ABSTRACT
Introduction
Spasticity is a common, debilitating symptom of multiple sclerosis (MS) with several treatment options including the cannabinoid-based treatment, nabiximols. The purpose of this review was to examine the existing clinical practice guidelines that direct the management of multiple-sclerosis-associated spasticity (MSS), to identify areas of similarity and divergence, and suggest where standardization and improvement may be obtained.
Areas covered
Published literature (PubMed), websites of relevant European Medical Associations and Health Technology Assessment bodies were systematically searched to identify guidelines describing the pharmacological management of MSS, focussing on European countries where nabiximols (Sativex® oromucosal spray) is approved. Sixteen publicly available guidelines were identified. Analysis was focused on, but not restricted to, the use of nabiximols in the wider context of the pharmacological treatment of MSS.
Expert Opinion/Commentary
We believe that currently MSS is insufficiently treated and this would be improved if a clear and detailed set of guidelines were available and implemented in daily practice. We would welcome the update and amalgamation of the existing guidelines by an international panel, using an evidence-based approach, into a single guideline that is more detailed and standardized in its approach to the initiation, monitoring and optimization of anti-spasticity drugs.
PLAIN LANGUAGE SUMMARY
People with multiple sclerosis often experience tight or stiff muscles and an inability to control those muscles. This is known as spasticity, which can have a devastating impact on a person’s ability to carry out their daily activities. In addition to physiotherapy, doctors can prescribe various medicines to improve spasticity; these are known as anti-spasticity treatments. Often, prescription choices are steered by guideline documents, written by medical experts. These documents contain important information such as when to prescribe, what to prescribe, how much to prescribe and how to measure how well the treatment is working. The purpose of this study was to examine whether the guidelines that guide the prescription of anti-spasticity treatments in people with multiple sclerosis in Europe, are fit for purpose for day-to-day medical practice. In particular, this article examines how the guidelines represent the newer cannabis-based treatment known as nabiximols, sold under the name Sativex oromucosal spray, which has become more widely available in many European countries over the last 10 years.
1. Introduction
Symptoms of multiple sclerosis (MS), such as spasticity, mobility impairment, cognitive dysfunction, fatigue, pain, bladder, bowel dysfunction, and others, are often inter-related and impact severely on the daily lives of individuals living with MS. However, the management of these symptoms remains a significant day-to-day challenge for them and physicians [Citation1]. Increased muscle tone leads to multiple sclerosis-associated spasticity (MSS), one of the most prevalent of MS symptoms, affecting around 80% of patients [Citation2,Citation3]. Severity can range from mild to severe, with around a third of individuals experiencing moderate or severe symptoms (defined as a score of ≥4 on the 0–10 numerical rating scale for spasticity, NRS-S) [Citation2]. Spasticity can persist despite the use of classic treatments [Citation2]. MSS can have a major impact on many aspects of daily MS patients’ quality of life [Citation1], with severity inversely associated with quality of life [Citation2], and is also a major driver of the financial burden of MS [Citation4]. Worsening spasticity can negatively impact other concomitant MS symptoms, for example, pain, bladder dysfunction, sleep disorders, and sexual dysfunction, which makes its management particularly relevant and its effective management has great potential to relieve this cluster of inter-related symptoms [Citation1].
As well as non-pharmacological treatments, for example, physiotherapy [Citation5,Citation6], a range of classic pharmacological anti-spasticity treatments can be prescribed to manage MSS [Citation7]. Well-established options include baclofen, benzodiazepines (e.g. diazepam), tizanidine, and dantrolene, all of which are available for over 30 years [Citation7]. Some alternative treatments, for example, tizanidine, not available in all countries and gabapentin (which is not specifically approved for the management of MSS and is mainly indicated for the treatment of neuropathic pain), are also becoming established in clinical practice [Citation7]. However, these treatments are not effective or are intolerable in a proportion of individuals, as reviewed by Beard et al. (2003) [Citation7]. Those who are non-responsive to, or who cannot tolerate these treatments can become eligible for the cannabinoid-based medicine, Sativex® oromucosal spray (GW/Jazz Pharmaceuticals). This option is currently licensed in some, predominantly European countries and despite not being approved in the USA, its United States Adopted Name (USAN), ‘nabiximols,’ has become a widely recognized generic term for Sativex oromucosal spray. Approximately 50–70% [Citation8–10] of individuals with inadequate response to previously prescribed anti-spasticity treatments have been shown to respond to this medicine. Another option for resistant spasticity, which has been shown to be effective, is the intrathecal baclofen subcutaneous pump, requiring a surgical implant of the pump and a catheter [Citation11,Citation12]. Complexity of implant and maintenance, risk of complications including toxicity and withdrawal [Citation13] limit the use of this option and this is typically reserved for the treatment of severe spasticity.
Although some aspects of the treatment of MS are well defined by international guidelines that cover the use of disease modifying drugs (DMDs) [Citation14,Citation15], guidelines for the management of MS-associated symptoms, including spasticity, are generally less well developed. Although guidelines and recommendations exist, they are normally developed for use in individual countries, and there has been no attempt, to date, to assess the degree of similarity or divergence between these different guidelines. Understanding the similarities and differences between available guidelines could facilitate clinical decision-making, highlight areas requiring further clarity and may be helpful in the future development of regional or global guidelines.
The objective of this review was to identify all relevant published European guidelines describing the use of pharmacological anti-spasticity treatments in the management of MSS, and to assess the degree of similarity and divergence between them, with the intention of informing the development of new evidence-based international recommendations to guide the proper use of pharmacological treatments, in the treatment of people with MSS. The focus was on how the use of nabiximols is recommended within the wider context of other anti-spasticity treatments.
