ABSTRACT
Introduction
Deep brain stimulation (DBS) is an emerging therapy for mood disorders, particularly treatment-resistant depression (TRD). Different brain areas implicated in depression-related brain networks have been investigated as DBS targets and variable clinical outcomes highlight the importance of target identification. Tractography has provided insight into how DBS modulates disorder-related brain networks and is being increasingly used to guide DBS for psychiatric disorders.
Areas Covered
In this perspective, an overview of the current state of DBS for TRD and the principles of tractography is provided. Next, a comprehensive review of DBS targets is presented with a focus on tractography. Finally, the challenges and future directions of tractography-guided DBS are discussed.
Expert Opinion
Tractography-guided DBS is a promising tool for improving DBS outcomes for mood disorders. Tractography is particularly useful for targeting patient-specific white matter tracts that are not visible using conventional structural MRI. Developments in tractography methods will help refine DBS targeting for TRD and may facilitate symptom-specific precision neuromodulation. Ultimately, the standardization of tractography methods will be essential to transforming DBS into an established therapy for mood disorders.
1. Introduction
Mood disorders are a leading cause of disability, morbidity, and mortality worldwide [Citation1]. In particular, major depressive disorder (MDD) is the most common psychiatric disorder, with lifetime prevalence estimates between 10–20% [Citation2,Citation3]. Depression manifests as recurrent episodes of diminished mood, loss of interest or pleasure, and other cognitive and neurovegetative symptoms that contribute to distress or functional impairment [Citation4,Citation5]. Psychotherapy and pharmacotherapy are the mainstays of management, however, up to 30% of patients develop treatment-resistant depression (TRD), typically defined as a failure of adequate trials of two established antidepressant medications [Citation6,Citation7].
Management of TRD is challenging and involves optimization of psychotherapy and pharmacotherapy. It may also include neuromodulation approaches such as electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), or vagus nerve stimulation (VNS) [Citation7,Citation8]. For patients who have failed conventional therapies, including ECT, deep brain stimulation (DBS) is an emerging and promising treatment option.
DBS is a routine therapy for neurological disorders such as Parkinson’s disease (PD), essential tremor (ET), dystonia, and epilepsy and uses surgically implanted intracranial electrodes or leads to modulate disorder-related brain networks by delivering chronic stimulation to specific brain areas [Citation9]. The current approach in DBS for TRD follows similar principles and is supported by an increasing understanding of the circuit mechanisms underlying depression. Stimulation of different brain areas has the potential to modulate networks involved in depression and include the subcallosal cingulate cortex (SCC), ventral tegmental area projection pathway (VTApp), ventral capsule/ventral striatum (VC/VS), lateral habenula (LHb), bed nucleus of the stria terminalis (BNST), and inferior thalamic peduncle (ITP).
Although DBS for TRD can be effective, response rates are variable and highlight the importance of target identification and lead placement. Given the complex and overlapping neural networks involved in depression and other neuropsychiatric disorders, effective DBS may require an emphasis on modulating structurally and functionally connected brain areas. Connectomic models of DBS may better explain DBS effects than traditional models based on focal modulation of brain targets. Developments in neuroimaging techniques over the past two decades have enabled the emergence of tractography-guided DBS, which has provided critical insights for strategically modulating brain networks and improving outcomes.
In this review, we provide an overview of tractography-guided DBS for mood disorders, with a focus on TRD. We begin by reviewing the current state of DBS for TRD. We follow this with an overview of the principles of tractography and how it is currently applied to DBS targeting in depression. Finally, we discuss how tractography-guided DBS can be refined and examine challenges and future directions for DBS for neuropsychiatric disorders.
2. DBS for depression
2.1. Brief history
Surgical treatment for psychiatric disorders appeared in the late 19th century with what was referred to as psychosurgery. Lobotomy was developed in the 1930s by Egas Moniz to alleviate psychiatric suffering [Citation10]. However, this approach had potentially devastating effects on patients’ intellect and personalities [Citation11] and thus the public developed negative attitudes toward psychosurgery. Lobotomy was mostly portrayed negatively in popular culture and in advocacy campaigns [Citation12].
In the 1950’s, less invasive and potentially reversible treatments were sought for psychiatric disorders. José Delgado, Carl Wilhelm Sem-Jacobsen, and Robert Heath independently explored the use of deep brain electrodes for stimulation and recording in patients with psychosis [Citation13]. Heath’s research faced considerable controversy because of its lack of rigorous methods and ethical considerations, and this ultimately led to the general distaste for brain stimulation in psychiatry until the 21st century.
Meanwhile, brain stimulation was investigated for movement disorders. In the 1960s, Sem-Jacobsen used chronic stimulation to identify potential lesion therapy sites for PD [Citation13]. Natalia Bekhtereva introduced the concept of chronic therapeutic electrostimulation in the thalamus and basal ganglia to treat movement disorders, including parkinsonism and dystonia [Citation14]. Throughout the 1980s, several cases of stimulation for movement disorders were published [Citation15,Citation16]. Ultimately, Alim-Louis Benabid’s team’s use of chronic ventral intermediate nucleus of the thalamus stimulation to treat tremor in PD brought attention to the potential of what is now known as DBS [Citation17].
Intracranial brain stimulation, which was initially used experimentally to treat psychiatric disorders, became increasingly studied for PD and ET. DBS was first approved by the United States Food and Drug Administration (FDA) in 1997 for ET and for tremor in PD. The success of DBS in treating ET, PD, and dystonia has sparked investigation into applications for other neurological and psychiatric disorders. A proof-of-principle pilot study on DBS for TRD was first conducted targeting the SCC [Citation18] and since, the field has expanded to explore neuromodulation of targets in related brain networks.
