ABSTRACT
Background
Although pre-clinical experiments associate cannabinoids with reduced depressive symptoms, there is a paucity of clinical evidence. This study aims to analyze the health-related quality of life changes and safety outcomes in patients prescribed cannabis-based medicinal products (CBMPs) for depression.
Methods
A series of uncontrolled cases from the UK Medical Cannabis Registry were analyzed. The primary outcomes were changes from baseline in the Patient Health Questionnaire-9 (PHQ-9), Generalized Anxiety Disorder-7 (GAD-7), Sleep Quality Scale (SQS), and EQ-5D-5 L at 1, 3, and 6 months. Secondary outcomes included adverse events incidence.
Results
129 patients were identified for inclusion. Median PHQ-9 at baseline was 16.0 (IQR: 9.0–21.0). There were reductions in PHQ-9 at 1-month (median: 8.0; IQR: 4.0–14.0; p < 0.001), 3-months (7.0; 2.3–12.8; p < 0.001), and 6-months (7.0; 2.0–9.5; p < 0.001). Improvements were also observed in GAD-7, SQS, and EQ-5D-5L Index Value at 1, 3, and 6 months (p < 0.050). 153 (118.6%) adverse events were recorded by 14.0% (n = 18) of participants, 87% (n = 133) of which were mild or moderate.
Conclusion
CBMP treatment was associated with reductions in depression severity at 1, 3, and 6 months. Limitations of the study design mean that a causal relationship cannot be proven. This analysis provides insights for further study within clinical trial settings.
Plain Language Summary
Depression is a highly prevalent mental health condition with approximately one in five people affected by at least one episode of depression in their lifetime. Two cardinal symptoms of depression are low mood and loss of interest. Since depression is such a debilitating condition, improving quality of life is an important part of treatment.
Antidepressant medications are currently an important part of treating depression, but the variability in their effectiveness means that there is a need for alternative treatments. Medicinal cannabis, which contains certain chemicals from the cannabis plant, has received growing interest as a potential novel treatment for depression. Due to the lack of clinical studies on the use of medicinal cannabis to treat depression, this study aims to assess the effects of medicinal cannabis on quality of life in patients suffering from depression.
The study included 129 patients treated with medicinal cannabis for depression at Sapphire Medical Clinics. The results showed that medicinal cannabis was associated with improvements in depression and anxiety symptoms, as well as health-related quality of life, and sleep quality after 1, 3, and 6 months of treatment. Although there were numerous adverse events in a small number of patients, most of these were mild or moderate. A major limitation is that this study cannot determine the extent to which medicinal cannabis is directly responsible for the improvements in depression symptoms that were observed. Future studies should focus on conducting clinical trials which can better evaluate the true treatment effects of medicinal cannabis for depression.
1. Background
Depression is a mental health condition that has been shown to be associated with impaired health-related quality of life (HRQoL) [Citation1]. This has been attributed to the symptoms of depression itself as well as additional impacts on social, occupational, and cognitive functioning [Citation1]. Poor HRQoL increases the likelihood of resistance to treatment, leads to an inability to perform occupational and social activities, and increases health-care costs [Citation1]. Therefore, there is a need to address HRQoL in people suffering from depression via a holistic mental health approach, including appropriate pharmacological treatments alongside psychological and social measures.
Antidepressant medications are a key component of depression treatment [Citation2]. Despite their widespread use, there is debate surrounding the efficacy of antidepressants [Citation3]. A recent meta-analysis demonstrated that although second-generation antidepressants were more effective than placebo, the summary effect sizes were mostly modest, with response rates around 50% [Citation3]. According to another meta-analysis, antidepressants have no or minimal effects in mild-to-moderate depression, whereas the effects were more substantial in very severe depression [Citation4]. Despite second-generation antidepressants displacing tricyclic antidepressants due to improved tolerability, adverse effects remain an issue an dropout rates are significantly higher among patients administered second-generation antidepressants in randomized controlled trials (RCTs) compared to placebo [Citation5].
There has subsequently been interest in exploring potential targets aside from monoamine reuptake inhibitors, such as the endocannabinoid system [Citation6]. It is a system that comprises cannabinoid receptors, endogenous cannabinoids (endocannabinoids), and enzymes that synthesize and degrade endocannabinoids [Citation7,Citation8]. Its main receptors are cannabinoid receptors type 1 (CB1) and type 2 (CB2). CB1 receptors are abundant in the brain and have a role in regulating neurotransmitter release, particularly gamma-aminobutyric acid and glutamate [Citation9]. In the central nervous system, CB2 receptors are located on microglial cells, astrocytes, and neurons and have been proposed to play a role in neuroprotection and regulation of emotional behavior [Citation9,Citation10].
(−)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and/or cannabidiol (CBD) are the main active pharmaceutical ingredients in cannabis-based medicinal products (CBMPs) [Citation11]. Δ9-THC is mainly responsible for the psychotropic properties of cannabis, such as euphoria, and acts as a partial CB1 and CB2 receptor agonist [Citation9]. CBD may act as a negative allosteric modulator of CB1 receptors, although there is controversy about the exact mechanism of CBD on cannabinoid receptors [Citation12]. However, it is accepted that CBD primarily acts through the inhibition of fatty acid binding ligands [Citation13]. This reduces the transportation of anandamide, an endogenous partial CB1 agonist, to fatty acid amide hydrolase, which leads to increased levels of anandamide and increased constitutive activation of CB1 receptors [Citation13]. By increasing endocannabinoid signaling via interaction with CB1 and CB2 receptors in the endocannabinoid system, CBMPs have been proposed as potential therapeutic compounds for the treatment of depression.