2. Methods
A systematic review was carried out to identify national clinical practice guidelines relating to the pharmacological management of MSS, with a focus on, but not limited to the use of nabiximols (Sativex oromucosal spray, GW/Jazz Pharmaceuticals) in MSS. The scope of the review was limited to public domain documents containing guidelines or recommendations, relating to the European countries of interest, namely those in which nabiximols is approved (Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Iceland, Ireland, Italy, Lichtenstein, Luxembourg, Netherlands, Norway, Poland, Portugal, Slovakia, Spain, Sweden, Switzerland, and the UK). The scope of the review was limited in this way for reasons of feasibility and to reflect the widespread regulatory approval of nabiximols in the European region. Our intention was to identify all relevant guidelines in the countries of interest that reflected the current care of people with MSS in those countries. We recognized that such guidelines may be published via a variety of avenues, for example, in an international (global) forum such as a peer-reviewed medical journal, but may also be available from other, national sources, for example, websites of relevant national medical associations and national Health Technology Assessment (HTA) bodies that determine reimbursement for medical treatments. A systematic review therefore searched for these three main streams. To identify relevant published literature, the following search was carried out on PubMed: (‘multiple sclerosis’[Title] AND (‘guideline’[Title] OR ‘recommendation’[Title] OR ‘consensus’[Title])) AND (spasticity). To identify relevant national or regional medical associations, a manual search of websites of national specialist relevant medical associations (those affiliated with the World Federation of Neurology, as identified from https://wfneurology.org/about-us/member-societies) for the countries of interest, was carried out. Finally, the HTA bodies of the European countries of interest were identified from the EU umbrella organization website (EUnetHTA, accessed via: https://www.eunethta.eu/about-eunethta/eunethtanetwork/). A manual search of the identified HTA websites, representing national (governmental) bodies, was carried out. Where multiple HTAs existed in an individual country, all relevant HTA body websites were searched. Documents identified by other means were also reviewed and included if relevant. All searches were carried out between 8 and 11 November 2021.
2.1. Inclusion/exclusion
Inclusion was limited to guidelines that were available in the public domain, relating to generalized MSS, those published within the last 10 years (2011 to 2021, inclusive). For the purposes of this review, ‘national guidelines’ were defined as any documents that included guidelines or recommendations published by national or international medical associations, or by national governmental bodies or in the published literature. Documents were included into the analysis regardless of whether they mentioned Sativex/nabiximols. Guidelines were included regardless of language of publication and with no prejudice with regard to the methodology used to generate them, and guidelines that were not clearly evidence-based and with poorly described methodologies were included along with documents based on high-quality methods. This broad inclusion was designed to capture all information available to medical practitioners in the countries of interest so that a complete understanding of available guidelines could be obtained.
Any published guidelines or recommendations documents, which represented a local, regional or institutional practice, were excluded. Any documents whose scope did not cover MSS were also excluded. Guidelines focussing exclusively on DMDs and guidelines related to spasticity in other conditions (e.g. spinal cord injury), or related only to non-pharmacological management (e.g. physiotherapy) alone, were also out of the scope of this review and were excluded. Where associations or bodies had published ‘update’ documents, the most recent, relevant document was reviewed.
All identified clinical guidelines were reviewed and the information relating to the following categories extracted into a pre-defined spreadsheet: source, country, authority, scope, methodological quality (whether evidence-based; whether developed using a formal methodology), and an overview of pharmacological pathway for treatment of MSS, details of target population and methods recommended for monitoring clinical response to treatment.
Points of divergence and convergence between the guidelines were explored. In particular, pre-specified research questions were explored which related to the following:
What patient pathways/steps relating to the prescription of anti-spasticity treatments are described by the existing guidelines?
For individuals being prescribed nabiximols, what patient characteristics are defined by the guidelines?
Are there definitions of clinical ‘response’ and ‘non-response’ to anti-spasticity treatments provided by the guidelines?
What concurrent treatments (pharmacological and non-pharmacological) are recommended in conjunction with nabiximols?
3. Findings
3.1. Availability of national guidelines
The search yielded 16 relevant documents in total (). Eleven European countries were represented; Austria [Citation16], Belgium [Citation17] (whose national neurological association adopted the guidelines published by the European Academy of Neurology), Czech Republic [Citation18], Denmark [Citation19], Germany [Citation20–22], Italy [Citation23], Netherlands [Citation24], Portugal [Citation25], Spain [Citation26,Citation27], Sweden [Citation28] and the UK [Citation29,Citation30], as well as one additional paper representing three medical associations: the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), the European Neurological Society (ENS), and the European Federation of Neurological Societies [EFNS]) [Citation31]. Details of the identified guidelines are shown in . Guidelines were published by a variety of organizations including HTA bodies (five documents from four countries) [Citation20,Citation25,Citation27,Citation29,Citation30], and medical associations (11 documents; six of which were identified from the website of the relevant association [Citation16–19,Citation21,Citation24] three of which were published as peer reviewed literature [Citation23,Citation26,Citation31] and two of which were identified via other means [Citation22,Citation28]).
Figure 1. Flow Chart.
*Manual search of websites of national specialist relevant medical associations identified from https://wfneurology.org/about-us/member-societies.
**Manual search of websites from national (governmental) HTA bodies that determine reimbursement for medical treatments; limited to European countries where nabiximols (Sativex oromucosal spray) is known to be reimbursed. Relevant HTAs identified from https://www.eunethta.eu/about-eunethta/eunethtanetwork/.
^Two relevant documents from UK's HTA body, NICE, were included.
DM, disease modification; HTA, Health Technology Assessment; NICE, National Institute for Health and Care Excellence; UK, United Kingdom.
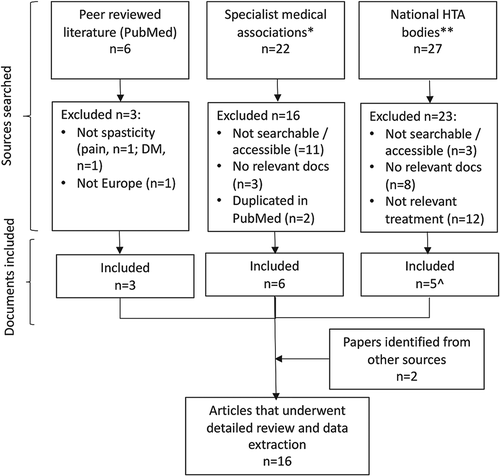
No relevant national guidelines were identified for the following European countries where Sativex oromucosal spray is currently approved: Finland, France, Iceland, Ireland, Lichtenstein, Poland, or Slovakia.
