancet. 1980;1(8171):777–778.
- Heinen F, Scheidt CE, Nickel T, et al. Spasmodic torticollis - a multicentre study on behavioural aspects II: signs, symptoms and course. Behav Neurol. 1996;9(2):81–88.
- Marciniec M, Szczepańska-Szerej A, Popek-Marciniec S, et al. Pain incidence in cervical dystonia is determined by the disease phenotype. J Clin Neurosci. 2020;79:133–136.
- Antonini A, Tinazzi M, Abbruzzese G, et al. Pain in Parkinson’s disease: facts and uncertainties. Eur J Neurol. 2018;25(7):917–e69.
- Marciniec M, Szczepańska-Szerej A, Kulczyński M, et al. Pain in cervical dystonia and the antinociceptive effects of botulinum toxin: what is currently known? Rev Neurosci. 2019;30(7):771-779.
- Lorentz IT, Subramaniam SS, Yiannikas C. Treatment of idiopathic spasmodic torticollis with botulinum toxin A: a double-blind study on twenty-three patients. Mov Disord. 1991;6(2):145–150.
- Pappert EJ, Germanson T. Botulinum toxin type B vs. type A in toxin-naïve patients with cervical dystonia: randomized, double-blind, noninferiority trial. Mov Disord. 2008;23(4):510–517.
- Quagliato EM, Carelli EF, Viana MA. A prospective, randomized, double-blind study comparing the efficacy and safety of type a botulinum toxins botox and prosigne in the treatment of cervical dystonia. Clin Neuropharmacol. 2010;33(1):22–26.
- Camargo CH, Teive HA, Becker N, et al. Botulinum toxin type A and cervical dystonia: a seven-year follow-up. Arq Neuropsiquiatr. 2011;69(5):745–750.
- Brin MF, Fahn S, Moskowitz C, et al. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov Disord. 1987;2(4):237–254.
- Kulisevsky J, Lleó A, Gironell A, et al. Bilateral pallidal stimulation for cervical dystonia: dissociated pain and motor improvement. Neurology. 2000;55(11):1754–1755.
- Cacciola F, Farah JO, Eldridge PR, et al. Bilateral deep brain stimulation for cervical dystonia: long-term outcome in a series of 10 patients. Neurosurgery. 2010;67(4):957–963.
- Castagna A, Albanese A. Management of cervical dystonia with botulinum neurotoxins and EMG/ultrasound guidance. Neurol Clin Pract. 2019;9(1):64–73.
- Trosch R, Misra P, Maisonobe P, et al. Geographic differences in pain perception in patients with cervical Dystonia (P1.033). Neurology. 2016;86:1–33.
- Consky E, Lang AE. Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M, editors. Therapy with bofulinum toxin. New York: Marcel Dekker; 1994:211-237.
- Comella CL, Perlmutter JS, Jinnah HA, et al. Clinimetric testing of the comprehensive cervical dystonia rating scale. Mov Disord. 2016;31(4):563–569.
- Cano SJ, Warner TT, Linacre JM, et al. Capturing the true burden of dystonia on patients: the cervical dystonia impact profile (CDIP-58). Neurology. 2004;63(9):1629–1633.
- Cano SJ, Hobart JC, Edwards M, et al. CDIP-58 can measure the impact of botulinum toxin treatment in cervical dystonia. Neurology. 2006;67(12):2230–2232.
- Müller J, Wissel J, Kemmler G, et al. Craniocervical dystonia questionnaire (CDQ-24): development and validation of a disease-specific quality of life instrument. J Neurol Neurosurg. 2004;75(5):749–753.
- EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN. 2005;113(1):9–19.
- Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105(12):214–219.
- Lund JP, Donga R, Widmer CG, et al. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69(5):683–694.
- Camargo CH, Cattai L, Teive HA. Pain relief in cervical Dystonia with botulinum toxin treatment. Toxins (Basel). 2015;7(6):2321–2335.
- Avenali M, De Icco R, Tinazzi M, et al. Pain in focal dystonias - A focused review to address an important component of the disease. Parkinsonism Relat Disord. 2018;54:17–24.
- Lobbezoo F, Tanguay R, Thon MT, et al. Pain perception in idiopathic cervical dystonia (spasmodic torticollis). Pain. 1996;67(2–3):483–491.
- Paracka L, Wegner F, Blahak C, et al. Sensory alterations in patients with isolated idiopathic Dystonia: an exploratory quantitative sensory testing analysis. Front Neurol. 2017;8:553.
- Tinazzi M, Valeriani M, Squintani G, et al. Nociceptive pathway function is normal in cervical dystonia: a study using laser-evoked potentials. J Neurol. 2012;259(10):2060–2066.