Despite numerous pre-clinical studies on the endocannabinoid system, there is a paucity of high-quality evidence on CBMPs in treating depression [Citation14,Citation15]. In particular, no RCTs have been conducted to date in this field [Citation14,Citation15]. Some RCTs studying the effects of CBMPs on chronic pain, neurological disease, and cannabis withdrawal have included changes in depression symptoms as a secondary outcome [Citation14,Citation15]. Such RCTs have generally shown that CBMPs do not have a significant effect on mood [Citation16–25]. Observational studies have produced differing results, with some concluding that CBMPs do not affect depression symptoms and others suggesting that CBMPs improve symptoms of depression [Citation26–30]. Several studies have also shown that prolonged illicit cannabis use is associated with an increased risk of depression and may negatively impact clinical recovery [Citation31,Citation32]. Illicit cannabis, which typically has high Δ9-THC levels, has been shown to increase the risk of psychiatric co-morbidity including psychosis, mood disorders, and suicidal ideation [Citation33–35]. Co-morbid anxiety and reduced sleep quality are also common in individuals with depression [Citation36,Citation37]. When present, both anxiety and impaired sleep are associated with reduced likelihood of achieving remission from depression [Citation38,Citation39]. Although some studies report positive effects [Citation40–42], other research suggests adverse effects of CMBPs on anxiety and sleep quality [Citation43,Citation44]. Overall, there is currently no high-quality evidence for the use of CBMPs to treat depression and the available evidence is conflicting.
Therefore, the primary aim of this study was to analyze the outcomes of patients prescribed CBMPs for the treatment of depression to assess the effects of CBMPs on HRQoL and to determine the safety of their use. The participants included in the study were enrolled in the UK Medical Cannabis Registry (UKMCR), which collects prospective data on health-related quality-of-life outcomes for patients in the UK treated with CBMPs [Citation45].
2. Methods
2.1. Study overview
A series of uncontrolled cases of patients prescribed CBMPs for depression were conducted using the UKMCR. Participants were prompted to complete questionnaires about patient-reported outcome measures (PROMs) at baseline and after 1 month, 3 months, and 6 months. This study did not require any formal ethical approval in accordance with the NHS Health and Research Authority and Research Ethics Committee guidance (Appendix A). The study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidance [Citation46].
2.2. Setting and participants
The UKMCR collects prospective pseudonymized data on patients treated with CBMPs and is privately owned by Sapphire Medical Clinics. To date, Sapphire Medical Clinics is the only clinic that requires patients to routinely register with the UK Medical Cannabis Registry. Sapphire Medical Clinics specializes in cannabis-based medicine and treats patients in the UK and Channel Islands, using remote consulting, with CBMPs produced according to Good Manufacturing Practice (GMP) criteria. In line with UK guidelines for prescribing CBMPs, participants were already diagnosed with treatment-resistant depression prior to commencement of CBMPs, having already completed an adequate trial of licensed pharmacological therapies [Citation47]. Individuals were recruited consecutively to the UK Medical Cannabis Registry prior to their initial consultation following the provision of informed consent. Individuals are subsequently screened to ensure they have been prescribed CBMPs. After an initial consultation with a clinician, clinicopathological information, comorbidities, drug and alcohol history, and medication history are entered. PROMs, clinical effectiveness measures, and adverse events questionnaires are remotely administered to patients via an online platform at baseline, 1 month, 3 months, 6 months, and then at 6-month intervals [Citation45].
The inclusion criteria for this study were patients aged greater than 18 years old with a primary diagnosis of depression and prescribed CBMPs for at least 1 month. The primary indication for treatment with CBMPs was determined via consultation with a clinician. Patients with other secondary and tertiary conditions that could be indications for prescribing CBMPs were also considered, provided that the primary indication was depression. Patients treated with CBMPs for other conditions with a secondary or tertiary indications of depression were excluded. Patients with no baseline PROMs were also excluded. There were no further exclusion criteria.
2.3. Data selection
Baseline questionnaires captured demographic data including age, sex, occupation, body mass index (BMI) calculated from height and weight, alcohol consumption, smoking history, and recreational cannabis use. Information about current antidepressant medication was recorded but patients who stopped taking antidepressants at the time of data extraction were excluded. Primary, secondary, and tertiary conditions that indicated a prescription for CBMPs were recorded. The incidence of comorbidities including those used in the Charlson comorbidity index, a measure that predicts ten-year mortality [Citation48], as well as hypertension, arthritis, epilepsy, and endocrine dysfunction was recorded. The Charlson comorbidity index was thereafter calculated for each participant.
CBMP prescription data were recorded at each follow-up including formulation, route of administration, Δ9-THC and CBD concentrations and doses, and cannabis strain. All CBMPs were manufactured according to GMP criteria [Citation47]. Adverse events were either self-reported by patients via an online reporting form at the point of resolution or recorded retrospectively during a consultation with a clinician and prior to completion of their PROMs. Participants could report their adverse events utilizing free text with no limits on the number of adverse events they could record. Adverse events were classified according to the Common Terminology Criteria for Adverse Events v4.0 [Citation49].
Self-reporting using PROMs is shown to be a valid and reliable method for determining changes in symptoms of depression [Citation50], anxiety [Citation51], and insomnia [Citation52] as well as the overall HRQoL [Citation53]. The following PROMs were collected at baseline and after 1 month, 3 months, and 6 months.
2.3.1. Patient Health Questionnaire-9 (PHQ-9)
PHQ-9 is a 9-item numerical rating scale (NRS) that assesses the presence and severity of depression. It scores nine different symptoms of depression each depending on how often they are experienced by patients on a scale of 0 (not at all) to 3 (nearly every day). This is used to generate a total score ranging from 0 to 27 where mild, moderate, moderately severe, and severe depression is defined as a total score ≥5, ≥10, ≥15, and ≥20, respectively, [Citation50]. The PHQ-9 has demonstrated excellent test–retest reliability and internal reliability with a Cronbach’s α between 0.86 and 0.89 [Citation50].
2.3.2. Generalized Anxiety Disorder-7 (GAD-7)
GAD-7 is a 7-item NRS that evaluates the presence and severity of generalized anxiety disorder. It involves seven different symptoms of anxiety being scored by patients each depending on how often they are experienced on a scale of 0 (not at all) to 3 (nearly every day). The total score ranges from 0 to 21 with mild, moderate, and severe anxiety being defined as a total score ≥5, ≥10, ≥15, respectively, [Citation51]. The GAD-7 has demonstrated high internal consistency with a Cronbach’s α greater that 0.82. In addition, the GAD-7 has also been validated for use in populations with depression [Citation54].