2.2. Depression as a network disorder
Mood disorders, and more broadly psychiatric disorders, are now understood as network disorders, with pathologic changes involving multiple brain areas and their interactions. The diagnosis of MDD based on the DSM-5 requires the presence of depressed mood and/or loss of interest [Citation19]. Network dysfunction is thought to underlie these core symptoms, with the affective network implicated in the perception of negative affect and sadness, and the reward network being involved in anhedonia [Citation18,Citation20]. The default mode network, frontoparietal network, and salience network have also been implicated in depression [Citation4]. Other depressive symptoms, such as psychomotor impairment, sleep issues, and loss of appetite, may be caused by varying degrees of dysfunction across these brain networks.
2.3. Overview of DBS targets and challenges
Although the exact mechanisms of DBS are not completely understood, stimulation is thought to disrupt abnormal information flow and help reestablish normal network activity [Citation21]. Based on the current understanding of pathologic circuitry, DBS targets for TRD aim to primarily modulate the affective network or reward network. The six targets that have been studied are the SCC, VTApp, VC/VS, LHb, BNST, and ITP. The SCC is implicated in emotional regulation [Citation22] and is a critical brain area for cognitive and emotional control. The VTApp is thought to connect the VTA to the VS and nucleus accumbens (NAcc), which are all key components of the reward network [Citation23]. The LHb is involved in regulating negatively motivated behaviors and reinforcement learning, and is an affective and cognitive hub [Citation24,Citation25]. The BNST is thought to play a role in anxiety, stress-response learning, and social behaviors [Citation26,Citation27]. The ITP may be involved in the reward network, given that it connects the mediodorsal thalamus to the orbitofrontal cortex (OFC) [Citation28].
Most studies on DBS for TRD have been case reports or open-label trials, with only a few randomized controlled trials (RCTs) involving the SCC, VC/VS, and VTApp. Response rates have ranged from 23.1% to 92% and remission rates have ranged from 9% to 75% [Citation29]. Overall, clinical outcomes have been variable, even in studies where the same target was used.
Factors that likely contribute to outcome variability include patient selection, DBS target identification and selection, the latency between stimulation to clinical benefit, and the optimization of stimulation parameters. Patient selection is critical to the efficacy of DBS for movement disorders and this principle is also imperative for psychiatric disorders. There is notable heterogeneity in depression with regard to clinical phenotypes and the patient experience. As such, the identification of factors that differentiate responders from non-responders would facilitate a more robust DBS candidacy evaluation process. Furthermore, patients with different symptoms and comorbidities may have distinct patterns of network dysfunction across multiple brain networks. Optimal DBS therapy will likely require consideration of patient-specific brain networks in order to individually tailor neuromodulation.
Unlike the almost immediate benefit observed in DBS for tremor, improvement with DBS for TRD can take weeks to months to occur. Acute behavioral responses during intraoperative stimulation of the SCC, VC/VS, and VTApp have been described, although the significance of these findings for lead placement and treatment response is unclear [Citation30–32]. Maximal benefit may appear after 6–12 months of continuous stimulation [Citation33], which could represent a latency to benefit or a need to optimize stimulation parameters. Optimal stimulation parameters can be difficult to identify due to the absence of immediate feedback when programming or a clear electrophysiological biomarker. Tractography can be used to help identify DBS targets, facilitate accurate lead placement, and anatomically guide DBS programming.
3. Tractography
3.1. Introduction to the structural connectome
It is now well accepted that DBS affects both local brain areas and distant connected brain areas [Citation9]. However, investigation of anatomical connections in the human brain have been historically limited to postmortem studies. Consequently, tracer studies in animal models, particularly in the macaque monkey, have provided considerable insight into the organization of human white matter pathways [Citation34]. The development of diffusion weighted magnetic resonance imaging (DWI) techniques over the past two decades has facilitated the noninvasive, in vivo investigation of human structural connectivity. These techniques collectively led to the conceptualization of the human connectome as a comprehensive map of the neural elements and connections of the brain [Citation35,Citation36]. Neuroimaging techniques have improved our understanding of neurological disease and connectomic approaches are being increasingly used to guide DBS targeting [Citation37].
Normalized connectomes are derived from connectivity data averaged across numerous participants and individualized connectomes are derived from participant specific data. Normalized connectomes can be used as a reference map to plan DBS targeting, whereas an individualized connectome can provide a personalized method for target selection. Targeted tractography, which reconstructs specific white matter tracts, provides the foundation for tractography-guided DBS.
3.2. Technical introduction
3.2.1. Diffusion weighted MRI
MRI uses a strong magnetic field and radiofrequency pulses to collect information about anatomic nuclei based on their magnetic properties. DWI of the human brain was first described in 1986 [Citation38] and is sensitive to the random translational diffusion or Brownian motion of molecules containing hydrogen nuclei [Citation39,Citation40]. As such, DWI measures the diffusion of water molecules in tissue. Diffusion is restricted by physical obstacles and serves as a proxy measure of tissue microstructure and architecture. In tissues where barriers to diffusion are few or not coherently oriented, for example a cerebrospinal fluid filled ventricle, diffusion is relatively similar in all directions and is isotropic [Citation39,Citation40]. In contrast, in white matter, where diffusion is directionally oriented because of barriers like the axon membrane, myelin sheath, microtubules, and neurofilaments [Citation41], diffusion occurs along one axis more than others and is anisotropic.