- Tinazzi M, Squintani GM, Bhatia KP, et al. Pain in cervical dystonia: evidence of abnormal inhibitory control. Parkinsonism Relat Disord. 2019;65:252–255.
- Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8(2):143–151.
- Hallett M. Pathophysiology of dystonia. J Neural Transm Suppl. 2006;70(Suppl):485–488.
- Poisson A, Krack P, Thobois S, et al. History of the ‘geste antagoniste’ sign in cervical dystonia. J Neurol. 2012;259(8):1580–1584.
- Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13(1):100–112.
- Shakkottai VG, Batla A, Bhatia K, et al. Current opinions and areas of consensus on the role of the cerebellum in Dystonia. Cerebellum. 2017;16(2):577–594.
- Brüggemann N. Contemporary functional neuroanatomy and pathophysiology of dystonia. J Neural Transm (Vienna). 2021;128(4):499–508.
- Prell T, Peschel T, Köhler B, et al. Structural brain abnormalities in cervical dystonia. BMC Neurosci. 2013;14:123.
- Brodoehl S, Wagner F, Prell T, et al. Cause or effect: altered brain and network activity in cervical dystonia is partially normalized by botulinum toxin treatment. Neuroimage Clin. 2019;22:101792.
- Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013;28(7):958–967.
- Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17(2):192–200.
- Konrad C, Vollmer-Haase J, Anneken K, et al. Orthopedic and neurological complications of cervical dystonia–review of the literature. Acta Neurol Scand. 2004;109(6):369–373.
- Duane DD. Spasmodic torticollis: clinical and biologic features and their implications for focal dystonia. Adv Neurol. 1988;50:473–492.
- Trosch RM, Espay AJ, Truong D, et al. Multicenter observational study of abobotulinumtoxinA neurotoxin in cervical dystonia: the ANCHOR-CD registry. J Neurol Sci. 2017;376:84–90.
- Mahajan A, Jankovic J, Marsh L, et al. Cervical dystonia and substance abuse. J Neurol. 2018;265(4):970–975.
- Brans JW, Lindeboom R, Snoek JW, et al. Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double-blind controlled trial. Neurology. 1996;46(4):1066–1072.
- Costa J, Espirito-Santo C, Borges A, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2005;1:CD003633.
- Rodrigues FB, Duarte GS, Marques RE, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2020;11(11):CD003633.
- Esquenazi A, Delgado MR, Hauser RA, et al. Duration of symptom relief between injections for abobotulinumtoxinA (Dysport(R)) in spastic paresis and cervical dystonia: comparison of evidence from clinical studies. Front Neurol. 2020;11:576117.
- Truong D, Duane DD, Jankovic J, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double-blind, placebo-controlled study. Mov Disord. 2005;20(7):783–791.
- Truong D, Brodsky M, Lew M, et al. Long-term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism Relat Disord. 2010;16(5):316–323.
- Poewe W, Burbaud P, Castelnovo G, et al. Efficacy and safety of abobotulinumtoxinA liquid formulation in cervical dystonia: a randomized-controlled trial. Mov Disord. 2016;31(11):1649–1657.
- Lew MF, Brashear A, Dashtipour K, et al. A 500 U/2 mL dilution of abobotulinumtoxinA vs. placebo: randomized study in cervical dystonia. Int J Neurosci. 2018;128(7):619–626.
- Hauser RA, Truong D, Hubble J, et al. AbobotulinumtoxinA (Dysport) dosing in cervical dystonia: an exploratory analysis of two large open-label extension studies. J Neural Transm (Vienna). 2013;120(2):299–307.
- Dashtipour K, Wietek S, Rubin B, et al. AbobotulinumtoxinA using 2-mL dilution (500 U/2-mL) maintains durable improvement across multiple treatment cycles. J Clin Mov Disord. 2020;7:8.
- Trosch RM, Misra VP, Maisonobe P, et al. Impact of abobotulinumtoxinA on the clinical features of cervical dystonia in routine practice. Clin Parkinsonism Relat Disord. 2020;3:100063.
- Marciniec M, Szczepańska-Szerej A, Rejdak K. Cervical dystonia: factors deteriorating patient satisfaction of long-term treatment with botulinum toxin. Neurol Res. 2020;42(11):987–991.
- Poewe W, Deuschl G, Nebe A, et al. What is the optimal dose of botulinum toxin A in the treatment of cervical dystonia? Results of a double blind, placebo controlled, dose ranging study using Dysport. German Dystonia Study Group. J Neurol Neurosurg Psychiatry. 1998;64(1):13–17.