2.3.3. Sleep Quality Scale (SQS)
SQS is a single-item NRS used to evaluate sleep quality. Patients are asked to rate their quality of sleep over the past 7 days on a scale of 0 (terrible) to 10 (excellent) [Citation52]. The SQS has demonstrated excellent concurrent validity with the Pittsburgh Sleep Quality Index, as well as construct validity and test–retest reliability [Citation52].
2.3.4. EQ-5D-5L
EQ-5D-5L is an instrument used to measure HRQoL and consists of two parts. The first part is an NRS used to assess HRQoL across five domains including mobility, self-care, usual activities, pain or discomfort, and anxiety or depression each on a scale ranging from 1 (no problems) to 5 (extreme problems) [Citation53]. A 5-digit code is then generated from these scores and mapped to EQ-5D-5L index values, which are calculated using methods described by van Hout et al. [Citation55], to standardize the health states to a UK population. This is the preferred methodology for calculating HRQoL by the National Institute of Health and Care Excellence whilst a UK validation study is also currently underway [Citation56]. An EQ-5D-5L index value of 1 represents full HRQoL and a score less than 0 represents an HRQoL worse than death. The second part of the EQ-5D-5L, which was not analyzed as part of this study, is a visual analog scale where patients rate their health on a scale of 0 (worst health imaginable) to 100 (best health imaginable) [Citation53].
2.3.5. Patient Global Impression of Change (PGIC)
PGIC is a single-item NRS that assesses a patient’s belief about treatment efficacy. It consists of a 7-point scale where patients rate their overall improvement since starting treatment on a scale ranging from 1 (no change or worse) to 7 (considerable improvement) [Citation57].
2.4. Outcomes measures
The primary outcomes of this study were changes in PHQ-9, GAD-7, SQS, and ED-5D-5L from baseline to 1, 3, and 6 months. In addition, median PGIC values were reported at each follow-up period. The secondary outcome was the incidence of adverse events.
2.5. Statistical analysis
Data about patient demographics, antidepressant medications, diagnoses, comorbidities, CBMP prescriptions, and adverse events were analyzed using descriptive statistics. Baseline PROMs were compared independently to PROMs at 1, 3, and 6 months to allow an analysis of patients with missing follow-up PROMs. The Shapiro–Wilk test was used to determine whether data sets were normally distributed. Statistical analysis was performed using the paired t-test for parametric data or the Wilcoxon signed-rank test for non-parametric data. Statistical significance was defined as p < 0.050. Unless otherwise stated, data were presented as mean (± standard deviation (SD)) for parametric data or median (interquartile range (IQR)) for non-parametric data. Subgroup analysis was performed by comparing PHQ-9 changes from baseline and the proportion of adverse events between subgroups using Mann-Whitney U tests. The subgroups were based on baseline anxiety (GAD-7 ≥ 5), cannabis status, severe baseline depression (PHQ-9 ≥ 20), and antidepressant prescriptions. A chi-square analysis was performed to analyze the association between Δ9-THC/CBD dose and the occurrence of adverse events. The data analysis was performed using Statistical Package for Social Sciences (SPSS) [IBM Statistics version 28 SPSS Inc].
3. Results
3.1. Patient data
Patients with a primary diagnosis of depression were extracted from the UKMCR and those without baseline PROMs or who were enrolled for less than 1 month were excluded. This resulted in 129 patients for final analysis and from this total, 107, 72, and 34 patients had PROMs recorded at 1 month, 3 months, and 6 months, respectively.
Demographic data about participants included in the study were analyzed (). The mean age was 35.6 (±11.1) years and the mean BMI was 26.9 (±6.3) kg/m2. Ninety-five patients (73.6%) were male and 34 patients (26.4%) were female. Regarding occupation, the category with the highest number recorded was ‘unemployed’ with 34 patients (26.4%).
Table 1. Baseline data about patient demographics and medical history (n = 129).
One hundred patients (77.5%) were either current or ex-smokers with a median pack-year history of 10.0 (4.0–20.0). The median weekly alcohol consumption was 1.0 (0.0–6.0) unit. One hundred and fifteen patients (89.1%) were either current or ex-users of recreational cannabis with a median exposure of 6.5 (2.0–20.0) cannabis gram years. Seventy-two patients (55.8%) were on antidepressant medication at the time of data extraction, 11 (8.5%) of whom were on 2 antidepressants. The most prescribed antidepressants among participants of this study were sertraline (n = 21, 16.3%), venlafaxine (n = 19, 14.7%), and citalopram (n = 14, 10.6%).
The highest recorded secondary and tertiary diagnoses were anxiety (n = 42, 32.6%) and insomnia (n = 8, 6.2%) respectively (). The median Charlson comorbidity index was 0.0 (0.0–0.0). The incidence of other comorbidities including hypertension (n = 6, 4.7%), arthritis (n = 4, 3.1%), epilepsy (n = 2, 1.6%), venous thromboembolism (n = 2, 1.6%), and endocrine dysfunction (n = 5, 3.9%) was recorded.
Table 2. Primary, secondary, and tertiary indications for cannabis-based medicinal products of study participants (n = 129).
3.2. CBMP dosing and mode of administration
CBMPs were prescribed via sublingual, oral, or vaporized routes of administration. Vaporized dry flower preparations alone were prescribed to 61 patients (47.3%), oral or sublingual oils alone were prescribed to 21 patients (16.3%) and 33 patients (25.6%) were prescribed both. The most prescribed vaporized dry flower preparation was Adven® 0%CBD/20% Δ9-THC hybrid flos (Curaleaf International, Guernsey, UK), and the most prescribed oral or sublingual oils were Adven® 20 mg/ml Δ9-THC full spectrum hybrid/indica oil and Adven® 50 mg/ml CBD broad spectrum oil (Curaleaf International, Guernsey, UK). The median daily initial Δ9-THC dose was 120.0 mg (100.0–200.0 mg) and the median daily initial CBD dose was 5.5 mg (0.0–100.0 mg). Among patients taking both preparations, the median daily Δ9-THC dose was 10.0 mg (0.0–20.0 mg) and 150.0 mg (100.0–200.0 mg) from oils and dry flowers, respectively. The median daily CBD dose was 100.0 mg (0.0–100.0 mg) and 0.5 mg (0.0–5.0 mg) from oils and dry flower, respectively, in patients taking both preparations.