For each voxel in the brain, diffusion is measured in at least three different directions (e.g. X, Y, and Z) using pairs of magnetic field gradient pulses. The first pulse labels hydrogen nuclei based on spatial location and the second pulse detects changes in location based on attenuation of the MRI signal as a result of diffusion that occurs in the time between the two pulses [Citation39]. Since diffusion is not uniform in all directions, mathematical models can be fit to estimate diffusion directionality and in the case of white matter, infer the orientation of fibers and tracts [Citation39,Citation40]. Various models have been developed to perform tractography, each with its advantages and disadvantages in both research and clinical settings. The diffusion tensor imaging (DTI) model is commonly used, including for DBS.
3.2.2. Diffusion tensor imaging
In DTI tractography, diffusion of water at each voxel of the brain is modeled by a tensor or ellipsoid shaped mathematical model [Citation42,Citation43] (). A tensor is characterized by 3 orthogonal eigenvectors, each representing an axis of diffusion, and 3 eigenvalues (λ1, λ2, λ3), each representing the magnitude of diffusion along a corresponding axis (). Accordingly, the shape of a tensor provides information about the direction and magnitude of diffusion in a voxel. A spherical tensor is consistent with isotropic diffusion and an elongated ellipsoid tensor is consistent with anisotropic diffusion.
Figure 1. (A) Diffusion tensor imaging (DTI) tractography models diffusion of water at each voxel using a tensor model, characterized by 3 orthogonal eigenvectors and their corresponding eigenvalues (λ1, λ2, λ3). The principal diffusion direction is the eigenvector with the largest eigenvalue, λ1, in this example. (B) Streamline tractography traces the principal diffusion directions of tensors through adjacent voxels to estimate underlying white matter tracts in 3-dimensional space.
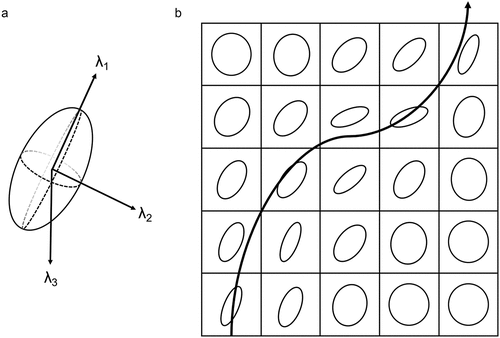
Several parameters are calculated from a tensor and help describe its shape. Fractional anisotropy (FA) measures the overall degree of anisotropy, with a value of 0 representing complete isotropy and a value of 1 representing complete anisotropy. The principal eigenvector corresponds with the largest eigenvalue and represents the principal axis or long axis of diffusion. Axial diffusivity (AD) measures diffusion along the long axis and radial diffusivity (RD) measures diffusion perpendicular to this axis. Mean diffusivity (MD) measures the magnitude of diffusion irrespective of direction.
In a process known as streamline tractography, streamlines are generated by tracing the principal diffusion direction of tensors through adjacent voxels to estimate underlying white matter tracts [Citation44] (). In deterministic tractography, streamlines are generated starting from specified seeds or voxels of interest and their propagation is stopped using arbitrary parameters that suggest that adjacent voxels do not belong to the trajectory. For example, FA thresholds suggest that an adjacent voxel is likely not in white matter and curvature thresholds suggest that an ongoing trajectory is likely not anatomically plausible or belongs to a different trajectory.
Although deterministic tractography is relatively straightforward, there is inherent uncertainty regarding the accuracy of modeled white matter pathways. Any given voxel is modeled by a single tensor and principal diffusion direction. However, a voxel may contain white matter fibers with different orientations. In addition, streamlines may be inaccurately stopped as they approach voxels with reduced FA as a result of the presence of gray matter or crossing fibers.
Probabilistic tractography methods attempt to account for uncertainty in trajectories by using a probability distribution of possible streamline orientations at each voxel [Citation45,Citation46]. Thousands of streamlines are then generated from a specified seed through the probability distributions of voxels to create a connectivity distribution, where the density of streamlines at any given voxel represents the probability of structural connectivity with the seed. Compared to deterministic tractography, probabilistic tractography provides qualitative information about the likelihood of connectivity and may be a more sensitive technique [Citation40], at the cost of higher computational demands and a higher rate of false positive reconstructions.
3.2.3. Principles of tractography-guided DBS
Tractography-guided DBS is motivated by the potential of structural connectivity to improve modulation of disorder-related networks and clinical outcomes. A central goal is the identification and direct targeting of white matter tracts using DBS leads. Direct tract targeting ideally requires a priori anatomical knowledge of tracts of interest and evidence to justify targeting a specific tract [Citation47]. Evidence can be obtained from studies of white matter tracts in the vicinity of DBS leads, which may provide data on which tracts may be modulated by DBS and whether modulation of specific tracts may be associated with clinical benefit. These studies are particularly helpful for depression, where work is ongoing to identify optimal DBS targets [Citation30,Citation48].
Currently, there are no standardized approaches to tractography-guided DBS. Tractography methods, including their testing and validation, are active areas of research. Technical considerations include data acquisition parameters, data preprocessing, tractography model and method, and region of interest (ROI) selection [Citation49]. Importantly, tractrography results are variable across scanning protocols and MRI scanners. Tractography for clinical purposes should be interpreted in the context of these methodological details, together with imaging findings and the clinical presentation.
Targeted tractography is preferred over whole-brain tractography for DBS targeting. Whole-brain tractography tends to produce false positive tracts [Citation50], which cannot be safely relied on for surgery. In targeted tractography, anatomically defined inclusion and exclusion ROIs are used to constrain streamlines to known anatomy and has been shown to produce accurate reconstructions reflective of underlying white matter tracts [Citation51]. Structural ROIs are used and can be complemented by functional ROIs derived from functional MRI (fMRI). Accurate reconstructions are critical to facilitate effective DBS and to avoid side effects.