- Yun JY, Kim JW, Kim HT, et al. Dysport and Botox at a ratio of 2.5:1 units in cervical dystonia: a double-blind, randomized study. Mov Disord. 2015;30(2):206–213.
- Dressler D, Bigalke H. Pharmacology of botulinum toxin drugs. In: Truong DMD, Dressler D, Hallett M, et al., editors. Manual of botulinum toxin therapy. Cambridge: Cambridge University Press; 2009;13-23.
- Filippi GM, Errico P, Santarelli R, et al. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993;113(3):400–404.
- Trompetto C, Currà A, Buccolieri A, et al. Botulinum toxin changes intrafusal feedback in dystonia: a study with the tonic vibration reflex. Mov Disord. 2006;21(6):777–782.
- Marciniec M, Szczepańska-Szerej A, Kulczyński M, et al. Pain in cervical dystonia and the antinociceptive effects of botulinum toxin: what is currently known? Rev Neurosci. 2019;30(7):771–779.
- Siongco PRL, Rosales RL, Moore AP, et al. Botulinum neurotoxin injections for muscle-based (dystonia and spasticity) and non-muscle-based (neuropathic pain) pain disorders: a meta-analytic study. J Neural Transm (Vienna). 2020;127(6):935–951.
- Park J, Park HJ. Botulinum toxin for the treatment of neuropathic pain. Toxins (Basel). 2017;9:9.
- Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol. 2014;119-120:39–59.
- Wheeler A, Smith HS. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology. 2013;306:124–146.
- Kim D-W, Lee S-K, Ahnn J. Botulinum toxin as a pain killer: players and actions in antinociception. Toxins (Basel). 2015;7(7):2435–2453.
- Delnooz CC, Pasman JW, Beckmann CF. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One. 2013;8(5):e62877.
- Rosales RL, Dressler D. On muscle spindles, dystonia and botulinum toxin. Eur J Neurol. 2010;17(Suppl 1):71–80.
- Feng L, Zhang Z, Malam Djibo I, et al. The efficacy of single-photon emission computed tomography in identifying dystonic muscles in cervical dystonia. Nucl Med Commun. 2020;41(7):651–658.
- Bergenheim AT, Nordh E, Larsson E, et al. Selective peripheral denervation for cervical dystonia: long-term follow-up. J Neurol Neurosurg. 2015;86(12):1307–1313.
- Cohen-Gadol AA, Ahlskog JE, Matsumoto JY, et al. Selective peripheral denervation for the treatment of intractable spasmodic torticollis: experience with 168 patients at the Mayo Clinic. J Neurosurg. 2003;98(6):1247–1254.
- Münchau A, Palmer JD, Dressler D, et al. Prospective study of selective peripheral denervation for botulinum-toxin resistant patients with cervical dystonia. Brain. 2001;124(Pt 4):769–783.
- Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255(6):881–884.
- Skogseid IM, Ramm-Pettersen J, Volkmann J, et al. Good long-term efficacy of pallidal stimulation in cervical dystonia: a prospective, observer-blinded study. Eur J Neurol. 2012;19(4):610–615.
- Ostrem JL, Racine CA, Glass GA, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76(10):870–878.
- Kim HJ, Jeon B. Arching deep brain stimulation in dystonia types. J Neural Transm (Vienna). 2021;128(4):539–547.
- Bradnam LV, Meiring RM, Boyce M, et al. Neurorehabilitation in dystonia: a holistic perspective. J Neural Transm. 2021;128(4):549–558.
- De Pauw J, Van der Velden K, Meirte J, et al. The effectiveness of physiotherapy for cervical dystonia: a systematic literature review. J Neurol. 2014;261(10):1857–1865.
- Franco JH, Rosales RL. Neurorehabilitation in dystonia (Chapter 14). In: Kanovsky P, Bhatia K, Rosales RL, editors. Dystonia and dystonic syndromes. Germany: Springer, GmbH; 2015. ISBN 978-3-7091-1515-2 ISBN 978-3-7091-1516-9 (eBook). DOI: https://doi.org/10.1007/978-3-7091-1516-9)
- Delnooz CC, Horstink MW, Tijssen MA. Paramedical treatment in primary dystonia: a systematic review. Mov Disord. 2009;24(15):2187–2198.
- Queiroz MA, Chien HF, Sekeff-Sallem FA, et al. Physical therapy program for cervical dystonia: a study of 20 cases. Funct Neurol. 2012;27(3):187–192.
- Tassorelli C, Mancini F, Balloni L, et al. Botulinum toxin and neuromotor rehabilitation: an integrated approach to idiopathic cervical dystonia. Mov Disord. 2006;21(12):2240–2243.