3.3. Patient reported outcome measures
Follow-up scores for PHQ-9, GAD-7, SQS, EQ-5D-5L, and PGIC at 1, 3, and 6 months were analyzed (). There were improvements at 1, 3, and 6 months for PHQ-9 (p < 0.001, p < 0.001, p < 0.001), GAD-7 (p < 0.001, p < 0.001, p = 0.015), SQS (p < 0.001, p < 0.001, p = 0.029), the EQ-5D-5L usual activities (p < 0.001, p < 0.001, p = 0.005) and anxiety/depression subscores (p < 0.001, p < 0.001, p < 0.001), and the EQ-5D-5L Index Value (p < 0.001, p < 0.001, p = 0.003). Statistically significant improvements were observed for the EQ-5D-5L mobility (p = 0.003, p = 0.011, p = 0.257), self-care (p = 0.009, p = 0.016, p = 0.589), and pain and discomfort (p < 0.001, p < 0.001, p = 0.090) subscores at 1 and 3 months. The median PGIC was 6.0 at 1, 3, and 6 months.
Table 3. Median (IQR) baseline and follow-up scores for PHQ-9, GAD-7, SQS, EQ-5D-5L, and PGIC at 1, 3, and 6 months.
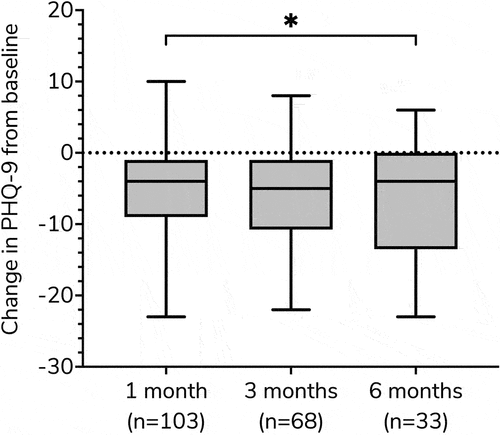
Median (IQR) PHQ-9 changes from baseline were −4.0 (−9.0, −1.0), −5.0 (−10.8, −1.0), and −4.0 (−13.5, 0.0) for 1, 3, and 6 months, respectively (). Improvement in PHQ-9 was greater at 6 months than at 1 month (p = 0.039). Clinically, significant reductions in PHQ-9 (≥5) [Citation58] were observed in 51 (49.5%), 35 (51.5%), and 16 (48.5%) of patients at 1, 3, and 6 months, respectively.
3.4. PHQ-9 subgroup analysis
Improvements in PHQ-9 were larger in individuals with baseline anxiety (GAD-7 ≥ 5), current or ex-users of illicit cannabis, and patients with severe baseline depression (PHQ-9 ≥ 20) at all follow-up intervals except the anxiety subgroup at 6 months (p < 0.050) (). There was no significant difference in PHQ-9 changes among patients currently prescribed antidepressants compared to those not prescribed antidepressants at all follow-up intervals ().
Table 4. Subgroup analysis of median (IQR) changes in PHQ-9 from baseline at 1, 3, and 6 months. Subgroups were compared using Mann-Whitney U tests.
3.5. Adverse events
Eighteen patients (14.0%) reported adverse events and there were 153 (118.6%) adverse events in total (). The most common adverse events were fatigue (n = 14, 10.9%) and insomnia (n = 13, 10.1%). As a proportion of the total number of adverse events, 49.7% (n = 76) were mild, 37.3% (n = 57) were moderate, and 13.0% (n = 20) were severe. There were no reported incidents of life-threatening or disabling adverse events. Subgroup analysis revealed no significant difference in the proportion of adverse events based on anxiety (p = 0.494), cannabis status (p = 0.096), depression severity (p = 0.659), or antidepressant prescriptions (p = 0.981). There was no significant association in the occurrence of adverse events between participants with a daily Δ9-THC dose greater than the median versus those with a daily Δ9-THC dose less than the median (χ2 = 1.73, p = 0.188). Similarly, there was no significant association between adverse events and CBD dose (χ2 = 0.334, p = 0.563).
Table 5. Adverse events recorded by participants (n = 129).
4. Discussion
This study analyzed a case series of patients with depression treated with CBMPs. Initiation of CBMP treatment was associated with statistically significant improvements in PHQ-9, GAD-7, and SQS at 1, 3, and 6 months (p < 0.050). All EQ-5D-5L subscores and the EQ-5D-5L Index Value improved at 1 and 3 months (p < 0.050). The improvement in the EQ-5D-5L usual activities and anxiety/depression subscores, and the EQ-5D-5L Index Value was also sustained at 6 months (p < 0.050). The incidence of adverse events was 153 (118.6%), the majority of which were either mild or moderate. Although changes in the outcome measures described were statistically significant, the use of a case series study design limits the extent to which a causal relationship can be determined.
The median decrease in PHQ-9 from baseline was 4.0, 5.0, and 4.0 after 1, 3, and 6 months, respectively. Clinically significant reductions in PHQ-9 (≥5) were observed in approximately 50% of patients across all follow-up intervals [Citation58]. This suggests that CBMPs could provide some symptomatic improvement for depression although the causality of this association cannot be determined for certain. The broad range in changes to PHQ-9 scores indicates that response to CBMPs varies on an individual basis and may reflect a degree of confounding bias.