4. Tractography-guided DBS for treatment resistant depression
4.1. Subcallosal cingulate cortex
The SCC lies ventral to the genu of the corpus callosum and is structurally connected to the medial frontal cortex, anterior and posterior cingulate cortex, medial temporal lobe, mediodorsal thalamus, hypothalamus, NAcc, and dorsal brainstem [Citation52–54]. The SCC does not have clear anatomical landmarks and has high inter-individual structural and functional variability.
Initial SCC DBS studies used anatomical landmarks, stereotactic coordinates, and microelectrode recording (MER) to place electrodes at the gray-white matter junction about halfway between the genu of the corpus callosum and the anterior commissure [Citation55]. In the first open-label study, 4 of 6 patients responded and 3 of 6 patients were in or near remission at 6 months [Citation18]. This cohort eventually included 14 additional patients, with a response rate of 60% and a remission rate of 33% at 1 year, and sustained results at 3–6 years [Citation56–58]. Other open-label studies reported response rates between 29% to 92% and remission rates between 35.3% to 58% [Citation59–61].
Although open-label studies were promising, the largest RCT to date, with 60 participants receiving active stimulation and 30 participants receiving sham stimulation, did not show a significant difference between the stimulation and sham groups at 6 months, with response rates of 20% and 17% and remission rates of 5% and 7% respectively [Citation33]. This trial was stopped by the sponsor after a midpoint futility analysis and posed a challenge to the use of DBS for psychiatric disorders. Interestingly, an improvement in response rate to 53% was observed at 18 months with open-label stimulation, which may have been partially due to programming changes, which were not permitted during the first 12 months. Other factors likely contributed to the lack of early response, including the consistency of lead placements and trajectories. Responders have not been differentiated from nonresponders based on the anatomical position of SCC leads [Citation55] and these data suggested that an individualized approach is likely required.
Tractography has helped optimize lead placement by identifying critical white matter tracts in the vicinity of the SCC (). Using probabilistic tractography from volumes of tissue activated by SCC DBS, 12 responders had shared involvement of pathways from the SCC to the medial prefrontal cortex (PFC) via the forceps minor and uncinate fasciculus, the rostral and dorsal cingulate cortex via the cingulum bundle, and the NAcc/VS via the fronto-striatal fibers [Citation48]. Non-responders who became responders after programming changes ultimately had stimulation of all 4 white matter tracts. When 11 patients were implanted using prospective individualized tractography using these 4 white matter tracts, the response rate was 72.7% and the remission rate was 54.6% at 6 months [Citation62]. This improved response suggested that individualized tractography may improve outcomes by identifying specific targets for effective DBS.
Figure 2. Tractography for subcallosal cingulate cortex (SCC) DBS. Effective SCC DBS involves concurrent stimulation of 4 white matter tracts: the forceps minor (red), the uncinate fasciculus (purple), the cingulum bundle (yellow), and the fronto-striatal fibres (not shown). These white matter tracts are used to guide lead placement for SCC DBS.
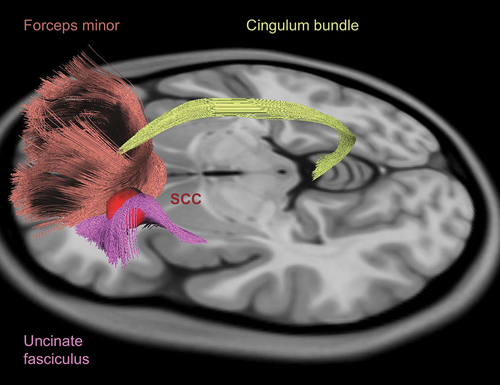
Further investigation is needed to understand inter-individual variability in SCC structural connectivity [Citation63] and to determine how different tracts contribute to DBS benefit. Studies have suggested that cingulum bundle stimulation may be associated with an early response [Citation64] and that cingulum bundle and forceps minor stimulation may be sufficient to achieve a clinical response.
4.2. Ventral tegmental area projection pathway
The VTApp is a tract identified by tractography that is thought to connect the VTA with the NAcc and PFC [Citation23]. Although the VTApp was originally referred to as the superolateral branch of the medial forebrain bundle (slMFB), it is distinct from the medial forebrain bundle, which is primarily characterized in rodents. The white matter anatomy underlying VTApp tractography has been a point of discussion in the literature and may involve intermixed tracts of the internal capsule [Citation65]. The VTApp is closely associated with the limbic hyperdirect pathway (IHDP) between the PFC and limbic subthalamic nucleus (STN) and the relative roles of these tracts in DBS for psychiatric disorders are unclear. The IHDP may be a distinct tract lateral and superior to the VTApp [Citation66]. In the anterior limb of the internal capsule (ALIC), the VTApp also travels immediately lateral to the anterior thalamic radiation (ATR), which connects the anterior and mediodorsal thalamus with the PFC and may be involved in mediating sadness [Citation67].
The VTApp is the only DBS target for TRD that is primarily guided by tractography because it cannot be readily identified by conventional structural MRI (). It is targeted as it exits from the VTA laterally, within a ‘therapeutic triangle’ defined by the mamillary body and mamillothalamic tract anteriorly, red nucleus posteriorly, and the STN and substantia nigra pars reticulata (SNr) laterally [Citation68]. Deterministic tractography from a white matter ROI immediately lateral to the VTA is used to visualize the VTApp for preoperative planning [Citation67,Citation68]. DBS implantation is also guided intraoperatively by MER to identify the neighboring red nucleus, STN, and SNr, and by macrostimulation to potentially identify side effects such as diplopia from oculomotor nerve stimulation [Citation68].
