ABSTRACT
Introduction
Alzheimer’s disease (AD) is characterized by a progressive decline in cognition and daily function, leading to a greater need for caregiver support. Clinical disease is segmented into a preclinical stage, mild cognitive impairment, and mild, moderate, and severe stages of Alzheimer’s dementia. Although AD trials enroll participants at various stages of illness, treatment efficacy is often assessed using endpoints based on measures of outcomes that are held fixed across disease stages. We hypothesize that matching the primary outcomes measured in the endpoint hierarchy to the stage of disease targeted by the trial will increase the likelihood of detecting true treatment benefits.
Areas covered
We discuss current approaches to assessing clinical outcomes in AD trials, followed by a consideration of how effect detection can be improved by linking the stage of AD to the endpoints that most likely reflect stage-specific disease progression.
Expert opinion
Failing to account for stage-specific relevance and sensitivity of clinical outcomes may be one factor that contributes to trial failures in AD. Given the history of failure, experts have begun to scrutinize the relevance and sensitivity of outcomes as a potentially modifiable barrier to successful trials. To this end, we present a framework for refining trial endpoint selection and evaluation.
1. Introduction
1.1. Alzheimer’s disease
Dementia is a chronic progressive syndrome characterized by deterioration in cognitive function associated with functional decline beyond what is expected from normal aging. Alzheimer’s disease (AD) accounts for 60–80% of all cases of dementia in older adults. Estimated 5.8 million people in the USA and 9.7 million people in Europe are currently living with AD and other forms of dementia [Citation1,Citation2].
AD is a neurodegenerative disease characterized by the accumulation of beta-amyloid (Aß) in the form of plaques, and the accumulation of hyperphosphorylated tau proteins in the form of neurofibrillary tangles, ultimately leading to neurodegeneration as manifested by progressive neuronal loss and cerebral atrophy [Citation3]. The pathologic changes of AD usually unfold in a predictable fashion, first affecting medial temporal structures, leading to memory decline [Citation4]. As AD pathology progresses, corresponding deficits in other cognitive domains emerge, often executive dysfunction followed by verbal disability. Cognitive ability is tracked through objective measures, including neuropsychological batteries, and subjective measures, including patient self-reported or informant/caregiver reported scales that assess changes in cognition, functional capacities (e.g. driving and finance management), quality of life (QoL), and mood.
While the field has increasingly recognized that the AD stage may influence treatment efficacy, the primary efforts have focused on moving to earlier stages (e.g. preclinical AD) in hopes that earlier intervention will facilitate successful disease modification. However, the outcomes measured in endpoint hierarchies (e.g. global cognition and function) are often not targeted to the stage of illness being studied. While many studies have succeeded in demonstrating group differences in biomarkers of AD, such as positron emission tomography (PET) and cerebrospinal fluid (CSF) based measures of amyloid and tau, progress has been slow in the evaluation of clinical outcomes, specifically in the use and development of clinically relevant measures of cognitive and functional status. The use of cognitive composites may improve the reliability of measurement but may not improve sensitivity to within-person change or to treatment effects, as discussed below.
1.1.1. Key terminology and aims
In this manuscript, we distinguish among outcomes, measures, and endpoints, with specific emphasis on linking all three to the stage of disease. The outcome is defined as the construct of interest (e.g. memory), while the measure is a specific instrument assessing the construct (e.g. the Free and Cued Selective Reminding Test of memory [FCSRT]) and the endpoint, which is the event/outcome that can be measured objectively to determine the impact of an intervention (e.g. change from baseline to the 18-month follow-up visit in the free recall score on active treatment vs. placebo) [Citation5].
We recommend a three-step process for trial design and endpoint selection. First, select a targeted stage of illness. Second, determine which outcomes are of the greatest clinical relevance to the targeted stage of illness. This is based on several factors, including the outcomes that matter the most to stakeholders, the outcomes most likely to change over the course of a clinical trial (say 12–36 months) in the absence of treatment, and, in part, based on outcomes most likely to be sensitive to treatment effects. The third step is to select the best available measure(s) for each outcome of interest. ‘Best’ is defined according to reliability, validity, and stage-specific sensitivity to change. This manuscript is focused on the first two steps of this process. Specifically, we link concept relevance and responsiveness to the AD stage and propose stage-specific employment of outcomes to maximize the sensitivity of measures/instruments used in defining endpoints. For example, memory is a construct that may be particularly relevant in preclinical AD, as memory decline has been shown to accelerate 7 years prior to dementia diagnosis, and several years prior to the diagnosis of mild cognitive impairment (MCI) [Citation6].
Here, we first present an overview of AD clinical staging. Second, we review the characteristic features of the clinical stages of AD. Third, we outline existing models that operationalize clinical AD staging definitions. Fourth, we link outcomes of import to AD stage and propose a hierarchy for relevance and sensitivity of outcomes by AD stage. This fourth section is divided into specific subsections: Cognition, Activities of Daily Living (ADLs), QoL, Psychological and Behavioral Problems, and Caregiver Support and Burden. The manuscript then presents the conclusions and clinical expert opinion.
2. Clinical stages of AD
AD typically progresses through well-defined stages characterized by stage-specific symptoms [Citation7–10]. In the preclinical phase, individuals appear cognitively normal without clinical symptoms, although there are measurable differences in the rates of decline in memory starting years before the diagnosis of dementia. In the preclinical stage, Aß, or both Aß and Tau, pathology are revealed by CSF studies, PET, and more recently by emerging blood-based biomarkers [Citation7,Citation11,Citation12]. For people on a trajectory to AD dementia, memory decline accelerates several years before the development of the next stage, often termed MCI. Broadly, MCI is characterized by cognitive impairment in one or more cognitive domains, including episodic memory, executive function, or verbal function, with little or no impact on independence in daily life [Citation13]. MCI has several operational definitions; Petersen and colleagues distinguish amnestic and non-amnestic MCI defined by performance that is usually 1.5 standard deviations below the mean of a normative reference group [Citation14–16]. Jak and Bondi do not distinguish MCI subtypes based on domains of cognitive impairment [Citation17]. The annual rate of conversion from MCI to overt dementia is 10% to 15% [Citation18–20]. In addition, persons with MCI back transition to normal cognitive performance at the rate of 10–40% per year; factors associated with back transition include transient effects on cognitive performance such as systemic illness, medications with cognitive toxicity or a poor night’s sleep prior to testing [Citation21]. Moreover, transitions from just below to just above a cut-score may occur due to day-to-day variation in cognitive performance.