3.2. General approaches to the use of anti-spasticity treatments in MSS
Multiple guidelines acknowledged that MSS itself may be helpful to affected individuals by facilitating mobility and counteracting muscle weakness; treatment is only necessary when spasticity becomes a problem [Citation19,Citation26,Citation27]. Once a decision has been made to treat the spasticity, guidelines often recommend that physicians should define a detailed management plan; this should include both pharmacological and non-pharmacological treatments and should define treatment goals. A personalized approach is recommended to achieve clinical benefits that are meaningful to each individual. This can depend on therapeutic history, personal preferences, specific complications experienced (pain and contractures, for example), the individual’s social and economic situation, as well as other symptoms associated with MSS, such as mobility, fatigue, or depression [Citation16,Citation21,Citation26,Citation28]. Examples of treatment goals include [Citation16,Citation19,Citation22,Citation28]:
Maintaining/achieving independence in everyday life
Improvement of gait and mobility
Reduction of clonus and frequency of spasms
Pain reduction
Facilitation of activities of daily living (e.g. hygiene)
Avoidance of secondary complications (e.g. contractures, pressure ulcers)
Minimization of psychological impact and improvement in quality of life
Both non-pharmacological and pharmacological interventions were widely acknowledged in the available guidelines. Some non-pharmacological interventions included working with the patient to identify triggers for spasticity and developing strategies to minimize these triggers [Citation22,Citation26,Citation28,Citation29]. The most commonly specified non-pharmacological treatment was exercise and physiotherapy, widely acknowledged to be extremely important as first-line interventions [Citation16,Citation17,Citation19,Citation22,Citation26,Citation28] with exercise referral schemes specifically mentioned only in some guidelines [Citation19,Citation21]. This reflects the widespread support for the use of physiotherapy in the management of MSS [Citation5,Citation6]. Although elsewhere there is recognition that other non-pharmacological treatments can be used, it has been acknowledged that more robust evidence and detail of these non-pharmacological treatment modalities is needed [Citation32].
It was acknowledged in some guidelines that if these measures are effective in controlling spasticity, then pharmacological treatment may not be necessary [Citation21,Citation22,Citation26].
3.3. What patient pathways relating to the prescription of anti-spasticity treatments are described by the existing guidelines?
If non-pharmacological approaches (outlined above) are not effective in controlling the spasticity, pharmacological treatment may be necessary. This is typically recommended in addition to the non-pharmacological interventions described above. When initiating pharmacological anti-spasticity treatments, the general approach is to ‘start low, go slow’ [Citation16,Citation19].
Treatment lines or treatment sequencing in the identified guidelines were typically not clear cut. The identified guidelines were reviewed; where a preferential order of treatment was suggested, this was interpreted as treatment lines. Preferred treatment lines were defined, or could be deduced, from 10 of the identified guidelines () [Citation16,Citation19,Citation20,Citation25–31]. In the remaining guidelines, each treatment was considered in isolation and not in the context of a treatment pathway or algorithm.
These treatment lines and sequencing varied between different guidelines; however, some similar themes were apparent.
Firstly, the majority of guidelines (where treatment lines were specified) recommended a range of treatments that can be used as a first-line treatment as monotherapy; this range of treatments always included baclofen and most commonly also included tizanidine. None of the guidelines offered guidance or recommendations on how to choose which of the first-line treatments to prescribe first.
Secondly, all guidelines that presented some element of treatment sequencing recommended the use of nabiximols at some point through the treatment lines, as an add-on treatment, once first-/second-line treatments had failed to provide enough control of spasticity and/or were not well tolerated at the intended therapeutic dose level (). In some guidelines, at least two first-line monotherapy options were recommended before nabiximols was recommended; for example, the German HTA body, G-BA, stipulated that at least two previous attempts to reduce spasticity should have been made, before nabiximols is prescribed, each with different oral anti-spasticity treatments, including at least baclofen or tizanidine, delivered at an optimum dose [Citation20].
Thirdly, most guidelines agreed that intrathecal baclofen should be considered as a final option if all other approaches had failed and if the degree of spasticity was sufficiently severe, thus reserving this option for people with MS who are refractory to any other option and who experience disabling spasticity.
Finally, the majority of guidelines acknowledged the need to switch between treatments if there was a lack of clinical response. However, only a minority of the guidelines formally recommended switching treatment due to lack of tolerability [Citation21,Citation31]. This is not currently aligned with real-world clinical practice where the lack of success of a treatment could be due to lack of tolerability, lack of clinical efficacy or other reasons, any of which could be a valid reason for switching.
Several differences between the guidelines were also apparent. Firstly, the number of options available as recommended first-line treatment varied. Only one guideline [Citation27] recommended baclofen only as an initial treatment with all the others recommending a range of options to baclofen as a first-line treatment, including tizanidine [Citation16,Citation20,Citation25,Citation26], gabapentin (acknowledging that the product is not indicated for spasticity, but for neuropathic pain) [Citation29], or multiple alternative monotherapy options [Citation19,Citation28,Citation31].
Secondly, some guidelines recommended dantrolene or benzodiazepines for the treatment of MSS [Citation28,Citation29,Citation31]. Others did not [Citation16,Citation25]. The reason for this was often unclear. This may be explained in some cases by the fact that benzodiazepines (including those with long-acting properties) are not formally indicated for the management of spasticity in some of European countries, and its use is not evidence-based.
Thirdly, the recommendations in the guidelines varied in terms of how many alternative monotherapies, or combinations of first-line treatments, should be tried before progressing to nabiximols. Nabiximols appeared as early as a second-line treatment [Citation19,Citation26,Citation28] and as late as a fourth-line treatment [Citation30].
Treatment algorithms were presented in five of the identified guidelines, all of which differed in terms of the details presented [Citation16,Citation19,Citation26,Citation28,Citation31]. These algorithms typically described an individual being prescribed either baclofen or another anti-spasticity treatment (most commonly tizanidine), in monotherapy, as a first-line treatment; if this was not successful, it was most commonly recommended that they could be switched to the other of the two options, again in monotherapy [Citation31]. If this also failed, some guidelines [Citation26,Citation29] mentioned that these first-line treatments may be prescribed in combination; no robust evidence supporting this approach was provided. An alternative was to prescribe first-line treatments in combination with nabiximols as an add-on treatment. A composite treatment algorithm that takes all available information from the identified guidelines is shown in , showing points of convergence and divergence between the treatment algorithms currently available in European recommendations.
Figure 2. Composite treatment algorithm showing points of similarity and difference between the recommended treatment of generalized MSS in published national guidelines.
The information in this composite algorithm is based on all of the identified guidelines that described treatment pathways or lines of treatment (as shown in [Citation16,Citation19,Citation20,Citation25,Citation26,Citation27,Citation28,Citation29,Citation30,Citation31]), not only those that formally developed an algorithm. This algorithm is limited to generalized spasticity; elements from the published guidelines that related to focal spasticity have been excluded.