An observational cohort study by Round et al. analyzing changes in PHQ-9 in a cohort of 5,103 patients prescribed CBMPs for various diagnoses reported a mean change in PHQ-9 of −0.20 (95%CI −0.26 to −0.14, p < 0.0001) over a median follow-up time of 196 days [Citation59]. Although the change in PHQ-9 was statistically significant, 95.1% of patients did not have a clinically significant change in their PHQ-9 and 92.7% did not change depression severity category [Citation59]. By contrast, approximately 50% of patients in the present study experienced a clinically significant change in PHQ-9. A potential reason for this difference is that Round et al. did not include patients with a confirmed diagnosis of depression and 23.1% of patients had baseline PHQ-9 scores less than 4 which indicates no symptoms of depression. These factors could have reduced the apparent effect of CBMPs on PHQ-9.
Another observational study by Martin et al. compared symptoms in CBMP users with anxiety or depression to controls both at baseline and after a mean follow-up of 14 months [Citation60]. Symptoms of anxiety and depression were assessed using the Hospital Anxiety and Depression Scale [Citation61]. At baseline, CBMP users (n = 368) had a lower mean depression HADS score (8.6 vs 10.8, p < 0.001) and were more likely to present below the clinical cutoff score compared to controls (n = 170) [Citation60]. During follow-up, controls who initiated CBMP treatment experienced a decrease in mean depression scores (−2.65, p < 0.001, n = 36) and had greater odds of dropping below the clinical cutoff score (OR 6.47, p = 0.01) [Citation60]. By contrast, controls who did not initiate CBMP treatment reported no significant change in mean depression scores (−0.22, p = 0.24) [Citation60]. These results support those of the present study and show that symptomatic changes are detectable in multiple validated PROMs.
However, some studies have suggested that daily cannabis use is associated with the development of depression [Citation62,Citation63]. Such studies have mainly analyzed adolescent populations that were not included in the present study. Also, it is unclear whether this is a direct pharmacological effect of cannabis or because of psychosocial factors associated with illicit drug use. A study by Lucatch et al. associates extended cannabis abstinence with an improvement in depressive symptoms [Citation64]. Lucatch et al. used a relatively small sample size (n = 11) and it is unclear whether these effects continue beyond the withdrawal phase. Another study by Cooke et al. reports no significant effect of cannabis abstinence on depression symptoms [Citation31]. Also, abstinence would not apply to the subpopulation who have never used illicit cannabis.
Several RCTs analyzing the efficacy of CBMPs for chronic pain or neurological conditions with changes in depression symptoms as a secondary outcome have been conducted [Citation16–25]. All such RCTs have found no significant effect of CBMPs on symptoms of depression compared to a placebo [Citation16–25]. A major limitation of these studies is that the patients included did not have a primary diagnosis of depression and changes in depression symptoms could be confounded by the effectiveness of CBMPs for the primary condition [Citation65]. Although evidence from current RCTs suggests that CBMPs are ineffective for treating depression, the antidepressant effects of CMBPs cannot be ruled out since no RCTs including patients with a primary diagnosis of depression have been conducted to date.
Theories about the antidepressant effects of CBMPs are centered around the actions of the endocannabinoid system and its interaction with other systems [Citation66]. Increased activation of CB1 receptor signaling in the prefrontal cortex and hippocampus has been linked to antidepressant effects [Citation66]. Increased endocannabinoid signaling has also been suggested to increase monoaminergic neurotransmission, decrease stress-induced activation of the hypothalamic-pituitary-adrenal axis, stimulate hippocampal neurogenesis, and increase expression of neurotrophins such as brain-derived neurotrophic factor [Citation66].
Subgroup analysis indicated that patients with baseline anxiety experienced greater PHQ-9 improvement at 1 and 3 months. This was expected due to the overlap between the symptoms of depression and anxiety [Citation67]. Unexpectedly, improvement in PHQ-9 was greater amongst current or ex-cannabis users compared to cannabis naïve patients. Alterations to the endocannabinoid system in long-term cannabis users, such as the development of pharmacological tolerance, would be expected to reduce the response to CBMPs [Citation68]. A possible explanation for this phenomenon could be the relatively small number of cannabis naïve patients in this cohort. However, current and ex-cannabis users may represent a group of patients who respond clinically to cannabis for symptoms of depression, and subsequently, achieve further benefit through utilizing CBMPs produced in accordance with GMP and prescribed by specialist conditions according to their clinical presentation. Severe baseline depression was associated with greater PHQ-9 improvement. According to an observational study by Rapin et al., CBMP use was associated with a significant decrease in symptoms of depression (p < 0.010, n = 279), especially with moderate-to-severe depression [Citation69]. There was no difference in change in PHQ-9 scores in those who were also concurrently prescribed anti-depressant medications at baseline. This may suggest that there is not synergistic effect of CBMPs with currently licensed antidepressants, despite the action of CBD at serotonin receptors [Citation70–72], suggesting other mechanisms of action may be more important for achieving a clinical effect in those with depression. However, this must of course be further studied in pharmacodynamic and pharmacokinetic studies.
The present study showed that treatment with CBMPs was associated with improvements in GAD-7, SQS, and the EQ-5D-5L Index Value at 1, 3, and 6 months (p < 0.050). The anxiolytic properties of CBMPs are supported by two RCTs that associated CBMPs with improvements in anxiety provocation tests [Citation40,Citation41]. According to an RCT by Walsh et al., CBMPs improved symptoms of insomnia and sleep quality in patients with chronic insomnia compared to placebo (Insomnia Severity Index −5.07 units, 95%CI −7.28 to −2.86, p = 0.0001, n = 23) [Citation42]. Although RCTs with larger cohorts are needed to confirm these results, there is preliminary evidence that CBMPs could have therapeutic potential for insomnia and poor sleep secondary to other comorbidities. According to a cross-sectional study conducted by Schlienz et al., CBMP users reported significantly better self-reported HRQoL (p < 0.001) compared to controls including lower anxiety and depression (p < 0.001) [Citation73]. Moreover, these effects of CBMPs on HRQoL are also demonstrated in analyses of patients with other conditions enrolled in the UKMCR [Citation74–76].