Figure 3. Tractography for ventral tegmental area projection pathway (VTApp) DBS. Lead placement for VTApp DBS is primarily guided by tractography. The target is the VTApp (orange) as it exits the ventral tegmental area (VTA) (green), bordered by the subthalamic nucleus (STN) (light purple) and substantia nigra pars reticulate (SNr) (dark purple) laterally, the red nucleus posteriorly (not shown), and the mammillary body anteriorly (not shown).
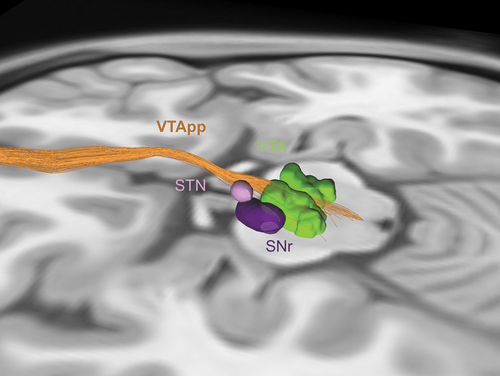
The first study of VTApp DBS included 7 patients, who all experienced an acute increase in appetitive motivation during intraoperative testing [Citation32]. By 1 week after stimulation, 4 patients achieved response criterion. Ultimately, 6 patients were responders and 4 patients achieved remission, with sustained benefit for up to 4 years [Citation69]. Another study showed a rapid response within 1 week in 3 of 4 patients [Citation70]. The one nonresponder had reduced structural connectivity between the VTApp and PFC. This cohort eventually included 10 patients, with 6 of 9 patients (1 patient withdrew from the study) maintaining response at 2 years [Citation71]. In a pilot RCT with an 8-week double-blind, sham-controlled phase followed by an open-label phase, all 16 patients achieved some clinical response [Citation72]. Interestingly, 10 patients responded within 1 week and 14 patients responded within 8 weeks, with no significant difference between the stimulation and sham groups. This acute antidepressant effect was attributed to micro-lesioning or placebo. At 12 months, 50% of patients were in remission.
VTApp DBS has been associated with a more rapid and consistent clinical response than other targets. This effect may be partly related to the use of individualized tractography for consistent targeting. Despite individualized targeting, there are patients who do not respond to stimulation, which suggests a need to consider other clinical and neuroimaging features to facilitate more nuanced target selection [Citation73]. The modulation of specific fibers within the VTApp may predict response [Citation70], but further investigation is needed to differentiate responders from nonresponders.
4.3. Ventral capsule/ventral striatum
The NAcc is a major component of the VS. It is located anterior to the posterior border of the anterior commissure and extends dorsolaterally into the putamen and dorsomedially into the caudate [Citation74]. The NAcc receives input from the cortex, VTA, amygdala, and hippocampus and it projects to the pallidum, substantia nigra, thalamus, VTA, BNST, habenula, and hypothalamus. The ALIC contains various white matter tracts, including tracts to the frontal pole, medial temporal lobe, cerebellum, NAcc, thalamus, hypothalamus, and brainstem [Citation53]. Tracts associated with the affective and reward networks are found within the ventral ALIC [Citation23].
This review refers to the VC/VS as a DBS target. However, the terms VC, ALIC, VS, and NAcc have been used interchangeably in the literature to designate a similar target, despite these structures being anatomically distinct. They have also been used to designate different targets. Variability in nomenclature, targeting, and lead trajectories can make it challenging to draw conclusions from VC/VS DBS studies. Many authors may refer to targets as ‘regions’ because of the lack of specificity in understanding the targets.
VC/VS DBS was first used for refractory obsessive-compulsive disorder (OCD) and was associated with improvement in mood [Citation75,Citation76]. The first study for TRD targeted the NAcc in 3 patients, who had an immediate antidepressant effect with stimulation and worsening of depression within 2 weeks after stimulation was discontinued [Citation20]. A subsequent study used the same targeting approach in 10 patients and observed a 50% response rate at 12 months [Citation77], and sustained results at 2–4 years [Citation78]. In another study of 4 patients, leads were implanted with the deepest contact in the NAcc, the next adjacent contact in the limbic caudate, and the 2 most superficial contacts in the associative caudate [Citation79]. These patients did not respond to NAcc or caudate stimulation during the first 9 months. With further programming optimization, 3 patients responded and 1 patient achieved remission in the following 6 months.
In a study of 15 patients, leads were implanted with the deepest contact in the VS, the next adjacent contact near the VC/VS junction, and the 2 most superficial contacts within the ALIC [Citation31]. The remission rate was 26.6% at 6 months and 53.3% at the last follow-up (mean of 14.9 months). The first VC/VS RCT used the same targeting approach in 30 patients and did not show a significant difference in response rate between active and sham stimulation during the initial 16-week controlled phase, with similar response rates during the subsequent open-label phase [Citation80]. Another RCT included 25 patients in a 52-week open-label trial, followed by a 12-week randomized, double-blind crossover phase with active or sham stimulation [Citation81]. Leads were implanted with the deepest contact in the NAcc and the other contacts in the VC. At the end of the open-label phase, the response rate was 40% and lower depression scores were associated with active stimulation compared to sham stimulation.