AD dementia is categorized into mild, moderate, and severe stages, with characteristic progression across those stages. In mild AD, people experience more widespread cognitive decline and reduced functional independence in instrumental ADLs (IADLs). Individuals may require assistance for activities such as managing finances and medication, driving, meal preparation, and home maintenance [Citation22]. Mild AD also presents with changes in mood, increased anxiety, and significant reductions in facets of QoL [Citation10,Citation23]. Continued and substantial cognitive decline characterizes moderate AD; dysphasia often advances and problem-solving regresses to an elemental level [Citation24]. Functional autonomy is lost, whereby individuals need assistance with basic ADLs (BADLs) such as eating, personal hygiene, dressing, and toileting [Citation25]. People with moderate AD can also experience psychosis, sleep disturbance and wandering [Citation24,Citation26]. In the severe stage of disease, individuals likely require considerable levels of care and may experience weight loss, dysphagia, and motor impairment [Citation27,Citation28].
The well-documented stage-specific progression of clinical outcomes supports the hypothesis that no single outcome (e.g. global cognition) is optimal for assessing treatment response across all stages of disease. Matching the outcomes and endpoints to the stage of illness may improve sensitivity to detecting true treatment effects. For example, caregiver burden is likely to be relatively low in the MCI and early AD phases; therefore, measures of caregiver burden may be relatively insensitive to treatment benefits during these stages. The disconnect between AD stage and selection of relevant outcomes and subsequent measures used to define trial endpoints may be one of the many factors that contribute to the abandonment or failure of 200+ clinical programs over the last decade [Citation29,Citation30]. Prior to 2003, only five therapies had been approved for the treatment of AD, all of which showed only a modest symptomatic improvement [Citation31]. Efforts to develop a disease-modifying therapy (DMT) have been largely unsuccessful; only one DMT (aducanumab) has been approved by the Food and Drug Administration (FDA) since 2003, though there are other treatments in late-stage development [Citation32]. Due to uncertainty of the true clinical benefit, reimbursement status for aducanumab in the USA is currently limited to patients enrolled in qualifying clinical trials [Citation33].
Past trial failures have been attributed to 1) diagnostic inaccuracy of patients enrolled into trials stemming from the use of clinical eligibility criteria in the absence of biomarkers, though this has been addressed by broad use of AD biomarkers; 2) inadequate understanding of the heterogeneity of AD that could include patient types more or less likely to decline in the absence of treatment or to respond to particular treatments [Citation34]; 3) inappropriate selection of therapeutic target or dosing regimen; and 4) repeated selection of outcomes and endpoints that are suboptimal for the stage of illness under investigation [Citation35,Citation36]. If the goal of treatment is to prevent further progression and the selected outcome(s) is incapable of reflecting progression in the targeted stage of illness, detecting treatment-related delays in clinically meaningful deterioration can be impossible.
Contemporary trials evaluating the effectiveness of anti-amyloid and/or anti-Tau therapies in AD often include a measure of cognition, or a composite measure of cognition, and daily function as primary endpoints; this is consistent with the current recommendations made by the FDA [Citation37–39]. Various supplementary outcomes (e.g. QoL, caregiver burden, and neuropsychiatric symptoms [NPS]) are often included as key secondary endpoints. We suggest that the outcomes selected for measurement in the endpoint hierarchy of contemporary trials may not be fully optimized for the stage of AD illness under study. Whereby outcomes predicted to become impaired in the relevant stages of disease are not appropriately selected, measured, or tested [Citation40]. For instance, BADLs are comprised of fundamental skills needed to manage very basic physical needs. The performance of BADLs is typically impacted during the later stages of disease (i.e. moderate to severe) [Citation41]. Therefore, arguably, the measurement of BADLs should be prioritized when selecting an endpoint aimed at assessing the impact of treatment on daily function in patients with moderate-to-severe disease. The use of a BADL endpoint(s) would be expected to present with uniformly high function in early AD and therefore is of little relevance when evaluating DMTs in early disease. If the outcome is not known from epidemiologic studies to change in a certain stage of disease, then that outcome is not a logical endpoint candidate for DMTs.
3. Biological AD staging models
Criteria for AD diagnosis and staging have evolved. The 1984 criteria for clinical diagnosis of AD focused on the overt dementia phase only and on deficits in cognitive ability and functional status as the central characteristics of AD; the absence of other explanations for cognitive impairment was part of the diagnostic criteria. At this time, definitive diagnosis based on biology was possible only through brain biopsy, which was almost never used, or brain autopsy [Citation42]. The emergence of biomarkers as indicators of underlying AD pathology motivated the development of revised staging criteria and diagnostic guidelines. In 2011, the National Institute on Aging and Alzheimer’s Association (NIA-AA) published research guidelines for the continuum from preclinical to dementia [Citation9,Citation43]. Imaging and CSF biomarker evidence was also integrated into the diagnostic formulations for use in research settings, bolstering the validity of preclinical AD and supporting a direct link between AD pathologic changes and cognitive impairment, improving diagnostic specificity.
More recent guidelines refined the biological definition of AD. In the NIA-AA 2018 research framework [Citation44], Aβ and pathologic tau biomarkers define AD disease status, whereas neurodegenerative/neuronal injury biomarkers and cognitive symptoms characterize the entire spectrum and stage-specific disease severity [Citation44]. The 2018 framework also proposed a six-stage clinical progression model to supplement the biomarker profiles for interventional trials, where each stage is characterized by the progressive severity of clinical symptoms. Contemporary randomized controlled trials (RCTs) often employ instruments such as the Mini-Mental State Examination (MMSE) or Clinical Dementia Rating Scale (CDR) global alongside biomarker evidence to classify disease stage, whereby score-based norms are applied to categorize participants into a stage of disease [Citation45–47].
4. Linking disease stage to the most relevant clinical outcomes
Substantial advances in AD staging have not been fully incorporated into the selection of stage-specific primary and secondary outcomes and trial endpoints. For example, changes in global cognitive function are often used as a primary endpoint, whereby the same assessment criterion or score is employed across different disease stages. Cognition is a complex, multi-faceted construct encompassing memory, executive function, problem-solving, and reasoning. Recent evidence suggests these facets of cognition are not uniformly impaired across the clinical stages of AD.
In addition to cognition, the key outcomes frequently evaluated as endpoints in randomized trials include daily function (i.e. ADLs), QoL, psychological and behavioral problems, and caregiver support and burden. We summarize evidence on the ‘typical’ progression of each of these key clinical outcomes and the relevance of changes in those outcomes to disease stage. Note that while we neither agree nor disagree with the precision of disease staging, given that these discrete boundaries are routinely applied to the disease continuum within the field, and trials will stratify in such a manner, we simply advocate linking endpoints to stage. We then present a theoretical endpoint framework linking the AD stage to the outcomes that are hypothesized to maximize detection of changes in patient status and sensitivity to treatment within the associated stages.