The adverse event incidence in the present study was 153 (118.6%) with 86.9% being mild or moderate. Subgroup analysis did not reveal any factors that increased susceptibility to experiencing adverse events. There is a wide variation in the incidence of adverse events from CBMPs reported in the literature. According to a systematic review by Wang et al., the overall adverse event incidence of CBMPs from the 23 RCTs included was 4779 (247.4%), 96.6% of which were non-serious adverse events [Citation77]. One reason for this higher total adverse event incidence could be that Wang et al. only included RCTs that used oral/oromucosal CBMPs, whereas most patients in the present study were using vaporized dry flower preparations. Although vaporized preparations result in more frequent respiratory and psychotropic side effects [Citation78,Citation79], surveys among CBMP users have shown that vaporized preparations have an overall more favorable side effect profile compared to oral preparations [Citation80]. Daily Δ9-THC and CBD doses did not affect the occurrence of adverse events although this should be interpreted with caution due to the relatively small number of participants that experienced adverse events.
4.1. Limitations
The present study was an observational case series which cannot determine causal relationships. The absence of a placebo results in an inability to isolate the genuine treatment effects of CBMPs [Citation81]. Lack of randomization and blinding are other sources of bias and result in an inability to control for unknown confounding factors [Citation82]. Confounding bias was a major limitation of the present study and could include factors such as the use of other antidepressants, additional illicit cannabis use, attrition, and heterogeneity in Δ9-THC and CBD concentrations. Data about individuals who began antidepressant use midway through the study and participants who changed antidepressant dose between follow-up periods were not collected which could introduce additional confounding bias. Self-reporting via PROMs is prone to recall bias and exaggeration of symptoms. Additionally, there was a lack of follow-up data since a large proportion of patients did not have PROMs recorded for 3 and 6 months and no long-term data was available after 6 months. Despite being a validated scale for patients with depression [Citation52], use of the SQS could impact the sensitivity of sleep quality measurements since it is a single-item scale. Selection bias was another issue since patients who received greater benefits from CBMPs were more likely to continue treatment and complete follow-up PROMs. There was also another element of selection bias since 68% of patients were current users of illicit cannabis which limits generalizability as these patients were likely to have had a positive prior experience. In the UK population, it is estimated that 68.7% of people have never used cannabis which is higher than the proportion of cannabis-naïve patients in our sample (10.9%), indicating that the participants of the present study may not be representative of the general UK population [Citation83]. In addition, only 26.4% of the participants were female which does not reflect the higher prevalence of depressive disorders in females [Citation84]. Also, the wide variation in daily Δ9-THC and CBD doses which could lead to inconsistent treatment effects if patients have not been adequately titrated. Due to the limitations of sample size, an analysis of the differences between the effects of CBD and Δ9-THC could not be performed. Moreover, sample size limitations prevented more in-depth subgroup analysis according to anxiety or depression severity as measured by the GAD-7 and PHQ-9, respectively, or indeed a multivariate regression analysis to control for additional patients and treatment-related factors. Adverse event data were only available for 6 months that did not allow long-term adverse events to be assessed.
5. Conclusion
This study reports that treatment with CBMPs was associated with improvements in PHQ-9 (p < 0.050) after 1, 3, and 6 months in a case series of patients with a primary diagnosis of depression on the UKMCR. This suggests that CBMPs could have antidepressant effects, although the limitations of the study design mean that a causal relationship cannot be proven. CBMP use was also associated with improvements in anxiety, sleep quality, and overall HRQoL (p < 0.050). The adverse event profile was comparable to that of previous literature. Future studies could focus on conducting controlled observational studies or pilot trials to determine the potential of CBMPs as a treatment for depression. Additionally, the differences between the effects of THC and CBD remain unclear.
Disclaimer
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Declaration of interest
M.H. Sodergren is a consultant hepatopancreatobiliary surgeon, a director at Sapphire Medical Clinics, and a consultant at Imperial College NHS Trust, London. He is a senior clinical lecturer at Imperial College London and Chief Medical Officer at Curaleaf International. S. Erridge is a junior doctor and undertakes paid consultancy work at Sapphire Medical Clinics. S. Erridge is an honorary clinical research fellow at Imperial College London. C. Holvey is the chief clinical pharmacist at Sapphire Medical Clinics. R. Coomber is a consultant orthopedic surgeon, a director at Sapphire Medical Clinics, and a consultant at St George’s Hospital, London. D.A. Riano Barros is a consultant psychiatrist at Sapphire Medical Clinics and The South London and Maudsley NHS Foundation Trust. U. Bhoskar is a consultant psychiatrist at Sapphire Medical Clinics. G. Mwimba is a consultant psychiatrist at Sapphire Medical Clinics and Lead Clinician at the National Inpatient Unit for Under 12s in Scotland. She is also a consultant psychiatrist in the Paediatric Liaison team for Greater Glasgow and Clyde NHS Board. K. Praveen is a consultant psychiatrist at Sapphire Medical Clinics. C. Symeon is a consultant psychiatrist at Sapphire Medical Clinics (London) and St George’s University Hospitals NHS Foundation Trust. S. Sachdeva-Mohan is a consultant psychiatrist at Sapphire Medical Clinics. J. Rucker is a consultant psychiatrist and a former director at Sapphire Medical Clinics (London). J Rucker is an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King’s College London. J Rucker is funded by a fellowship (CS-2017–17-007) from the National Institute for Health Research (NIHR). S. Erridge, C. Holvey, R.Coomber, J.J.Rucker & M.H.Sodergren are the founding clinicians of Sapphire Medical Clinics, which is the first clinic registered with the CQC to evaluate patients for medical cannabis in England. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Study conception and design: S. Mangoo, S. Erridge, C. Holvey, R. Coomber, J.J. Rucker, M.H. Sodergren. Acquisition of data: S. Mangoo, S.Erridge, C.Holvey, R.Coomber, D.A.Riano Barros, U.Bhoskar, G.Mwimba, K.Praveen, C.Symeon, S.Sachdeva-Mohan, J.J.Rucker. Analysis and interpretation of data: S.Mangoo, S.Erridge, M.H.Sodergren. Drafting of manuscript: S.Mangoo, S.Erridge, M.H.Sodergren. Critical revision: S.Mangoo, S.Erridge, C.Holvey, R.Coomber, D.A.Riano Barros, U.Bhoskar, G.Mwimba, K.Praveen, C.Symeon, S.Sachdeva-Mohan, J.J.Rucker, M.H.Sodergren. All authors have contributed to and approved the final manuscript. All conditions as previously stated by the ICMJE have been met.