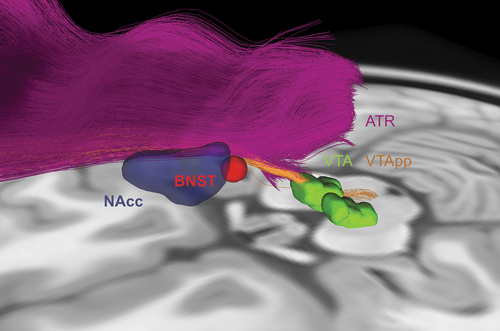
VC/VS DBS typically uses stereotactic coordinates to target the NAcc, but there is no standardized trajectory for the lead to pass through the internal capsule region. A lack of reliable targeting is a major limitation of VC/VS DBS, which is apparent in its variable clinical outcomes. Similar to SCC DBS, VC/VS DBS may be optimized by the identification of critical white matter tracts (). Tractography suggests that responders have common tracts lateral and posterior to the VS [Citation82]. Differentiating the VTApp, lHDP, and ATR in the ALIC may further improve targeting. Postoperative probabilistic tractography has shown that stimulation rarely involved both the VTApp and ATR, and that stimulation closer to these tracts in the ventral ALIC was associated with greater clinical benefit [Citation83]. In addition, structural and functional connectivity of the VC/VS with the PFC and OFC may predict clinical outcomes [Citation84]. Overall, these findings suggest that tractography has the potential to optimize DBS targeting in the region of the VC/VS.
Figure 4. Tractography for ventral capsule/ventral striatum (VC/VS) and bed nucleus of the stria terminalis (BNST) DBS. VC/VS and BNST DBS are currently performed using stereotactic coordinates to target the NAcc (blue) and BNST (red) respectively, with the lead passing through the internal capsule. These targets are in close proximity to each other and to white matter tracts associated with depression-related networks, such as the ventral tegmental area projection pathway (VTApp) (orange) and anterior thalamic radiation (ATR) (purple). As such, there is potential for tractography to guide lead placement in this region.
4.4. Lateral habenula
The habenula is a small, 30 mm3 nucleus () located dorsomedial to the thalamus posteriorly at the base of the third ventricle and consists of the LHb and medial habenula [Citation85]. The LHb receives inputs from the basal ganglia and limbic system through the stria medullaris, and has reciprocal connections with the pineal gland and serotonergic and dopaminergic areas, including the raphe nuclei and VTA [Citation24].
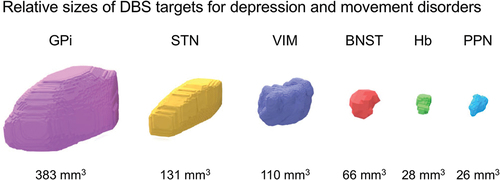
Figure 5. Size comparison of nuclear DBS targets for treatment-resistant depression (TRD) and movement disorders. Estimated volumes vary based on the source atlas, methodology, and neuropathology. In addition, volumes gradually decrease with normal aging and neurodegenerative diseases. Smaller nuclei are more challenging to target for DBS. In TRD, the identification of the bed nucleus of the stria terminalis (BNST) and habenula (Hb) is aided by high-resolution structural MRI. Of note, these nuclei are small relative to the size of a DBS lead and potential volume of tissue activated. GPi, globus pallidus interna; STN, subthalamic nucleus; VIM, ventral intermediate nucleus of the thalamus; PPN, pedunculopontine nucleus.
The first 2 patients with LHb DBS received DBS targeting the stria medullaris adjacent to the LHb [Citation86,Citation87]. Both patients responded to stimulation, experienced relapses within 1 week after stimulation was stopped, and achieved remission within 12 weeks after restarting stimulation. Another patient with LHb DBS had an acute antidepressant effect and had improvement at 12 weeks [Citation88]. The largest cohort of LHb DBS included 1 patient with MDD and 6 patients with bipolar disorder (BP), with improvement in depressive symptoms in 6 of 7 patients after 1 month of stimulation and sustained improvement at 12 months in 3 patients who continued to receive treatment in the study [Citation89].
Although the literature on LHb DBS is limited, the available evidence suggests that it may be a promising target for TRD. Given the small size of the habenula, its identification relies primarily on high-resolution anatomical MRI with at least 3 Tesla. The LHb can be further localized using susceptibility-weighted imaging and quantitative susceptibility mapping [Citation90]. Unlike other DBS targets for TRD, direct targeting using structural imaging may be sufficient for the LHb. Tractography provides limited additional information, considering the low resolution of DWI and the small size of the stria medullaris. Furthermore, the volume of tissue activated by a DBS lead in or near the LHb would be relatively large compared to the LHb and stria medullaris and would encompass both structures. Ultimately, the requirement and availability of high-resolution MRI may limit the adoption of LHb DBS in clinical practice.
4.5. Bed nucleus of the stria terminalis
The BNST is a small nucleus () that is in the vicinity of the VC/VS, medially adjacent to the ALIC, and above and below the anterior commissure [Citation26,Citation27]. Although studies of human BNST anatomy are limited, it has connections to the amygdala, hypothalamus, OFC, and medial PFC [Citation91].
BNST DBS developed from VC/VS DBS for OCD, as a gradual posterior shift from the VC/VS toward the BNST tended to provide better outcomes [Citation92,Citation93]. In OCD, BNST/ALIC DBS improved coexisting depression and anxiety [Citation93]. Studies on BNST DBS for TRD have involved heterogeneous cohorts with psychiatric comorbidities, including anxiety disorders, dependent personality disorder, and anorexia nervosa [Citation94–96]. In a study of 7 patients comparing BNST/ALIC DBS with ITP DBS, all patients had BNST/ALIC stimulation at 3 years of follow-up, with 5 patients responding and 2 patients achieving remission [Citation94]. In another study of 5 patients, BNST DBS was associated with sustained remission in 2 patients at 6–7 years of follow-up and response in 2 other patients [Citation97]. Two other patients had improvement with BNST DBS at up to 12 months [Citation95,Citation96].