4.1. Cognition
There is a relatively predictable progression of specific cognitive loss beginning in the preclinical AD stage, with impaired memory function that is noticeable yet subtle or, with impaired decision-making or judgment [Citation43]. Episodic memory loss, often observed in MCI, is characterized by difficulty recalling personal experiences and recent or past events [Citation48]. Episodic memory impairment is usually followed by subtle executive dysfunction (e.g. failures on tests such as response inhibition and dual tasking) and initial deficits in perceptual speed or in abstract thinking [Citation49,Citation50]. Devan and colleagues found that low scores on tests of episodic memory (delayed recall), semantic fluency (category naming for animals), processing speed (the Wechsler Adult Intelligence Scale-Revised [WAIS-R] [Citation51]; Digit Symbol), and judgment (Picture Arrangement) predicted transition to mild AD 2–3 years later [Citation52]. A considerable amount of literature demonstrates that performance on tests of episodic memory show accelerated decline about 7 years prior to the diagnosis of dementia and that executive dysfunction shows accelerated decline about 3 years before diagnosis on average [Citation6,Citation53].
The transition to mild AD is characterized by cognitive impairment in key domains that are sufficiently severe to limit daily functional activities. In mild AD, cognitive dysfunction expands from deficits in episodic memory to at least one other domain, such as executive function, language, or visuospatial ability. In contrast to MCI, mild AD occurs with significant deficits in orientation to place and time, as well as in planning and executing a sequence of actions. Dysphasia may become more pronounced [Citation54]. Progression to moderate and severe AD is marked by a substantial failure in memory systems, a loss of sense or awareness of time relationships, and progression to aphasia with minimal ability to communicate [Citation24]. Associated processes such as judgment and problem-solving, both verbal and visual, are reduced to a basic level.
Onset and progression from MCI to mild AD and beyond is mediated by a sequential loss of ability in specific facets of cognition with an increasing impact on ability to manage independently. Therefore, a change in global cognitive performance is unlikely to be an optimal primary endpoint for all stages of disease. We recommend selecting trial endpoints from among the set of cognitive facets that are most relevant and responsive to the stage of illness of eligible participants defining the trial population. Outcome measures characterizing these facets of cognition should be selected on the basis that the content of the instrument items/domains is consistent with the facets of cognition that are clinically relevant to the stage of disease under study.
Changes from baselines in the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) total score is routinely used as a primary endpoint in RCTs, including trials targeting the MCI stage [Citation55]. Not all facets of cognition are impaired in early MCI; accordingly, some tasks included in the ADAS-Cog are more clinically relevant than others during this stage of disease. A recent study using item response theory analyses indicates that only 4 of 11 tasks are informative for assessing cognitive function in MCI: word recall (2 tasks), word recognition (1 task), and self-awareness (1 task) [Citation54]. This demonstrates the importance of selecting an endpoint that incorporates the most clinically relevant outcomes to the stage of disease under study. This, in turn, will subsequently maximize the sensitivity of the chosen instrument to measure clinically relevant changes.
In the context of an RCT, therefore, we posit that outcomes of memory, learning, and executive function are likely to be relevant and sensitive facets within an MCI population. Endpoints evaluating changes in these outcomes would be expected to reflect the efficacy of an effective DMT targeted to early stages. Ideal instruments measuring these facets will separately assess memory storage and retrieval (like the FCSRT) [Citation6,Citation56] or word recall and recognition (e.g. California Verbal Learning Tests and Card Rotation Test).
The facets of memory, executive function, language, visuospatial ability, as well as global cognition are likely to be optimal endpoints for mild to severe AD, as dysfunction occurs across multiple facets of cognition at these stages of disease. To improve the reliability and range of coverage across a broad spectrum of cognitive ability, several cognitive composites have been suggested, as exemplified by the Preclinical Alzheimer’s Cognitive Composite 5 [PACC5] and Neuropsychological Test Battery [NTB] [Citation57–60]. These composites Z-score transform and average scores on multiple cognitive tests. One disadvantage of this approach may be that the tests, which are averaged may differ in sensitivity across stages of overt dementia. Therefore, attention must still be paid to the selection of these tests in relation to the stage of disease. It is important to note that variation within-person across cognitive domains has been shown to predict incident dementia, even after taking the level of cognitive performance into account. This demonstration that early disease variability among cognitive domains predicts dementia indicates that composite measures may obscure domain differences of clinical relevance [Citation61].
4.2. Activities of daily living (ADLs)
Impairment of ADLs is typically a consequence of noticeable progressive cognitive impairment. The decline in AD-mediated cognitive abilities precedes and predicts the onset of functional (i.e. ADLs) impairment [Citation62]. The progression of functional impairment is also thought to be mediated by the development of NPS [Citation63]. IADLs and BADLs are the dominant outcomes used in AD trials to evaluate ADLs. IADLs encompass assessments in the management of finances and medication, driving, meal preparation, household chores, and shopping, while BADLs assess the ability to bathe, dress, toilet, and eat independently [Citation64]. The performance of these ADLs varies in the level of cognitive load required to complete the task, and, as such, different ADLs are subject to impairment at different stages of AD. In general, IADL tasks require greater cognitive function compared with BADL tasks; therefore, the performance of IADLs is sensitive to the initial cognitive decline that occurs in the earlier AD stages. Limited evidence suggests subtle deficits in IADLs can occur in MCI, specifically in more complex activities (i.e. managing paperwork) [Citation65]. Yet, this finding varies with patient age and the instruments used [Citation66,Citation67].
Significant impairment in IADLs is associated with the transition from MCI to mild AD. Advancing cognitive dysfunction (i.e. memory loss, executive dysfunction, and reduced visuospatial processing) mediates the reduced ability to independently handle finances and medication, use the telephone and household appliances, and schedule appointments [Citation62,Citation68]. Depression has also been found to predict erosion in the performance of IADLs [Citation69]. Conversely, physical functioning is considered to be a key driver of basic ADLs; and as such, erosion of BADLs is most common in later disease stages [Citation70]. There appears to be some overlap in IADL and BADL loss as dementia progresses through the mild-to-moderate stages. On average, caregivers report that patients’ loss of the ability to independently bathe and dress precedes the inability to manage toileting, eating, and grooming [Citation71,Citation72]. Autonomy for eating, toileting, and ambulation is often observed prior to the onset of moderate-to-severe disease stages.
Alongside cognition, functional impairment is detailed in regulatory guidance as an important outcome for evaluation as efficacy endpoints in AD trials [Citation37,Citation73]. Similar to cognition, function is a multi-faceted construct for which a sequential loss of ability to independently perform IADLs and BADLs is generally observed. Therefore, change in global function is not an optimal primary endpoint for all stages of disease. When selecting an endpoint(s) to evaluate the treatment effect on the outcome of functional ability, we encourage researchers to consider which specific facets of ADLs are most clinically relevant to the stage of disease under study. In the context of an RCT, we recommend that endpoints of IADL ability are likely clinically relevant in late MCI to mild AD populations. The selected instrument measuring this facet should include items/domains that capture IADL tasks (e.g. Alzheimer’s Disease Cooperative Study Activities of Daily Living – Mild Cognitive Impairment [ADCS-ADL-MCI] or the Functional Activities Questionnaire [FAQ]) [Citation74,Citation75]. Using a composite measure of both IADLs and BADLs (e.g. ADCS-ADL) as an endpoint would feasibly introduce measurement insensitivity at the MCI and mild AD stages [Citation76]. In contrast, endpoints of global function (i.e. items of IADLs and BADLs) are likely to be of greater clinical relevance for moderate-to-severe AD stages.