![Figure 2. Composite treatment algorithm showing points of similarity and difference between the recommended treatment of generalized MSS in published national guidelines.The information in this composite algorithm is based on all of the identified guidelines that described treatment pathways or lines of treatment (as shown in Table 1 [Citation16,Citation19,Citation20,Citation25,Citation26,Citation27,Citation28,Citation29,Citation30,Citation31]), not only those that formally developed an algorithm. This algorithm is limited to generalized spasticity; elements from the published guidelines that related to focal spasticity have been excluded.](/cms/asset/964d027c-e364-4cdb-827f-b482fbccbc81/iern_a_2075263_f0002_oc.jpg)
Table 1. Available national/regional guidelines describing clinical practice relating to treatment of MSS.
3.3.1. What is needed
There is a need to investigate the ideal sequencing and combination of interventions so that a definitive treatment algorithm can be developed. An integrative evidence-based approach should be adopted that evaluates the benefits of anti-spasticity treatments in the wider context of previous anti-spasticity treatments and sequencing. Reasons for switching other than lack of effect response, for example lack of tolerability, need to be explored and acknowledged. The inclusion of treatments with only a low level of supporting evidence should be considered more formally; for example, for those without proper supportive studies of MSS (such as dantrolene) or combinations of first-line treatments (e.g. baclofen + tizanidine), the need for development of a clinical consensus or formal clinical investigations should be considered.
Once developed, a definitive treatment algorithm needs to be clinically validated; no treatment algorithms have been validated to date [Citation23].
3.4. How the use of nabiximols is addressed in the guidelines
Despite nabiximols being approved in all of the countries explored in this review, it was not mentioned in two of the identified national guideline documents [Citation18,Citation24]. The reasons for the omission were not clear. In all other identified guidelines, the use of nabiximols was mentioned; however, not all guideline documents provided formal recommendations that related directly to nabiximols. Several guidelines recommended nabiximols to be considered as a second-line option for the treatment of MS-associated spasticity [Citation19,Citation26,Citation28]. Formal recommendations relating to nabiximols (those that are evidence-based and/or were developed using a formal methodology) were presented in four of the guideline documents [Citation17,Citation23,Citation26,Citation31]. These recommendations were based on the efficacy shown in a series of high-quality studies and are shown in .
Table 2. Sativex-related recommendations in the context of Ms-associated spasticity, as provided in the national guidelines.
3.4.1. For people being prescribed nabiximols, what patient characteristics are defined by the guidelines?
The guidelines typically recommended use of nabiximols in-line with the label, where the description of the target population reflects that of the study population of people with MSS enrolled in pivotal studies that informed the original authorization of nabiximols in the EU. In all cases where this is specified, the use of nabiximols is limited to individuals with moderate-to-severe MSS [Citation16,Citation20,Citation25,Citation30] and who have not responded adequately to other anti-spasticity medication [Citation16,Citation25,Citation30,Citation31]. Several guidelines did not specify either details regarding the severity of MSS [Citation19,Citation21,Citation23,Citation26] or previous treatment [Citation19,Citation21,Citation23]. This is despite the fact that there is a clear target population for whom treatment with Sativex is suitable.
Although several papers stipulated that nabiximols were to be used only in individuals with ‘moderate-to-severe spasticity,’ this term was defined in only two of the guidelines reviewed [Citation17,Citation25]. In one guidelines document, the definition of moderate or severe spasticity was defined as having an NRS-S ≥4 [Citation25]. This is consistent with the inclusion criteria of seminal primary publications [Citation8,Citation9] and the product characteristics for nabiximols, as provided in the label for Sativex oromucosal spray. In the second document, the definition of severe spasticity was defined, as constant bilateral support required to walk 20m without resting (extended disability scale score [EDSS] >6.0) or higher, disability. This paper also highlighted that no uniform definition of ‘severe’ MSS exists [Citation17].
Although there was wide consensus that nabiximols should only be prescribed after failed prior anti-spasticity treatments, details of which specific prior treatments were only defined by two guidelines [Citation25,Citation31]. The first guidelines document recommended that nabiximols should only be prescribed in individuals who had already received ‘treatment with two (simultaneous) anti-spasticity drugs, including baclofen and/or oral tizanidine, stable for at least three months, without adequate symptom relief’ [Citation25]. The second guidelines document specified that nabiximols could be used ‘in MS patients with spasticity and a suboptimal therapeutic response or poor tolerance to oral drugs (baclofen, tizanidine, and gabapentin)’ [Citation31].
3.4.1.1. What is needed?
Clarity is required on the specific characteristics of people who are eligible for treatment with nabiximols, specifically:
A better definition of the severity of MSS by using specific and validated and reliable spasticity scales, to help to assign the appropriate treatment to specific individuals.
Clarification of the minimum or optimal number of ‘first-line’ treatments that a patient should have been attempted before nabiximols can be considered.
3.4.2. What definitions of clinical ‘response’ and ‘non-response’ to nabiximols and other anti-spasticity treatments are provided by the guidelines?
As a general observation, the guidelines seldom offered clear definitions of clinical response to any anti-spasticity treatments. Where clear information was provided, this was always related to treatment with nabiximols and never to any other anti-spasticity treatment, meaning that, other than for nabiximols, there was no agreed and recommended criteria by which to assess clinical response to treatment. This is probably because in many countries, continued access to nabiximols, as a cannabinoid-based treatment, is highly regulated according to strict criteria, in a way that is not the case for other treatment types. Currently, treatment switches or initiation of an add-on treatment, like nabiximols, are largely based on the physician and patient’s interpretation of there being a lack of clinical response, which is currently highly subjective and largely undefined by the national guidelines.
The first consideration when measuring clinical response is choosing an appropriate measurement tool to use. The clinical tools and scales used to evaluate spasticity vary in terms of inter-observer reliability, so patient-centered outcomes may be preferred. Only six of the identified guidelines documents [Citation16,Citation21,Citation25,Citation26,Citation28,Citation30] recommended the use of a specific validated measurement tool(s) or technique(s), for measuring spasticity, sometimes recommending multiple options. Recommended tools included:
0–4 modified Ashworth Scale (MAS) [Citation16,Citation21,Citation26,Citation28]
0–10 Numeric Rating Scale for Spasticity (NRS-S) [Citation16, Citation25, Citation26, Citation30]
Tardieu [Citation16,Citation21]
REPAS (MAS-based resistance to passive movement scale) [Citation16,Citation21]
Spasm frequency scale [Citation26]
Spasm frequency [Citation28]
These scales all vary considerably in their usability and application; the MAS requires an explorer to mobilize the patients’ different muscles and rate the resistance to repeated movement on a scale of 0 to 4. The 0–10 NRS-S reflects the own patients’ perception of their muscle rigidity. The Tardieu scale is similar to the MAS scale structure. Spasm frequency and severity add to a broader view of the muscle impairment, but this is less often reported.