Overall, BNST DBS may be promising for TRD, particularly if there is coexisting anxiety. BNST DBS is currently performed using indirect targeting with stereotactic coordinates, with the lead along the ALIC. However, the boundaries of the BNST are difficult to identify with conventional structural MRI given its small size and proximity to other nuclei. It is also in close proximity to depression-related white matter tracts, such as the VTApp, lHDP, and ATR (). As such, tractography of the ALIC and its component tracts has potential to improve DBS targeting and outcomes in the region of the BNST and VC/VS. In this region, there may be a single optimal target for TRD or multiple targets to address different symptoms.
4.6. Inferior thalamic peduncle
The ITP is small white matter tract in the prethalamic area posterior to the anterior commissure, measuring no more than a few millimeters in diameter. It connects the mediodorsal thalamus and OFC and is thought to be connected to the ascending reticular activating system [Citation28].
The first patient with ITP DBS had improvement in depressive symptoms and was ultimately explanted after 3 years without relapse [Citation98,Citation99]. The contribution of a lesion effect is unclear. The largest cohort compared ITP DBS with BNST/ALIC DBS in 7 patients and found no significant difference between targets [Citation94]. However, only 1 of 7 patients preferred ITP DBS over BNST/ALIC DBS and continued with ITP DBS at 8 years of follow-up.
The effectiveness of ITP DBS for TRD is less clear compared to the other targets. ITP DBS has been performed using indirect targeting using stereotactic coordinates and is difficult to further refine. Although the ITP is a white matter tract, tractography may provide limited additional information, particularly given its small size.
5. Refining tractography-guided DBS
5.1. Methodological caveats and limitations
Tractography infers anatomy based on the diffusion of water and its accuracy is influenced by every methodological step, from data acquisition, to modeling, to interpretation. DWI has low spatial resolution, typically 2 mm isotropic voxels at 3 Tesla, and a low signal-to-noise ratio (SNR) [Citation100,Citation101], which limits the accuracy of inter- and intra-voxel reconstructions. Resolution and SNR can be improved by increasing scan time, the number of scans, or magnetic field strength. However, scan time should be balanced with patient comfort and other clinical restrictions, and ultra-high field MRI facilities are not readily accessible.
Artifacts lead to errors in reconstruction and major addressable sources of noise in DWI include eddy currents and motion [Citation102]. Eddy currents are electrical currents induced by changing magnetic fields that are particularly pronounced in DWI because of the large amplitude gradient pulses used. The effect of eddy currents can be reduced by hardware modifications, such as shielding gradient coils, using bipolar gradient waveforms, and correction in data preprocessing. DWI is sensitive to motion, and head motion or transmitted motion from cardiac pulsation and respiration create artifacts. Although head position can be realigned in data preprocessing, this does not account for motion-induced artifacts, such as change in shim, dynamic field fluctuations, and slice-wise signal variations. As such, head motion is best addressed by optimizing comfort during scanning.
DTI tractography is commonly used, given its compatibility with widespread MRI technology, commercial availability of pulse sequences, relatively quick data acquisition times, and straightforward data processing methods. However, its inability to resolve different fiber orientations within a voxel is well recognized and limits reconstruction accuracy. High angular resolution diffusion imaging (HARDI) [Citation103] acquires images using a larger number of gradient directions to increase angular resolution and facilitates improved tract modeling.
Sophisticated tractography algorithms that improve the accuracy of tractography include Q-ball imaging [Citation104], diffusion spectrum imaging (DSI) [Citation105], and composite hindered and restricted model of diffusion (CHARMED) [Citation106], among others. These techniques are limited by the availability of MRI technology, longer acquisition times, and intensive computation compared to DTI. Importantly, tractography results are variable across and within tractography methods [Citation107] and inferences about underlying white matter structure should be interpreted with caution [Citation108]. Overall, there is a need for validation and standardization of tractography methods in clinical practice.
Knowledge of human white matter anatomy and tractography validation is largely inferred from animal models and postmortem studies [Citation34]. Despite concerns about the accuracy of tractography, the visualization of major white matter tracts with DTI has been consistent with postmortem human dissections [Citation109,Citation110]. Using a priori knowledge to guide targeted tractography reduces false positive reconstructions [Citation51].
Tractography for DBS targeting needs to balance sensitivity and specificity to identify white matter tracts of interest. Ultimately, an important discussion for the development of tractography-guided DBS involves the acceptable degree of accuracy for target identification and lead placement. Developments in tractography methods will improve the accuracy of tractography, but whether this will be associated with clinically meaningful changes in DBS targeting is unclear.
5.2. Individual structural connectivity
Tractography generated from large datasets, such as the Human Connectome Project [Citation111], can be used as reference maps for DBS targeting. Advantages of large datasets include improved SNR, the use of advanced MRI technology, and standardization with common sources of data. However, normalized tractography is insufficient for precise, patient-specific DBS targeting because of inherent inter-individual variability in brain anatomy. As with other applications of DBS, millimeter differences in stimulation location for depression can result in different brain areas being modulated and differences in clinical benefit [Citation112]. Furthermore, datasets for normalized connectomes are typically collected from healthy controls and are not representative of neurological or psychiatric disorders. In datasets for specific disorders, accounting for disease heterogeneity would be difficult and they may not be reflective of individual disease states.
As demonstrated by SCC and VTApp DBS, individualized tractography enables lead placement to be guided by patient-specific anatomy [Citation62,Citation68,Citation70,Citation113]. Individualized tractography is particularly useful for targeting white matter tracts with interindividual variability and for targeting structures that are not visible using conventional structural MRI, such as the VTApp. Investigation of which white matter tracts are in the vicinity of DBS leads and which tracts are associated with effective DBS will likely further inform targeting using individualized tractography [Citation64,Citation82,Citation112]. In particular, tractography has the potential to improve DBS in the region of the VC/VS and BNST. VC/VS as a target is poorly defined and may involve the BNST, which itself is difficult to visualize with structural MRI.