4.3. Quality of life (QoL)
QoL represents a subjective expression of general well-being and can directly or indirectly encompass elements of cognitive functioning, ADLs, social behavior, emotions, and mood. Cognitive deterioration, functional impairment, and depression appear to be strongly associated with a diminished QoL in people with AD [Citation77,Citation78]. Reductions in QoL begin early, often expressed among those with MCI prior to AD diagnosis [Citation79,Citation80]. Individuals with MCI have reported worse general health, lower vitality, and greater mood disturbances [Citation81]. In addition, MCI-status has been associated with reduced social participation and intimacy [Citation82]. Transition to mild AD brings further decrements in overall QoL; specifically, facets of QoL, such as overall life satisfaction and reduced autonomy in daily activities, are of greater relevance at this stage due to the development of significant functional impairment [Citation83].
Due to the subjective nature of QoL, it is generally agreed that any evaluation of this concept should aim to include the perception of the individual under study. However, people with AD tend to demonstrate a decreased self-awareness of their deficits, otherwise known as anosognosia [Citation84,Citation85]. People transitioning to the moderate-to-severe stages of dementia are often too impaired to accurately complete the subjective ratings themselves. This makes evaluating changes in QoL in AD populations with significant impairment psychometrically unreliable and invalid, and such measurement challenges functionally preclude evaluation of patient reported QoL beyond early AD stages. One problematic solution to this measurement issue has been the use of proxy ratings (i.e. relatives or caregivers) of QoL in later stages of AD; however, proxy appraisals can be heavily influenced by the burden and psychological well-being of the proxy reporter [Citation86]. As such, proxy rating should not be considered as a substitute for patient reporting. Given this consideration and the period of maximal QoL erosion in early AD stages, evaluating the outcome of patient reported QoL as an endpoint in AD trials would only be logical in preclinical, MCI, and mild AD stages.
Taken together, these studies indicate that the outcome of patient reported QoL is likely only relevant and sensitive during transition from normal to MCI or from MCI to mild AD. Therefore, a DMT expected to succeed early in AD would likely demonstrate a lower rate of QoL erosion in the period from preclinical to mild AD, with the comparator associated with greater erosion in QoL over the same period. The selected instrument should include items/domains of key facets of QoL such as vitality, autonomy, social participation, and life satisfaction, which are relevant to early-stage disease. For example, the Dementia Quality of Life (DEMQOL) and Quality of Life in Alzheimer’s Disease (QoL-AD) measures assess variations of these outcomes and are validated for use in AD populations [Citation87,Citation88]. It must be noted that these tools were developed for use in overt dementia populations, and therefore, their utility in preclinical or MCI populations may be limited. Efforts have been made to develop novel QoL tools for use in MCI (e.g. Mild Cognitive Impairment Questionnaire; MCQ [Citation89]); however, more research is needed.
4.4. Psychological and behavioural problems
A majority of persons with AD will develop one or more NPS, such as depression, anxiety, apathy, hallucinations, delusion, social or sexual disinhibition, sleep disturbances, aggression, or agitation, any of which have a profound impact on both the patient and caregiver(s) QoL [Citation90,Citation91]. These symptoms are recognized as key components of AD and are often expressed to varying degrees throughout the disease course [Citation91]. Some symptoms manifest during MCI and progress in parallel with deterioration in cognitive functioning [Citation92]; perhaps in part because preclinical AD pathogenic processes (e.g. amyloid metabolism, tau hyperphosphorylation, and mitochondrial dysfunction) are shared with some NPS [Citation93,Citation94]. Evidence suggests that NPS is an expression of underlying neurodegenerative brain disease and not solely a patient’s secondary response to their disease.
Depressive symptoms are found to precede and predict the onset of MCI and are associated with reductions in hippocampal volume [Citation95]. In MCI, the most common and impactful symptoms include depression, anxiety, irritability, apathy, and sleep disturbances [Citation96]. Depression is the most frequently reported NPS in AD and is also associated with cognitive decline and an increased risk of progression to overt dementia [Citation93,Citation97,Citation98]. The transition from MCI to AD is characterized by the possible presence of additional NPS such as hallucinations, delusions, disinhibition, euphoria, appetite/eating disorders, aberrant motor behavior, and agitation [Citation99]. These additional symptoms are more commonly observed in mild AD onwards; the temporal shift in symptomology being potentially mediated by the progressive decline in cognition, functional autonomy, and motor skills. For example, it has been found that psychotic symptoms (delusions, hallucinations) and agitation are strongly associated with accelerated functional impairment (both IADLs and BADLs), greater caregiver burden, and a reduced ability to communicate needs [Citation100,Citation101].
The proposed staging framework would predict that evaluating changes in NPS outcomes should consider which specific outcomes are predicted to be relevant at the stage of disease under study. In the context of an RCT, we posit that endpoints focusing on oncomes of depression, anxiety, irritability, apathy, and sleep disturbances are likely to be relevant and sensitive within MCI populations. As such, employing specific neuropsychiatric assessment scales aimed at measuring these relevant outcomes is likely ideal. For example, the Cornell Scale for Depression in Dementia (CSDD [Citation102]) to measure depression. It must be noted that the availability of dementia-specific depression scales or general NPS tools developed specifically for MCI is very limited, and therefore, should be a focus of future research to ensure NPS outcomes are being optimally evaluated. Within mild AD to severe AD stages, the NPS outcomes relevant and sensitive to MCI remain clinically relevant and sensitive. In addition, outcomes of psychotic symptoms, appetite/eating disorders, aberrant motor behavior, and agitation are clinically relevant and, therefore, would also be useful to consider as measured endpoints. Measures that include items/domains that capture a more comprehensive range of NPS outcomes are likely to be more clinically relevant and sensitive to change at the mild-to-moderate disease stages. For example, the Neuropsychiatric Inventory (NPI) and Behavioural Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD) [Citation103,Citation104].
4.5. Caregiver support and burden
Relief from burden is a persistent unmet need for those giving care to persons with AD. Caregiver burden is associated with the following risks to persons with AD: premature institutionalization, abuse, or reduced social and familial engagements [Citation105]. There is some evidence that suggests that individuals with MCI may require some level of assistance and support from family members or professional caregivers [Citation106]. However, as the disease becomes more prominent and restrictive, the more time the caregiver spends providing support, and subsequently, the greater the burden [Citation107]. The onset of significant loss of ADLs and/or NPS such as depression, psychosis, and aggression in the person with AD generally are contributing precursors to caregiver burden onset [Citation108]. Accordingly, it has been argued that the evaluation of caregiver burden in AD is optimal when the patient population progresses through the mild to severe stages of disease.