In the identified guidelines, the most commonly recommended tools were the NRS-S [Citation16,Citation25,Citation26,Citation30], and the MAS [Citation16,Citation21,Citation26,Citation28] both recommended by four guidelines. Some guidelines recommended several different measurement tools without ranking them, leaving the choice up to the preference of the clinician.
Details about the timepoints at which spasticity should be measured were described in detail by four of the guidelines () [Citation25,Citation26,Citation30,Citation31]. All four publications that specified the timepoint at which response to nabiximols should be assessed recommended an initial four-week timepoint [Citation25,Citation26,Citation30,Citation31] in line with the product label. No guidelines made any specific recommendations about having a periodic reassessment of the safety or efficacy of anti-spasticity treatments although guidelines from the UK made a general recommendation that medication should be reviewed annually after the optimal dose has been reached [Citation29] and guidelines from Germany recommended that the effect of anti-spasticity drugs should be checked ‘regularly’ [Citation22]. For clinical response to be assessed, it is necessary to use a means of measuring the degree of spasticity both before treatment begins and at appropriate timepoints once treatment has commenced; however, the need to establish a pre-treatment baseline was often not stated, despite this being a basic tenet of clinical science.
Table 3. Recommendations relating to the measurement and monitoring of clinical response to nabiximols.
Details of how to interpret changes in the degree of spasticity were provided in three guidelines [Citation25,Citation26,Citation30] (). All agreed that clinical response to treatment with nabiximols should be defined as greater than 20% reduction in spasticity, however only 2/3 stipulated the 0–10 NRS as the specific tool to use [Citation25,Citation30], with the third guidelines document mentioning several measurement options [Citation26]. Although a detailed review of the comparative precision of these measurement tools is outside the scope of this review, in our opinion, whereas a 20% change in the 0–10 NRS score may signify clinical response, a 20% change in other measures of spasticity (e.g. spasm frequency, Penn’s or MAS) may not. Furthermore, the recommended 20% or greater change in NRS-S should only be viewed as an ‘early’ response, which represents the minimum clinically important difference (MCID) [Citation33] and is consistent with the European label of nabiximols (Sativex oromucosal spray). This definition of early clinical response may be a useful benchmark that could be more widely adopted in clinical practice. However, over longer-term treatments, it should be acknowledged a 30% improvement (or greater) in NRS-S from baseline, for example after 12-weeks, is desirable – this has been identified as the ‘clinically important difference (CID) or, threshold for identifying a ‘responder’ [Citation33]. These details, relating to MCID and how this relates to the longer-term clinical response to nabiximols, were lacking from all of the identified guidelines. We believe that guidelines are needed that more clearly define a detailed and standardized approach to help clinicians interpret the efficacy of anti-spasticity treatments. This will drive a wider adoption of the most precise and best validated measurement tool(s).
In terms of a definition of when to stop treatment with nabiximols (with the presumption that patients would be switched to another treatment to manage their symptoms), only two guidelines offered a recommendation [Citation26,Citation31]. The European guidelines [Citation31] recommended that nabiximols be discontinued if there is ‘no significant symptom improvement’ after 4 weeks; the required degree of symptom improvement was not defined or clarified any further. Spanish guidelines were clear that if the threshold for clinical response was not reached at the end of the first 4 weeks of treatment, then nabiximols should be discontinued; for any individual with less than a 20% improvement in spasticity (potentially measured using a variety of measures, see ) discontinuation of treatment with nabiximols would be recommended. No recommendations or criteria were provided to describe the circumstances under which other anti-spasticity treatments would be stopped.
To conclude this section, for nabiximols and only where details were provided, it was recommended to measure clinical response using the NRS-S, where acceptable level of response was defined by a reduction of >20% (denoting an improvement in spasticity) after 4 weeks of treatment (). The guidelines reviewed did not make any recommendations about the measurement and interpretation of clinical response to other anti-spasticity treatments.
3.4.2.1. What is needed
To enable the reliable interpretation of clinical response, guidelines should specify a scale (or scales) to monitor changes in spasticity. Although it was outside the scope of this review to identify and promote one specific scale, it was identified that the ideal scale(s) should be validated, patient-focussed and easy to apply in daily clinical practice (for example, 0–10 NRS-S or spasm count), as opposed to the more complex and less patient-centered options, for example, the MAS. This consistent approach is needed to improve the consistency of reporting outcomes in this area, which are currently highly variable. This monitoring of the response to anti-spasticity treatments should also include the measurement of associated symptoms, such as pain, bladder dysfunction, and sleep disorder. Routine assessments of response to treatment should be expanded to all types of anti-spasticity treatments to improve objectivity. When to assess clinical response is currently under-explored, as is the need for periodic reassessment, guidelines should provide clearer instructions on when to measure response.
3.4.3. What concurrent treatments (pharmacological and non-pharmacological) are recommended by the existing guidelines, in conjunction with nabiximols?
Nabiximols can be used as an ‘add-on’ treatment; however, no information is available to describe which of the classic antispastic treatments or physiotherapy exercises nabiximols can be added to, for greatest effect. The nabiximols clinical trials and daily life practice studies have not shown differences in response after considering some different underlying antispastic treatments to which nabiximols can be added [Citation34]. Conversely, concomitant physiotherapy has been shown to improve the response rates [Citation35].
Furthermore, it is possible that when nabiximols is used as an add-on, it may permit a reduction in dosage of other drugs needed to reduce spasticity and associated symptoms. Most of the guidelines did not stipulate the need for periodic medical reviews that could potentially provide an opportunity to reduce the number or dosage of treatments taken in combination.
3.4.3.1. What is needed?
Further studies of spasticity and associated symptoms polypharmacy and dosage would be helpful to understand the number and types of treatments being given in addition to nabiximols. Periodic medical reviews to fine-tune the dosage of concurrent treatments should also be recommended so that people with MSS can achieve the highest degree of symptom control with the lowest polypharmacy and dosage. Recommendations are needed to define how frequently these reviews take place and what format they should take (e.g. in-person or telephone consultations). Publication of the resulting data would allow analysis and interpretation of how the addition of an anti-spasticity treatment to an existing regime affects longer-term polypharmacy and dose.