References of interest
Reference 9: This is a systematic review exploring important research on the effects of cannabinoids on the brain drawing on experiments performed in animal models.
Reference 14: This is one of the first clinically focused systematic review analyzing the use of medicinal cannabis for a broad range of psychiatric disorders.
Reference 15: This is a comprehensive systematic review and meta-analysis examining the evidence for medical cannabis in the treatment of mental health disorders.
Reference 45: This is the first analysis of the outcomes of the initial cohort of patients enrolled on the UK Medical Cannabis Registry.
Reference 77: This systematic review is one of the first studies analyzing the safety of medicinal cannabis by evaluating adverse events.
Additional information
Funding
References
- Shumye S, Belayneh Z, Mengistu N. Health related quality of life and its correlates among people with depression attending outpatient department in Ethiopia: a cross sectional study. Health Qual Life Outcomes. 2019 Nov 8;17(1):1–9.
- Osuch E, Marais A. The pharmacological management of depression. Dialogues Clin Neurosci. 2005;7(3):191–205.
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018 Apr 7;391(10128):1357–1366.
- Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010 Jan 6;303(1):47–53.
- Sharma T, Guski LS, Freund N, et al. Drop-out rates in placebo-controlled trials of antidepressant drugs: a systematic review and meta-analysis based on clinical study reports. Int J Risk Saf Med. 2019;30(4):217–232.
- Serra G, Fratta W. A possible role for the endocannabinoid system in the neurobiology of depression. Clin Pract Epidemiol Ment Health. 2007 Nov 19;3(1):25.
- Lu HC, MacKie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016 Apr 1;79:516–525.
- Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016 Aug;77:1050–1064.
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47.
- Morcuende A, García-Gutiérrez MS, Tambaro S, et al. Immunomodulatory Role of CB2 Receptors in Emotional and Cognitive Disorders. Front Psychiatry. 2022;13:866052.
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006 Jan;147(S1):S163–71.
- Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br J Pharmacol. 2015 Oct;172(20):4790–4805.
- Deutsch DG. A Personal Retrospective: elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs). Front Pharmacol. 2016 Oct 13;7:370.
- Sarris J, Sinclair J, Karamacoska D, et al. Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry. 2020 Jan 16;20(1):24.
- Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019 Dec 1;6(12):995–1010.
- Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014 Mar;71(3):281–291.
- Podda G, Constantinescu CS. Nabiximols in the treatment of spasticity, pain and urinary symptoms due to multiple sclerosis. Expert Opin Biol Ther. 2012 Nov;12(11):1517–1531.
- Trigo JM, Soliman A, Quilty LC, et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. PLoS One. 2018 Jan 31;13(1):e0190768.
- Aragona M, Onesti E, Tomassini V, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol. 2009 Feb;32(1):41–47.
- Moreno JL L-S, García Caldentey J, Trigo Cubillo P, et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J Neurol. 2016 Jul;263(7):1390–1400.
- Ball S, Vickery J, Hobart J, et al. The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess. 2015 Feb;19(12):1–187.
- Rog DJ, Nurmikko TJ, Friede T, et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005 Sep 27;65(6):812–819.
- Wade DT, Makela P, Robson P, et al. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004 Aug;10(4):434–441.
- Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010 Oct;81(10):1135–1140.
- Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003 Nov 8;362(9395):1517–1526.
- Wang Y, Jean Jacques J, Li Z, et al. Health outcomes among adults initiating medical cannabis for chronic pain: a 3-month prospective study incorporating Ecological Momentary Assessment (EMA). Cannabis. 2021 Oct;4(2):69–83.
- Fusar-Poli P, Allen P, Bhattacharyya S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010 May;13(4):421–432.
- Kudahl B, Berg ME, Posselt CM, et al. Medical cannabis and cannabis-based medicine show both potential efficacy and potential harms: cross-sectional comparison with controls on self-rated and interviewer-rated outcomes within the Danish pilot program on medical cannabis. Complement Ther Clin Pract. 2021 Nov;45:101476.
- Abelev S, Warne LN, Benson M, et al. Medicinal cannabis for the treatment of chronic refractory pain: an investigation of the adverse event profile and health-related quality of life impact of an oral formulation. Med Cannabis Cannabinoids. 2022;5(1):20–31.
- Mazza M. Medical cannabis for the treatment of fibromyalgia syndrome: a retrospective, open-label case series. J Cannabis Res. 2021 Feb 17;3(1):4.
- Cooke ME, Gilman JM, Lamberth E, et al. Assessing changes in symptoms of depression and anxiety during four weeks of cannabis abstinence among adolescents. Front Psychiatry. 2021;12:689957.
- Bahorik AL, Leibowitz A, Sterling SA, et al. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J Affect Disord. 2017;213:168–171.
- Martinotti G, Di Iorio G, Sepede G, et al. Cannabis use and psychosis: theme introduction. Curr Pharm Des. 2012;18(32):4991–4998.
- Ricci V, Martinotti G, Ceci F, et al. Duration of untreated disorder and cannabis use: an observational study on a cohort of young Italian Patients Experiencing Psychotic Experiences and Dissociative Symptoms. Int J Environ Res Public Health. 2021;18(23):12632.
- Martinotti G, Di Lorio G, Tedeschi D, et al. Prevalence and intensity of basic symptoms among cannabis users: an observational study. Am J Drug Alcohol Abuse. 2011 Mar;37(2):111–116.
- Fava M, Rankin MA, Wright EC, et al. Anxiety disorders in major depression. Compr Psychiatry. 2000 Apr;41(2):97–102.
- Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008 Sep 30;10(3):329–336.
- Almeida OP, Draper B, Pirkis J, et al. Anxiety, depression, and comorbid anxiety and depression: risk factors and outcome over two years. Int Psychogeriatr. 2012 Oct;24(10):1622–1632.
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10(4):473–481.
- Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacol. 2011 May;36(6):1219–1226.
- Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011 Jan;25(1):121–130.
- Walsh JH, Maddison KJ, Rankin T, et al. Treating insomnia symptoms with medicinal cannabis: a randomized, crossover trial of the efficacy of a cannabinoid medicine compared with placebo. Sleep. 2021 Nov 12;44(11):zsab149.
- Nicholson AN, Turner C, Stone BM, et al. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol. 2004 Jun;24(3):305–313.
- Hser YI, Mooney LJ, Huang D, et al. Reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality, but not quality of life. J Subst Abuse Treat. 2017;81:53–58.
- Erridge S, Salazar O, Kawka M, et al. An initial analysis of the UK Medical Cannabis Registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021 Sep;41(3):362–370.
- Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007 Nov;85(11):867–872.
- Case P. The NICE guideline on medicinal cannabis: keeping pandora’s box shut tight? Med Law Rev. 2020 May 1;28(2):401–411.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015 Nov;1(8):1051–1059.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–1097.
- Snyder E, Cai B, DeMuro C, et al. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018 Nov 15;14(11):1849–1857.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736.
- Johnson SU, Ulvenes PG, Øktedalen T, et al. Psychometric properties of the general anxiety disorder 7-item (GAD-7) scale in a heterogeneous psychiatric sample. Front Psychol. 2019;10:1713.
- Van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012 Jul-Aug;15(5):708–715.
- National Institute for Health and Care Excellence (NICE). Position statement on use of the EQ-5D-5L value set for England 2019 [ updated October 2019; cited 2022 Jul 11th]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l
- Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10(4):S73.
- McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord. 2010 Dec;127(1–3):122–129.
- Round JM, Lee C, Hanlon JG, et al. Changes in patient health questionnaire (PHQ-9) scores in adults with medical authorization for cannabis. BMC Public Health. 2020 Jun 23;20(1):987.
- Martin EL, Strickland JC, Schlienz NJ, et al. Antidepressant and anxiolytic effects of medicinal cannabis use in an observational trial. Front Psychiatry. 2021 Sep 9;12:729800.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983 Jun;67(6):361–370.
- Patton GC, Coffey C, Carlin JB, et al. Cannabis use and mental health in young people: cohort study. BMJ. 2002 Nov 23;325(7374):1195–1198.
- Hengartner MP, Angst J, Ajdacic-Gross V, et al. Cannabis use during adolescence and the occurrence of depression, suicidality and anxiety disorder across adulthood: findings from a longitudinal cohort study over 30 years. J Affect Disord. 2020 Jul 1;272:98–103.
- Lucatch AM, Kloiber SM, Meyer JH, et al. Effects of extended cannabis abstinence in major depressive disorder. Can J Addict. 2020;11(3):33–41.
- Li H, Ge S, Greene B, et al. Depression in the context of chronic diseases in the United States and China. Int J Nurs Sci. 2018 Nov 29;6(1):117–122.
- Hill M, Gorzalka B. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009 Dec;8(6):451–458.
- Zbozinek TD, Rose RD, Wolitzky-Taylor KB, et al. Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depress Anxiety. 2012 Dec;29(12):1065–1071.
- Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev. 2018;93:1–25.
- Rapin L, Gamaoun R, El Hage C, et al. Cannabidiol use and effectiveness: real-world evidence from a Canadian medical cannabis clinic. J Cannabis Res. 2021 Jun 23;3(1):19.
- Rock EM, Bolognini D, Limebeer CL, et al. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol. 2012;165:2620–2634.
- Silvestro S, Schepici G, Bramanti P, et al. Molecular targets of cannabidiol in experimental models of neurological disease. Molecules. 2020 Nov 7;25:5186.
- Papagianni EP, Stevenson CW. Cannabinoid regulation of fear and anxiety: an update. Curr Psychiatry Rep. 2019 Apr 27;21:38.
- Schlienz NJ, Scalsky R, Martin EL, et al. A cross-sectional and prospective comparison of medicinal cannabis users and controls on self-reported health. Cannabis Cannabinoid Res. 2021;6:548–558.
- Ergisi M, Erridge S, Harris M, et al. An updated analysis of clinical outcome measures across patients from the UK Medical Cannabis Registry. Cannabis Cannabinoid Res. 2022 Jan 24 [cited 2022 Aug 3]. Avilable from: https://doi.org/10.1089/can.2021.0145
- Ergisi M, Erridge S, Harris M, et al. UK Medical Cannabis Registry: an analysis of clinical outcomes of medicinal cannabis therapy for generalized anxiety disorder. Expert Rev Clin Pharmacol. 2022;18:1–9.
- Harris M, Erridge S, Ergisi M, et al. UK Medical Cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for chronic pain conditions. Expert Rev Clin Pharmacol. 2021;31:1–13.
- Wang T, Collet JP, Shapiro S, et al. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008 Jul 17;178:1669–1678.
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010 Oct 5;182:E694–701.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: an individual patient data meta-analysis. J Pain. 2015;16:1221–1232.
- Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids—an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199–210.
- Finniss DG, Kaptchuk TJ, Miller F, et al. Placebo effects: biological, clinical and ethical advances. Lancet. 2010 Feb 20;375:686–695.
- Akobeng AK. Understanding randomised controlled trials. Arch Dis Child. 2005 Aug;90:840–844.
- Hindocha C, Brose LS, Walsh H, et al. Cannabis use and co-use in tobacco smokers and non-smokers: prevalence and associations with mental health in a cross-sectional, nationally representative sample of adults in Great Britain, 2020. Addiction. 2021;116:2209–2219.
- Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol Bull. 2017 Aug;143(8):783–822.