Given that caregiver burden is experienced across the AD stage continuum, it is paramount to collect this information as part of interventional trials aimed at all stages of AD. However, the significant relationship between caregiver burden and disease-related impairment indicates that this outcome is expected to be more clinically relevant and sensitive in the later stages of disease. In the context of AD RCTs, effective treatments would be expected to delay the onset of caregiver burden or maintain the level of caregiver burden without further progression. Instruments measuring caregiver burden in AD include the Caregiver-Perceived Burden Questionnaire (CPBQ) and the Zarit Burden Interview (ZBI) [Citation109,Citation110].
5. Conclusion
No single outcome or measurement of an outcome is universally optimal as an endpoint for assessing treatment response at every stage of AD. By looking at the pattern of progression of key clinical outcomes (i.e. cognition, QoL, and daily function) in relation to the AD stage continuum, we hypothesize that the most relevant and sensitive endpoints will be based on outcomes that erode as a function of disease within and across the specific AD stages enrolled into a given RCT.
We propose that there is a need for an operational framework that connects the disease stage to the most relevant and sensitive clinical outcomes for applications in research in a particular stage or set of stages. Based on a review of the literature and ratification of findings with expert clinical opinion, summarizes our proposed staging framework for optimizing the evaluation of outcomes in AD trials. This framework explicitly links key outcomes (e.g. facets of cognition) with different stages along the AD continuum to guide endpoint selection.
Figure 1. Endpoint staging framework in AD: linking outcome to the most clinically relevant stage of disease.
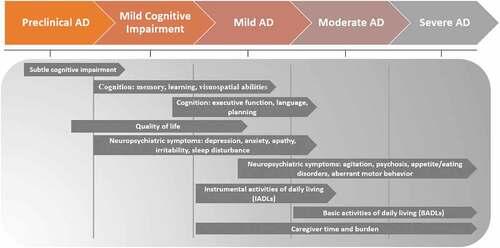
In contemporary trial designs [Citation111], the clinical endpoints frequently include total scale scores of ‘de facto’ ‘Gold standard’ outcome measures (e.g. ADAS-Cog total score); these endpoints are often held fixed across the trial population regardless of disease stage. We have demonstrated that this approach does not comport with the natural progression of AD and, therefore, is suboptimal for assessing the true treatment effect of DMTs in interventional studies. This staging framework () was developed to provide guidance on the selection of optimal trial endpoints specific to the stage of disease under study. By selecting endpoints based on outcomes and measures that are clinically relevant to the target stage of disease, we argue that measurement sensitivity and specificity will be increased. However, it should be noted that doing so could introduce heterogeneity in endpoints across trials, thereby confounding research synthesis. The tension between endpoint precision and research synthesis, however, could easily be resolved by introducing an endpoint random effect in meta-analytic models. This would enable research synthesis while preserving stage-specific endpoint sensitivity and precision.
Furthermore, this staging framework can guide hypothesis-driven primary and post-hoc analysis of trial endpoints. Specifically, this framework could be employed within two common analytical approaches: stratification and covariates. Studies that enroll patients at various stages of disease often evaluate treatment effects in the total sample and within pre-defined stratified subgroups (i.e. MCI vs. Mild AD; Mild AD vs. Moderate AD). This framework can be implemented to identify the appropriate target disease stage(s) for which to evaluate the proposed endpoints in, based on clinical relevance and sensitivity to change in the outcomes of interest. This approach requires a sufficient sample size to ensure that stratified estimates of efficacy are robust; if this cannot be achieved, conducting the analysis by adjusting for relevant covariates is a more powerful statistical approach. Staging covariates can be defined by using the framework to pre-specify a target disease stage (i.e. the stage predicted to be sensitive to a given endpoint) and a reference disease stage (i.e. all stages predicted not to be sensitive to a given endpoint). This enables researchers to appropriately account for the effect of the disease stage on the magnitude of treatment effect.
Given that most of the endpoints considered are various clinical outcome assessments (COAs), including, but not limited to, patient-reported outcomes (PROs) clinician-reported outcomes (ClinRO), observer-reported outcomes (ObsRO), or performance outcomes (PerfO) and that the context of endpoint deployment we have focussed on is RCTs, it is only logical to link this proposed framework to existing regulatory frameworks. In the case of such COAs, the FDA has established a regulatory framework for confirming that endpoints are appropriate for evaluating the planned effect within the population of interest. As outlined in Draft guidance 3 [Citation112], the division of COAs (DCOA) state COAs are appropriate endpoints if they are demonstrated to be fit-for-purpose in a given context of use (COU). Broadly, fit-for-purpose means that the COA has both content and psychometrically validity and responsiveness; and COU means the planned or intended population in which the COA will be deployed, and the effect of intervention evaluated. The proposed staging framework outlined here (See ) is consistent with this regulatory guidance. In our proposed framework, we would maintain the regulatory definition of fit-for-purpose, though the definition of COU would shift to the specific stage of AD in which the endpoints will be evaluated. Therefore, we view our proposed staging framework as a logical extension of the framework established by DCOA for any COA endpoint.
This is a theoretical research framework; it is not to be implemented into clinical practice as a diagnostic criterion or guideline. Moreover, it was developed based on a series of well-supported hypotheses, but these hypotheses need to be tested in application to establish the practical value of the framework. The proposed framework can be operationalized in both observational cohort studies and interventional trials. In a forthcoming piece of post-hoc analysis work, we test the hypotheses laid out in this manuscript by conditioning efficacy on trial participant AD stage (mild and moderate AD). This analysis has been conducted using the data from a phase 2 clinical trial of AD that included a COA-based co-primary endpoint (ADAS-Cog 12 and ADCS-ADL); the primary pre-specified analysis did not condition efficacy on stage.
Currently, there is substantial scientific debate surrounding the optimal trial design for novel AD therapies. With this proposed framework, we aim to supplement this discussion and encourage new avenues of research using a stage-specific approach for the evaluation of AD outcomes. It must be noted that this proposed framework is based on typical AD progression. We acknowledge that AD progression can be heterogeneous depending on several patient characteristics, such as age at onset, gender, level of education, and presence of genetic markers [Citation44,Citation113]. These considerations can be investigated within the proposed staging framework.
6. Expert opinion
Across the spectrum of illness, people can live with AD for decades. Consequently, clinical trials do not typically follow individuals across the full preclinical and clinical course of illness. Clinical trials typically run from 1 to 3 years and, out of necessity, focus on one or two stages of illness. A ‘stages of illness’ approach to enrollment aligns with the levels of prevention model as defined in public health. Primary prevention studies target asymptomatic individuals who screen negative for disease, even in its earliest stages; though primary prevention is desirable, large sample sizes and benign treatment are required for long-term exposure of large numbers of people [Citation114–116]. Strategies used for primary prevention of AD include cardiovascular risk reduction, better nutrition, physical and cognitive activities. Emerging evidence supports the benefits of primary prevention strategies for AD. In secondary prevention of AD, we identify individuals in the earliest stages of illness through cognitive screening, biomarker assessments, neuroimaging, or combinations of these approaches. As indicated in , enrolled individuals may be in the preclinical stage, with biological but not cognitive evidence of early disease, or in the MCI stage where some degree of subjective and objective cognitive impairment are required. There have been multiple secondary prevention trials enrolling people with MCI designed to delay the onset of dementia. The A4 study exemplifies efforts to enroll individuals with preclinical disease based on the presence of amyloid and the absence of cognitive impairment [Citation117]. In tertiary prevention, the goal is to slow or stop disease progression, prevent complications, or reduce disability in persons with well-established diseases.