3.5. Relationship between guidelines and reimbursement
In Western countries, reimbursement for a new pharmaceutical treatment is most often obtained shortly after its approval. Conversely, the inclusion of a medicine in formal treatment guidelines often only happens years after product approval. In some cases, reimbursement criteria can drive the content of guidelines. For example, the Italian medicines agency (AIFA) requested the e-Registry of all people treated with nabiximols (Sativex oromucosal spray) in an ad-hoc website requiring details about past, failed anti-spasticity medication history, initial response (as per 0–10 NRS-S scale), and continuation of response to gain reimbursement from 2013 to 2020, and this was the context in which the Italian national guidelines publication was developed (2019). In such scenarios, the importance of well conducted, reliable, and targeted HTA assessments is clearly important. The inverse is also true in that the development of robust clinical guidelines (that state which treatments a clinician should use to gain the most clinical benefit) can potentially be used as a persuasive tool to encourage HTA bodies to formally assess treatments that are currently unfunded, with a view to gaining reimbursement and improving access to treatments.
Lack of reimbursement for nabiximols can be a major barrier to its use; in some countries, experts or medical associations may recommend the use of a product based on clinical trial results but without reimbursement, it is unlikely to be of widespread benefit to people with MSS. This is the situation in some European countries currently (e.g. Sweden). In contrast, other countries support the reimbursement of nabiximols following detailed assessments by the national HTA body [Citation25,Citation30]. Harmonization of guidelines between countries may be able to identify treatment regimens most likely to benefit affected individuals, but without widespread reimbursement, differences in the abilities of patients to access treatments are likely to persist. Some guidelines rely extensively on the reimbursement requirements in their own healthcare system; for example, in Austria [Citation16] and Portugal [Citation25] the reimbursement of nabiximols is limited to what is defined as ‘medically justified individual cases’ requiring extra paperwork; but, ultimately, the profile of an eligible individual is the same as the one reflected on the approved label (i.e. people with MS, in order to improve moderate-to-severe spastic symptoms, who are not responding adequately to any other antispastic therapy, and who have observed clinically significant symptom relief through trial of nabiximols).
4. Geographic and global relevance of these findings
This analysis focussed on European guidelines, to reflect the more widespread regulatory approval of nabiximols in this region. Nabiximols is approved in several non-European countries including Israel, Colombia, Brazil, Chile, Peru, Canada, Australia, and New Zealand. To the best of our knowledge, these countries have not yet published any national guidelines covering the clinical use of nabiximols. One published guideline document from the USA recommended that ‘clinicians might offer Sativex oromucosal cannabinoid spray (nabiximols), where available, to reduce symptoms of spasticity, pain, or urinary frequency’ with a recommendation level of B (the second highest level) [Citation36]. This is despite nabiximols not yet being approved in the USA [Citation36]. Comparison of the similarities and differences between European guidelines and those published in other global regions is outside the scope of this review but may form the basis for future investigation.
The findings in this report are relevant to many of these other countries given the similarities in health-care systems and the overall approaches to the management of people with MSS and are of particular relevance in any countries or regions in the process of developing guidelines for this indication.
5. Conclusions
Use of nabiximols in the management of MSS is an important addition to the available therapeutic options and is recommended by relevant European guidelines, however, the details informing its use are not always well reflected by these guidelines. The primary intent of this review was to identify gaps and inconsistencies in existing guidelines and to inform the development of additional evidence-based international recommendations to guide the proper use of nabiximols in the treatment of people with MSS, in the context of a wider treatment pathway.
The guidelines reviewed here often do not make a formal distinction between first-line ‘classic’ antispastic treatments, such as baclofen or tizanidine, and often include medications, which do not have an approved indication for spasticity management (e.g. gabapentin); this makes the guidelines difficult to interpret and adopt rigorously in everyday clinical practice. Decisions to prescribe these treatments are often made on an individual basis, leaving the guidelines largely redundant. While clinical practice tailored toward the individual is a positive thing, this should be done in the context of clear guidance based on evidence and a broad consensus of opinion, which should clearly identify and encourage use of the most effective treatments and guide physicians away from less effective options or those with less supporting or limited data. Thorough assessment of all the clinical evidence was beyond the scope of this analysis but could well form the basis for future development of more detailed guidelines.
We would welcome the amalgamation and update of the existing guidelines by an international panel, validating the final structure and details considering the high-quality published evidence into one broadly applicable guideline, more detailed in its approach to the prescription and monitoring of physiotherapy and anti-spasticity drugs, including nabiximols. This new guideline could fill the gaps in the current guidance as identified in this analysis.
6. Expert opinion
This analysis highlights the similarities and differences between numerous clinical guidelines relating to the use of anti-spasticity treatments and can be used to inform future recommendations. We believe that MSS is currently not recognized or diagnosed early enough and is under-treated, and this would be improved if a clear and detailed set of guidelines were available globally.
In our opinion, based on our clinical experience, individuals who respond poorly to first-line treatments (most commonly baclofen) are likely to respond poorly to the other ‘classic’ treatments listed in the guidelines as first-line options. In clinical practice, we find that time spent rotating patients through several different ‘first-line’ monotherapy options and combinations is not time well spent and that individuals who respond poorly to first-line anti-spasticity treatments may benefit from earlier addition of nabiximols. We consider this to be a proactive approach to the management of MSS.
We carried out this review to support future development of guidelines and to enable a more detailed and standardized approach to the development future of guidelines. The majority of the identified guidelines emphasize treatment discontinuation based primarily on efficacy. This leaves a gap with respect to the management of individuals with tolerability issues who are unable to adhere to the prescribed regimen. We suggest that future clinical guidelines are needed to also specifically identify tolerability, as a valid reason for discontinuing anti-spasticity treatments. To facilitate this, routine and standardized assessments of both response and tolerability to any anti-spasticity treatments should be performed. Future studies in this area should also investigate the reasons and frequency of switching between different anti-spasticity treatments to inform this aspect of the treatment pathway. Add-on treatments potentially offer an advantage, in that they may support the use of lower dosages of the first-line treatment, thus improving tolerability.
We felt that the existing guidelines have several limitations that hamper their clinical utility; they do not answer the following questions:
How long should each treatment be administered before an individual can be reliably classed as a responder/non-responder?
What is the best treatment sequence? How many different treatments should be tried before progressing to nabiximols add-on?