- Hu W, Rundle-Gonzalez V, Kulkarni SJ, et al. A randomized study of botulinum toxin versus botulinum toxin plus physical therapy for treatment of cervical dystonia. Parkinsonism Relat Disord. 2019;63:195–198.
- Kaňovský P, Rosales R, Otruba P, et al. Contemporary clinical neurophysiology applications in dystonia. J Neural Transm (Vienna). 2021;128(4):509–519.
- Angelakis E, Liouta E, Andreadis N, et al. Transcranial alternating current stimulation reduces symptoms in intractable idiopathic cervical dystonia: a case study. Neurosci Lett. 2013;533:39–43.
- Bradnam LV, Frasca J, Kimberley TJ. Direct current stimulation of primary motor cortex and cerebellum and botulinum toxin a injections in a person with cervical dystonia. Brain Stimul. 2014;7(6):909–911.
- Lizarraga KJ, Al-Shorafat D, Fox S. Update on current and emerging therapies for dystonia. Neurodegener Dis Manag. 2019;9(3):135–147.
- Fietzek UM, Nene D, Schramm A, et al. The role of ultrasound for the personalized botulinum toxin treatment of cervical dystonia. Toxins (Basel). 2021;13(5):365.
- Kaymak B, Gürçay E, Ata AM, et al. Ultrasound imaging and guidance in the management of cervical dystonia: a caveat on the compartmentalization of sternocleidomastoid muscle. Parkinsonism Relat Disord. 2017;43:127–128.
- Esquenazi A, Delgado MR, Hauser RA, et al. Duration of symptom relief between injections for abobotulinumtoxinA (Dysport®) in spastic paresis and cervical dystonia: comparison of evidence from clinical studies. Front Neurol. 2020;11:576117.
- Colosimo C, Charles D, Misra VP, et al. group IICs. Cumulative effects of long-term treatment with abobotulinumtoxinA in cervical dystonia: findings from a prospective, observational study. J Neurol Sci. 2020;416:117015.
- Jost WH Torticaput versus torticollis: clinical effects with modified classification and muscle selection. Tremor and other hyperkinetic movements (New York, NY). 2019;9:DOI: https://doi.org/10.7916/tohm.v0.647.
- Jost WH, Biering-Sørensen B, Drużdż A, et al. Preferred muscles in cervical dystonia. Neurol Neurochir Pol. 2020;54(3):277–279.
- Demetrios M, Khan F, Turner-Stokes L, et al. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst Rev. 2013;6:Cd009689.
- Jankovic J, Truong D, Patel AT, et al. Injectable daxibotulinumtoxina in cervical dystonia: a phase 2 dose-escalation multicenter study. Mov Disord Clin Pract. 2018;5(3):273–282.
- A phase 2 study to evaluate the safety and efficacy of ABP-450 in the treatment of cervical dystonia. NCT04849988. Available from https://clinicaltrials.gov/ct2/show/NCT04849988. Last accessed Sept 2021.
- Kim D-Y, Kim JM. Safety and efficacy of prabotulinumtoxinA (Nabota®) injection for cervical and shoulder girdle myofascial pain syndrome: a pilot study. Toxins (Basel). 2018;10(9):355.
- Chaddock JA, Marks PM. Clostridial neurotoxins: structure-function led design of new therapeutics. Cell Mol Life Sci. 2006;63(5):540–551.
- Tehran DA, Pirazzini M. Novel botulinum neurotoxins: exploring underneath the iceberg tip. Toxins (Basel). 2018;10(5):190.
- Foster KA. Engineered toxins: new therapeutics. Toxicon. 2009;54(5):587–592.
- Rummel A, Mahrhold S, Bigalke H, et al. Exchange of the H(CC) domain mediating double receptor recognition improves the pharmacodynamic properties of botulinum neurotoxin. Febs J. 2011;278(23):4506–4515.
- Wang J, Zurawski TH, Bodeker MO, et al. Longer-acting and highly potent chimaeric inhibitors of excessive exocytosis created with domains from botulinum neurotoxin A and B. Biochem J. 2012;444(1):59–67.
- Guo J, Pan X, Zhao Y, et al. Engineering clostridia neurotoxins with elevated catalytic activity. Toxicon. 2013;74:158–166.
- Wang D, Zhang Z, Dong M, et al. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry. 2011;50(14):2711–2713.
- Foster K, Chaddock J. Targeted secretion inhibitors-innovative protein therapeutics. Toxins (Basel). 2010;2(12):2795–2815.
- Stancombe PR, Masuyer G, Birch-Machin I, et al. Engineering botulinum neurotoxin domains for activation by toxin light chain. Febs J. 2012;279(3):515–523.