Table 1. Relationship of stage of disease to type of prevention and goals of treatment.
The field has defined stages meticulously and developed trial designs for primary, secondary, and tertiary prevention. There have also been tremendous advances in the use of neuroimaging and biomarkers, in both CSF and blood, as eligibility criteria and as outcomes [Citation44,Citation111,Citation118,Citation119]. However, these advances have not been matched by advances in our measures of objective or subjective cognitive status and decline, or in measures of QoL and functional status. One of the greatest challenges we currently face in AD research is ensuring that the instruments and subsequent trial endpoints are appropriate for the stage of disease being studied. In many ways, the relevance and measurement issues presented, as well as the strategies we have advocated to overcome such issues, may also be interpreted as a synthesis of emerging bench research with observations in RCTs. For example, recent bench work has demonstrated that AD stage progression is associated with distinct elements and specific expressions of Tau isoforms, and these stage-specific expressions have been proposed as stage-specific interventional targets [Citation120].
From a clinical perspective, this implies that persons living with AD and their caregivers may continue to see trial failures until, and unless, biology/drug development, trial design, and measurement are appropriately linked to the disease stage. Many RCT failures in AD can be characterized by the shifting of similar designs with similar endpoints to earlier stages of illness. The use of biomarkers and imaging to define preclinical disease is a tremendous advance but might be supported by further refinements of clinical outcomes and endpoint assessments. While multiple drugs have demonstrated amyloid clearance from the brain, the corresponding slowing of cognitive and functional decline has not been robust [Citation121].
Article highlights
Alzheimer’s disease (AD) typically progresses through well-defined stages. Whereby clinical disease is segmented into a preclinical stage, mild cognitive impairment, and mild, moderate, and severe stages of Alzheimer’s dementia. Each stage of the disease is characterized by stage-specific symptoms.
In contemporary clinical trials of AD, treatment efficacy is often evaluated using primary and secondary endpoints based on measures of outcomes that are held fixed across different disease stages. Repeated selection of outcomes and endpoints that are suboptimal for the stage of illness under investigation can result in measurement issues and, ultimately, trial failure.
There have been tremendous advances in using neuroimaging and biomarkers, in both cerebrospinal fluid and blood, as outcomes. However, these advances have not been matched by advances in our measures of objective or subjective clinical outcomes (e.g. cognitive status, quality of life, and function).
One of the greatest challenges we currently face in AD research is ensuring that the instruments and subsequent trial endpoints are appropriate for the stage of disease being studied. In this paper, we present strategies we have advocated to overcome such issues.
Declaration of interest
R Lipton serves on the editorial boards of Neurology and Cephalalgia and is a senior advisor for Headache. He has received research support from the National Institutes of Health. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He has reviewed for the National Institute on Aging and National Institute of Neurological Disorders and Stroke; serves as consultant, advisory board member, or has received honoraria or research support from AbbVie, Amgen, Biohaven, Dr. Reddy’s Laboratories, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Teva, Vector, and Vedanta Research. He receives royalties from Wolff’s Headache, eighth edition (Oxford University Press, 2009), and Informa. He holds stock options at Biohaven and Ctrl M. W Stewart serve as a consultant for Grifols and Amgen. L Podger, D Serrano, and F Barnes, are employees of OPEN Health Group. W Rodriguez, M Runken, and D Gomez-Ulloa, are employees of Grifols. The development of this manuscript was sponsored by Grifols SSNA. All authors met the ICMJE authorship criteria. Neither honoraria nor any other form of payment was made for authorship. Financial arrangements of the authors with companies whose products may be related to the present manuscript are listed in the Disclosure Statement, as declared by the authors. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank E. Anne Davis for the thoughtful scientific insights and expertise provided in the initial discussions of the work presented in this manuscript.
Additional information
Funding
References
- Alzheimer’s Association As. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387.
- Europe A. Dementia in Europe Yearbook 2019: estimating the prevalence of dementia in Europe. Alzheimer Europe. 2019.
- Scheltens P, Blennow K, Breteler M, et al. Alzheimer’s disease. Lancet (Lond Engl). 2016;388(10043):505–517.
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259.
- Food and Drug Administration. Incorporating clinical outcome assessments into endpoints for regulatory decision making. 2018 https://www.fda.gov/media/132505/download.
- Grober E, Hall CB, Lipton RB, et al., Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14(2):266–278. .
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Focus. 2013;11(1):96–106.
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269.
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021 Mar;17(3):327–406.
- Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323.
- Schindler SE, Bateman RJ. Combining blood-based biomarkers to predict risk for Alzheimer’s disease dementia. Nature Aging. 2021;1(1):26–28.
- Ribeiro F, de Mendonça A, Guerreiro M. Mild cognitive impairment: deficits in cognitive domains other than memory. Dement Geriatr Cogn Disord. 2006;21(5–6):284–290.
- Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308.
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194.
- Petersen RC, Caracciolo B, Brayne C, et al. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214–228.
- Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatric Psychiatry. 2009;17(5):368–375.
- Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135.
- Farias ST, Mungas D, Reed BR, et al. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157.
- Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129–140.
- Malek-Ahmadi M. Reversion from mild cognitive impairment to normal cognition. Alzheimer Dis Assoc Disord. 2016;30(4):324–330.
- Brown PJ, Devanand D, Liu X, et al. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer's disease. Arch Gen Psychiatry. 2011;68(6):617–626.
- Hoe J, Hancock G, Livingston G, et al. Changes in the quality of life of people with dementia living in care homes. Alzheimer Dis Assoc Disord. 2009;23(3):285.
- Feldman H, Woodward M. The staging and assessment of moderate to severe Alzheimer disease. Neurology. 2005;65(6 suppl 3):S10–S17.
- Winblad B, Gauthier S, Åström D, et al. Memantine benefits functional abilities in moderate to severe Alzheimer’s disease. J Nutr Health Aging. 2010;14(9):770–774.
- Moran M, Lynch C, Walsh C, et al. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6(4):347–352.
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16(3):391–460.
- Franx BAA, Arnoldussen IAC, Kiliaan AJ, et al. Weight loss in patients with dementia: considering the potential impact of pharmacotherapy. Drugs Aging. 2017 Jun;34(6):425–436.
- Anderson RM, Hadjichrysanthou C, Evans S, et al. Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet. 2017;390(10110):2327–2329.
- Atri A. Current and Future Treatments in Alzheimer’s Disease. Semin Neurol. 2019 Apr;39(2):227–240.
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? Pharm Ther. 2010;35(4):208.