What is the best tool to measure response and how should response (or lack of) be objectively defined?
If a treatment seems to be working, how often should outcomes be reassessed?
We suggest that development of future guidelines should:
Be evidence-based
Follow an established and rigorous methodology for developing guidelines (e.g. GRADE)
Follow formal guidelines for the publication of clinical practice recommendations (e.g. the AGREE guidelines) [Citation37]
Be multi-national in scope
Include definitions of fundamental key terms and scales, such as ‘moderate’ or ‘severe spasticity’; ‘non-response’ and ‘clinical response’
Provide more detail on the practical aspects of prescribing
Be validated via clinical research
We believe that within the next 5 years, we will see a shift toward proactive treatment of spasticity, including earlier use of nabiximols. We also anticipate more standardized monitoring of spasticity and associated symptoms such as pain, muscle cramps, bladder dysfunction, sleep disorders and other MS spasticity-related symptoms.
Article highlights
A systematic review identified 16 guidelines documents that provided recommendations on the pharmacological management of multiple-sclerosis-related spasticity (MSS) throughout Europe.
There were many similarities and differences between these guidelines with scope for consolidation.
The identified guidelines did not address a number of relevant, pre-specified questions that are directly applicable to everyday practice. These included:
How long should each treatment be administered before an individual can be reliably classed as a responder/non-responder?
What is the best way to measure response?
How should response (or lack of response) be defined?
Which is the best treatment sequence? How many different treatments should be tried before progressing to nabiximols add-on?
If a treatment seems to be working, how often should outcomes be reassessed?
In the opinion of the authors, this area would benefit from an update and amalgamation of the existing guidelines by an international panel, using an evidence-based approach, into a single guideline, more detailed in its approach to the initiation, monitoring, and optimization of anti-spasticity drugs in MS. Validation of the final structure and details of these guidelines via clinical research is also needed.
Author contributions
All authors adhered to the ICMJE guidelines for authors and Good Publication Practice guidelines for pharmaceutical companies (GPP3) [Citation38]. F.J. Carod-Artal, C. Gasperini, C. Vila-Silvan, P. Adjamian and M. Bagul, contributed to the analysis and interpretation of the data, critically revising drafts, and have approved the final version. All authors agree to be accountable for all aspects of the work.
Declarations of interest
F.J. Carod-Artal: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that I have previously participated in an advisory board sponsored by GW Pharmaceuticals Ltd, part of Jazz Pharmaceuticals (October 2021).
C. Gasperini: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that I have previously received fee as speaker or advisory board from Almirall (EU commercializer of Sativex oromucosal spray).
C. Vila-Silvan: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that I am a full-time employee of Almirall SA, EU commercializer of Sativex oromucosal spray.
P. Adjamian: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that I am a full-time employee of GW Pharmaceutical Ltd, part of Jazz Pharmaceuticals.
M. Bagul: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that I am a full-time employee of GW Pharmaceutical Ltd, part of Jazz Pharmaceuticals.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Geolocation information
The clinical practice guidelines reviewed in this article all relate to European nations but are believed to be clinically and epidemiologically relevant to other countries who may develop such guidelines in future.
Acknowledgments
Editorial assistance was provided by Helen Farrington, pH Medical Communications Ltd and Jenny Smith, JMS Medical Writing Services Ltd and funded by GW Pharmaceuticals, part of Jazz Pharmaceuticals.
Data availability statement
Findings in this review were all drawn from publicly available information, as referenced within the text of the publication. No additional data repository is necessary.
Additional information
Funding
References
- Fernandez O, Costa-Frossard L, Martínez-Ginés ML, et al. Integrated management of multiple sclerosis spasticity and associated symptoms using the spasticity-plus syndrome concept: results of a structured specialists’ discussion using the workmat(®) methodology. Front Neurol. 2021;12:722801. PubMed PMID: 34646229; PubMed Central PMCID: PMCPMC8503561. eng.
- Rizzo MA, Hadjimichael OC, Preiningerova J, et al. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004Oct;10(5):589–595. PubMed PMID: 15471378; eng.
- Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int J MS Care. 2013 Fall;15(3):146–158. PubMed PMID: 24453777; PubMed Central PMCID: PMCPMC3883021. eng.
- Svensson J, Borg S, Nilsson P. Costs and quality of life in multiple sclerosis patients with spasticity. Acta Neurol Scand. 2014Jan;129(1):13–20. PubMed PMID: 23683163; eng.
- Etoom M, Khraiwesh Y, Lena F, et al. Effectiveness of physiotherapy interventions on spasticity in people with multiple sclerosis: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2018Nov;9711:793–807. PubMed PMID: 29794531; eng.
- Khan F, Amatya B, Bensmail D, et al. Non-pharmacological interventions for spasticity in adults: an overview of systematic reviews. Ann Phys Rehabil Med. 2019Jul;624:265–273. PubMed PMID: 29042299; eng.
- Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7(40):iii, ix–x, 1–111. PubMed PMID: 14636486; eng.
- Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex((®))), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011Sep;189:1122-1131. PubMed PMID: 21362108; eng.
- Markovà J, Essner U, Akmaz B, et al. Sativex(®) as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. 2019Feb;1292:119–128. PubMed PMID: 29792372; eng.
- Patti F, Messina S, Solaro C, et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry. 2016Sep;879:944–951. PubMed PMID: 27160523; PubMed Central PMCID: PMCPMC5013116. eng
- Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989 Jun 8;320(23):1517–1521. PubMed PMID: 2657424; eng.
- Ochs G, Struppler A, Meyerson BA, et al. Intrathecal baclofen for long-term treatment of spasticity: a multi-centre study. J Neurol Neurosurg Psychiatry. 1989Aug;528:933–939. PubMed PMID: 2487035; PubMed Central PMCID: PMCPMC1031830. eng
- Romito JW, Turner ER, Rosener JA, et al. Baclofen therapeutics, toxicity, and withdrawal: a narrative review. SAGE Open Med. 2021;9:20503121211022197. PubMed PMID: 34158937; PubMed Central PMCID: PMCPMC8182184. eng.
- Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. PubMed PMID: 29352526; eng, Eur J Neurol. 2018 Feb;25(2):215–237.
- Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of neurology. Neurology. 2018 Apr 24;90(17):777–788. PubMed PMID: 29686116; eng.