- Cummings J, Lee G, Zhong K, et al. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dementia. 2021;7(1):e12179.
- Database MC. Decision Memo. National Coverage Determination for Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease. 2022.
- Ezzati A, Davatzikos C, Wolk DA, et al. Application of predictive models in boosting power of Alzheimer’s disease clinical trials: a post hoc analysis of phase 3 solanezumab trials. Alzheimer's Dementia. 2022;8(1):e12223.
- Gauthier S, Albert M, Fox N, et al., Why has therapy development for dementia failed in the last two decades? Alzheimers Dement. 2016;12(1):60–64. .
- Mangialasche F, Solomon A, Winblad B, et al. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9(7):702–716.
- United States Food and Drug Administration. Guidance for industry. Early Alzheimer’s disease. Developing drugs for the treatment (draft guidance) Feb, 2018.
- Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–1704.
- Ostrowitzki S, Lasser RA, Dorflinger E, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res Ther. 2017;9(1):1–15.
- Banks SJ, Qiu Y, Fan CC, et al. Enriching the design of Alzheimer’s disease clinical trials: application of the polygenic hazard score and composite outcome measures. Alzheimer's Dementia. 2020;6(1):e12071.
- Giebel CM, Sutcliffe C, Stolt M, et al. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: a European study. Int Psychogeriatr. 2014 Aug;26(8):1283–1293.
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS‐ADRDA work group* under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279.
- Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562.
- Huang L-K, Chao S-P, C-J H. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020;27(1):1–13.
- O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65(8):1091–1095.
- National Institute for Health and Clinical Excellence Draft scope for the appraisal of donepezil, galantamine, rivastigmine & memantine for the treatment of mild to moderate Alzheimer’s disease issue date: September 2009.
- Pause BM, Zlomuzica A, Kinugawa K, et al. Perspectives on episodic-like and episodic memory. Front Behav Neurosci. 2013;7:33.
- Fabrigoule C, Rouch I, Taberly A, et al. Cognitive process in preclinical phase of dementia. Brain. 1998;121(1):135–141.
- Matsuda O, Saito M. Multiple cognitive deficits in patients during the mild cognitive impairment stage of Alzheimer’s disease: how are cognitive domains other than episodic memory impaired? Int Psychogeriatr. 2009;21(5):970–976.
- Wechsler D. WAIS-R: Wechsler adult intelligence scale-revised. New York N.Y: Psychological Corporation, [1981] ©1981; 1981.
- Devanand D, Folz M, Gorlyn M, et al. Questionable dementia: clinical course and predictors of outcome. J Am Geriatr Soc. 1997;45(3):321–328.
- Hall CB, Ying J, Kuo L, et al. Estimation of bivariate measurements having different change points, with application to cognitive ageing. Stat Med. 2001;20(24):3695–3714.
- Ueckert S, Plan EL, Ito K, et al., Improved utilization of ADAS-cog assessment data through item response theory based pharmacometric modeling. Pharm Res. 2014;31(8):2152–2165. .
- Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer’s disease assessment scale–cognitive subscale (ADAS-Cog): modifications and responsiveness in pre-dementia populations. a narrative review. J Alzheimers Dis. 2018;63(2):423–444.
- Grober E, Sanders AE, Hall C, et al. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24(3):284.
- Delis DC, Kramer JH, Kaplan E, et al. CVLT: California verbal learning test-adult version: manual. San Antonio, TX: Psychological corporation; 1987.
- Wilson JR, De Fries J, Mc Clearn G, et al. Cognitive abilities: use of family data as a control to assess sex and age differences in two ethnic groups. Int J Aging Hum Dev. 1975;6(3):261–276.
- Papp KV, Rentz DM, Orlovsky I, et al. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimer's Dementia. 2017;3(4):668–677.
- Harrison J, Minassian SL, Jenkins L, et al. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64(9):1323–1329.
- Holtzer R, Verghese J, Wang C, et al. Within-person across-neuropsychological test variability and incident dementia. Jama. 2008 Aug 20;300(7):823–830.
- Zahodne LB, Manly JJ, MacKay-Brandt A, et al. Cognitive declines precede and predict functional declines in aging and Alzheimer’s disease. PLoS One. 2013;8(9):e73645.
- Delgado C, Vergara RC, Martínez M, et al. Neuropsychiatric symptoms in Alzheimer’s disease are the main determinants of functional impairment in advanced everyday activities. J Alzheimers Dis. 2019;67(1):381–392.
- Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. the Alzheimer’s disease cooperative study. Alzheimer Dis Assoc Disord. 1997;11(2):S33–9.
- Dubbelman MA, Jutten RJ, Tomaszewski Farias SE, et al. Decline in cognitively complex everyday activities accelerates along the Alzheimer’s disease continuum. Alzheimers Res Ther. 2020 Oct 29;12(1):138.
- Burton CL, Strauss E, Bunce D, et al. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55(5):570–581.
- Allaire JC, Gamaldo A, Ayotte BJ, et al. Mild cognitive impairment and objective instrumental everyday functioning: the everyday cognition battery memory test. J Am Geriatr Soc. 2009 Jan;57(1):120–125.
- Tabira T, Hotta M, Murata M, et al. Age-related changes in instrumental and basic activities of daily living impairment in older adults with very mild Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2020 Jan-Apr;10(1):27–37.
- Politis AM, Alexopoulos P, Vorvolakos T. May neuropsychiatric symptoms be a potential intervention target to delay functional impairment in Alzheimer’s disease? Int Psychogeriatr. 2020;32(6):689–691.
- Cahn-Weiner DA, Farias ST, Julian L, et al. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007 Sep;13(5):747–757.
- Fields JA, Machulda M, Aakre J, et al. Utility of the DRS for predicting problems in day-to-day functioning. Clin Neuropsychol. 2010 Oct;24(7):1167–1180.
- Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: a UK study. Aging Ment Health. 2015 Jan;19(1):63–71.
- United States Food and Drug Administration. Guidance for industry. Alzheimer’s disease. Developing drugs for the treatment of early stage disease (draft guidance) Feb, 2013.
- Pedrosa H, De Sa A, Guerreiro M, et al. Functional evaluation distinguishes MCI patients from healthy elderly people—the ADCS/MCI/ADL scale. J Nutr Health Aging. 2010;14(8):703–709.
- Pfeffer RI, Kurosaki TT, Harrah CH Jr., et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982 May;37(3):323–329.
- Ard MC, Galasko DR, Edland SD. Improved statistical power of Alzheimer clinical trials by item-response theory: proof of concept by application to the activities of daily living scale. Alzheimer Dis Assoc Disord. 2013 Apr-Jun;27(2):187–191.
- Bowling A, Rowe G, Adams S, et al. Quality of life in dementia: a systematically conducted narrative review of dementia-specific measurement scales. Aging Ment Health. 2015 Jan;19(1):13–31.
- Torisson G, Stavenow L, Minthon L, et al. Reliability, validity and clinical correlates of the Quality of Life in Alzheimer’s disease (QoL-AD) scale in medical inpatients. Health Qual Life Outcomes. 2016;14(1):1–8.