- Baumhackl U, Berger T, E C. ÖMSB (Österreichische Multiple Sklerose Bibliothek): evidenzbasierte Informationen zu Allen Aspekten der MS für Betroffene sowie Ärzte und Ärztinnen und Angehörige medizinischer Gesundheitsberufe [ÖMSB Austrian multiple sclerosis library: evidence-based information on all aspects of MS for those affected as well as doctors, relatives and medical health professionals]. 2020. cited2021 Sept 30]. Available from: https://www.oegn.at/wp-content/uploads/2020/05/OEMSB_2020.pdf
- Solari A, Giordano A, Sastre-Garriga J, et al. EAN guideline on palliative care of people with severe, progressive multiple sclerosis. Eur J Neurol. 2020Aug;278:1510–1529. PubMed PMID: 32469447; eng
- Society CN. Clinical standard for diagnosis and treatment of multiple sclerosis and neurimyelitis optica 2021. [cited 2021 Nov]. Available from: https://www.czech-neuro.cz/archiv/data/M/w/u/0031-rs-doporuceni.pdf
- Rasmussen P, Jensen H. Generelt on behandling af MS-symptomatisk. 2015. [cited 2021 Sept 29]. Available from: https://neuro.dk/wordpress/nnbv/generelt-om-symptomatisk-ms-behandling/
- Bundesausschuss G. [Decision of the Federal Joint Committee: an amendment to the medicines directive (AMRL): annex XII - resolutions on the benefit assessment of drugs with new ones active ingredients according to § 35a sgb v - extract from Cannabis sativa L., folium cum flore (Active ingredient combination from Delta-9-Tetrahydrocannabinol and cannabidiol) ( Reassessment after the deadline). BAnz AT 30112018 B6. 2018.
- Platz T, J. Wissel, E. Donauer. Therapie des spastischen Syndroms, S2k-Leitlinie, 2018, in: deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie. 2021. [cited 2021 Sept 29]. Available from: www.dgn.org/leitlinien
- DGN, KKNMS. Guidelines for diagnosis and therapy of MS. 2014. [cited Jan 2021]. Available from: http://www.kompetenznetz-multiplesklerose.de/wp-content/uploads/2016/02/dgn-kknms_ms-ll_20140813.pdf
- Comi G, Solari A, Leocani L, et al. Italian consensus on treatment of spasticity in multiple sclerosis. Eur J Neurol. 2020Mar;273:445–453. PubMed PMID: 31652369; eng
- Federation of Medical Specialists. [Multiple sclerosis]. 2021. [cited Nov 2021]. Available from: https://richtlijnendatabase.nl/richtlijn/multiple_sclerose_ms/startpagina_-_multiple_sclerose_ms.html
- Infarmed. [Public report on the evaluation of the request for the submission of medicune for human use] Relatoria publico de avaliacao do perido de comparticipacao de medicamento para uso humano: DCI – delta-9-tetrahidrocanabinol (THC PFV) + Canabidiol (CBD PFV). Prep de Fármacos Vegetais, ext Cannabis sativa. 2021. [cited 2021 Sept 30]. Available from: https://www.infarmed.pt/documents/15786/1437513/Relat%C3%B3rio±p%C3%BAblico±de±avalia%C3%A7%C3%A3o±do±medicamento±Sativex±2019/c055642c-92fe-4e84-9da6-f72f3a9c0e06?version=1.0.
- Oreja-Guevara C, Montalban X, de Andrés C, et al. Consensus document on spasticity in patients with multiple sclerosis. Grupo de Enfermedades Desmielinizantes de la Sociedad Española de Neurología. Rev Neurol. 2013 Oct 16;57(8):359–373. PubMed PMID: 24081891; spa.
- (AQuAS) CAfHQaA. Practice guide care clinic to people with multiple sclerosis. 2012. [cited Nov 2021]. Available from: https://aquas.gencat.cat/web/.content/minisite/aquas/publicacions/2012/pdf/gpc_esclerosi_multiple_aiaqs2012ca_completa.pdf
- Svenska MS Sällskapet JL. Spasticitet Metodboken [Spasticity methodbook] 2019. [cited 2021 Sept 30 2021]. Available from: http://www.mssallskapet.se/wp-content/uploads/2019/03/Spasticitet.pdf
- NICE. Multiple sclerosis: management of multiple sclerosis in primary and secondary care. CG186. 2019. [cited 2021 Sept 29]. Available from: www.nice.org.uk/guidance/cg186
- NICE. Cannabis-based medicinal products. NICE guideline NG144. HYPERLINK “C:\Users\User\Desktop\Clients\PH Medical Communica- tions Ltd\GWP042_Sativex guidelines_narrative review www.nice.org.uk\guidance\ng144” 2019. [cited 2021 Sept 30]. Available from: www.nice.org.uk/guidance/ng144
- Otero-Romero S, Sastre-Garriga J, Comi G, et al. Pharmacological management of spasticity in multiple sclerosis: systematic review and consensus paper. Mult Scler. 2016Oct;2211:1386–1396. PubMed PMID: 27207462; eng
- Amatya B, Khan F, La Mantia L, et al. Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database Syst Rev. 2013Feb;282:Cd009974. PubMed PMID: 23450612; eng
- Farrar JT, Troxel AB, Stott C, et al. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008May;30(5):974–985. PubMed PMID: 18555944; eng
- Amjad F, Boster A, Carod Artal J,R, et al. Post hoc analysis of nabiximols efficacy by concomitant medication use in 2 enriched placebo-controlled randomized clinical trials (RCTs). 2022; Presented at ACTRIMS, 2022 Feb 24-26; Florida, [Cited 2022 Mar 8]. Available from: https://www.abstractsonline.com/pp8/#!/10495/presentation/700
- Grimaldi AE, De Giglio L, Haggiag S, et al. The influence of physiotherapy intervention on patients with multiple sclerosis-related spasticity treated with nabiximols (THC:CBD oromucosal spray). PloS one. 2019;14(7):e0219670. PubMed PMID: 31361750; PubMed Central PMCID: PMCPMC6667203. eng.
- Yadav V, Bever C Jr., Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014 Mar 25;82(12):1083–1092. PubMed PMID: 24663230; PubMed Central PMCID: PMCPMC3962995. eng.
- Brouwers MC, Kerkvliet K, Spithoff K. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ (Clin Res Ed). 2016 Mar 8;352:i1152. PubMed PMID: 26957104; PubMed Central PMCID: PMCPMC5118873. eng.
- Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015 Sep 15;163(6):461–464. PubMed PMID: 26259067; eng.