- Ezzati A, Zammit AR, Katz MJ, et al. Health-related quality of life, cognitive performance, and incident dementia in a community-based elderly cohort. Alzheimer Dis Assoc Disord. 2019 Jul-Sep;33(3):240–245.
- Bárrios H, Narciso S, Guerreiro M, et al. Quality of life in patients with mild cognitive impairment. Aging Ment Health. 2013;17(3):287–292.
- Weiss EM, Papousek I, Fink A, et al. Quality of life in mild cognitive impairment, patients with different stages of Alzheimer's disease and healthy control subjects. Neuropsychiatr. 2012;26(2):72–77.
- Hussenoeder FS, Conrad I, Roehr S, et al. Mild cognitive impairment and quality of life in the oldest old: a closer look. Qual Life Res. 2020 Jun;29(6):1675–1683.
- Kahle-Wrobleski K, Ye W, Henley D, et al. Assessing quality of life in Alzheimer’s disease: implications for clinical trials. Alzheimers Dement (Amst). 2017;6(1):82–90.
- Landeiro F, Mughal S, Walsh K, et al. Health-related quality of life in people with predementia Alzheimer’s disease, mild cognitive impairment or dementia measured with preference-based instruments: a systematic literature review. Alzheimer’s Res Ther. 2020 November 18;12(1):154.
- Ecklund-Johnson E, Torres I. Unawareness of deficits in Alzheimer’s disease and other dementias: operational definitions and empirical findings. Neuropsychol Rev. 2005 Sep;15(3):147–166.
- Karlawish JH, Casarett D, Klocinski J, et al. The relationship between caregivers’ global ratings of Alzheimer’s disease patients’ quality of life, disease severity, and the caregiving experience. J Am Geriatr Soc. 2001 Aug;49(8):1066–1070.
- Smith S, Lamping D, Banerjee S, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess. 2005;9(10):1–iv.
- Logsdon RG, Gibbons LE, McCurry SM, et al. Quality of life in Alzheimer’s disease: patient and caregiver reports. Aging Ment Health Aging. 1999;5. 21–32.
- Dean K, Jenkinson C, Wilcock G, et al. The development and validation of a patient-reported quality of life measure for people with mild cognitive impairment. Int Psychogeriatr. 2014 Mar;26(3):487–497.
- Savaskan E, Bopp-Kistler I, Buerge M. Therapy guidelines for the behavioural and psychological symptoms of dementia. Praxis (Bern 1994). 2014;103(3):135–148.
- Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. Bmj. 2015 Mar 2;350(mar02 7):h369.
- Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012 May;69(5):493–498.
- Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004 Aug;61(8):1290–1293.
- Canevelli M, Adali N, Cantet C, et al. Impact of behavioral subsyndromes on cognitive decline in Alzheimer’s disease: data from the ICTUS study. J Neurol. 2013;260(7):1859–1865.
- Sundermann EE, Katz MJ, Lipton RB. Sex differences in the relationship between depressive symptoms and risk of amnestic mild cognitive impairment. Am J Geriatric Psychiatry. 2017;25(1):13–22.
- Pocnet C, Antonietti J-P, Donati A, et al. Behavioral and psychological symptoms and cognitive decline in patients with amnestic MCI and mild AD: a two-year follow-up study. Int Psychogeriatr. 2015;27(8):1379–1389.
- Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Jama. 2002 Sep 25;288(12):1475–1483.
- Roberto N, Portella MJ, Marquié M, et al. Neuropsychiatric profiles and conversion to dementia in mild cognitive impairment, a latent class analysis. Sci Rep. 2021;11(1):1–9.
- Van der linde RM, Dening T, Stephan BC, et al. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. 2016;209(5):366–377.
- Ballard CG, O’Brien JT, Coope B, et al. Psychotic symptoms in dementia and the rate of cognitive decline. J Am Geriatr Soc. 1997 Aug;45(8):1031–1032.
- Cohen-Mansfield J. Behavioral and psychological symptoms of dementia. 2015.
- Alexopoulos GS, Abrams RC, Young RC, et al. Cornell scale for depression in dementia. Biol Psychiatry. 1988 Feb 1;23(3):271–284.
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):10S–16S.
- Reisberg B, Borenstein J, Salob SP, et al. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry. 1987;48 Suppl:9–15.
- Balardy L, Voisin T, Cantet C, et al. Predictive factors of emergency hospitalisation in Alzheimer’s patients: results of one-year follow-up in the REAL. FR Cohort. J Nutr Health Aging. 2005;9(2): 112–116
- McIlvane JM, Popa MA, Robinson B, et al. Perceptions of illness, coping, and well-being in persons with mild cognitive impairment and their care partners. Alzheimer Dis Assoc Disord. 2008;22(3):284–292.
- Mohamed S, Rosenheck R, Lyketsos CG, et al. Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am J Geriatric Psychiatry. 2010;18(10):917–927.
- Dauphinot V, Delphin-Combe F, Mouchoux C, et al. Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: a cross-sectional study. J Alzheimers Dis. 2015;44(3):907–916.
- Erder MH, Wilcox TK, Chen W-H, et al. A new measure of caregiver burden in Alzheimer’s disease: the caregiver-perceived burden questionnaire. Am J Alzheimer’s Dis Other Dementias®. 2012;27(7):474–482.
- Hébert R, Bravo G, Préville M. Reliability, validity and reference values of the Zarit Burden Interview for assessing informal caregivers of community-dwelling older persons with dementia. Canadian Journal on Aging / La Revue Canadienne du Vieillissement. 2000;19(4):494–507.
- Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group. Lancet Neurol. 2021 Jun;20(6):484–496.
- Food and Drug Administration. Methods to identify what is important to patients & select, develop or modify fit-for-purpose clinical outcomes assessments. https://www.fda.gov/downloads/Drugs/NewsEvents/UCM620708.pdf.
- Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18(5):421–430.
- Sabbagh MN, Perez A, Holland TM, et al. Primary prevention recommendations to reduce the risk of cognitive decline. Alzheimers Dement. 2022 Jan 13;18(8):1569–1579.
- Assunção SS, Sperling RA, Ritchie C, et al. Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer’s disease. Alzheimers Res Ther. 2022 Apr 19;14(1):54.
- Grober E, Lipton RB, Sperling RA, et al. Associations of stages of objective memory impairment with amyloid PET and structural MRI: the A4 study. Neurology. 2022 Mar 29;98(13):e1327–e1336.
- Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014 Mar 19;6(228):228fs13.
- Teunissen CE, Verberk IM, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66–77.
- Hampel H, Cummings J, Blennow K, et al. Developing the ATX (N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol. 2021;17(9):580–589.
- Wesseling H, Mair W, Kumar M, et al. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell. 2020;183(6):1699–1713. e13.
- Food and Drug Administration. FDA grants accelerated approval for Alzheimer’s drug. 2021. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