Although individualized tractography theoretically provides greater targeting accuracy than normalized tractography, these two approaches have not been directly compared. One notable drawback of individualized tractography is lower SNR. Longer scans or multiple scans per individual produce more reliable results, but may not always be clinically feasible. An acceptable degree of reconstruction accuracy for individualized tractography needs to be determined.
5.3. Functional neuroimaging
Functional ROIs can be used alongside structural ROIs to constrain streamline generation in targeted tractography [Citation114,Citation115] and can be identified using task-based fMRI or resting-state fMRI (rs-fMRI). There are challenges to identifying ROIs for depression using task-based fMRI. Brain areas associated with depression such as the anterior cingulate cortex and dorsolateral PFC are activated by a variety of cognitive and affective tasks [Citation116] and there are no universally accepted tasks for functional localization. Furthermore, activation of an area during one task does not necessarily indicate that the area is actually involved in depression. In contrast, functional connectivity in healthy adults is readily identifiable and reproducible with rs-fMRI [Citation117]. Using rs-fMRI to identify functional ROIs from brain networks such as the default mode network, frontoparietal network, or affective network may be a feasible alternative to task-based localization.
fMRI can also be used to explore the in vivo effects of DBS on brain networks. Recently, different stimulation parameters for DBS for epilepsy were shown to be associated with different activation patterns using fMRI [Citation118,Citation119]. Similar to DBS for TRD, DBS for epilepsy lacks immediate clinical feedback to confirm effective lead placement or guide programming. fMRI has the potential to provide insight into the network mechanisms of DBS and may provide an imaging-based biomarker to help optimize DBS. Overall, the use of fMRI to complement tractography-guided DBS for TRD remains an open area of investigation.
6. Tractography-guided DBS for other mood and psychiatric disorders
Several studies on DBS for TRD included patients with TRD in context of BP and targeted the SCC [Citation56,Citation59], VTApp [Citation32], VC/VS [Citation31], and LHb [Citation89]. In a cohort of 7 BP patients and 10 MDD patients, SCC DBS was similarly effective for treating depression in BP and MDD, although only 3 of 7 patients completed follow-up at 2 years [Citation59]. In 6 BP patients with LHb DBS, 5 of 6 patients had improvement in depressive symptoms at 1 month [Citation89]. One patient had an increase in mania symptoms in the first month, which resolved, and another patient had an acute manic episode 2 months after surgery. Other studies on TRD have included, at most, one patient with BP [Citation31,Citation32,Citation56].
DBS for TRD shares many targets with other psychiatric disorders. In 2009, the FDA granted a humanitarian device exemption for ALIC DBS for refractory OCD. The broader VC/VS, BNST, VTApp, LHb, and ITP have also been studied as targets for OCD [Citation120,Citation121]. Studies on DBS for anorexia nervosa have involved the SCC, VTApp, VC/VS, and BNST as targets [Citation122]. VC/VS DBS, particularly of the NAcc, has been studied for substance use disorders [Citation123]. Overall, the application of tractography-guided DBS for targets in other psychiatric disorders is similar to MDD. Future investigation may reveal critical nuances in tract targeting of specific symptoms of different disorders, such as anxiety.
7. Conclusion
Tractography can ultimately improve clinical outcomes in DBS for mood disorders through the process of identifying critical white matter tracts for modulation and by guiding accurate lead placement. Targeted tractography is especially valuable when precise targeting of white matter tracts is required and when white matter structures cannot be targeted using conventional structural MRI technology. As tractography becomes increasingly used in studies of DBS for TRD, it will continue to inform how depression-related networks can be more effectively modulated by DBS and this will directly impact the refinement of DBS targeting approaches.
8. Expert opinion
Tractography is being increasingly used to investigate and guide targeting for DBS for mood disorders. Thus, tractography-guided DBS is poised to become an essential approach in the emerging field of psychiatric neurosurgery. As tractography methods continue to develop alongside advances in DBS for TRD, important issues have emerged, including the accuracy of tractography and the refinement of DBS targets for TRD.
Research on tractography methods has emphasized improving reconstruction accuracy to better reflect underlying white matter anatomy. The ability to resolve distinct white matter tracts will further facilitate an understanding of which pathways contribute to depression-related brain networks and how their modulation can be associated with clinical effects. Using the location of DBS leads, contacts, and volumes of tissue activated for seed-based tractography has been successful in the development of tractography-guided SCC DBS [Citation48,Citation112]. This technique can be applied to other DBS targets for TRD and more detailed tractography reconstructions can help identify new white matter tracts of interest. Of note, although tractography accuracy is important for precisely mapping connectivity and understanding the anatomy of potential DBS target regions, accuracy may be less meaningful for the clinical delivery of stimulation. Volumes of tissue activated by stimulation are on the order of millimeters, which is typically greater than the resolution of DWI. Furthermore, DBS cannot selectively stimulate specific fibers in a given volume of tissue. Consequently, tractography for DBS guidance in clinical practice will likely be less sophisticated than tractography used in research settings.
DBS targets for TRD need to be well-defined and reproducibly associated with clinical benefit for DBS to become an established therapy for TRD. To date, tractography has been critical for improving outcomes in SCC DBS [Citation62,Citation113] and for the implementation of VTApp DBS [Citation68]. Tractography will continue to have an important role in refining DBS targeting for TRD. Tractography is particularly useful for understanding inter-individual variability in white matter anatomy, which likely contributes to variability in DBS response [Citation63,Citation124], and for understanding the differential contribution of white matter tracts in the vicinity of DBS leads [Citation64]. These aspects provide a roadmap for further improvement of SCC DBS. DBS targeting in the region of the VC/VS and BNST may be better defined from determining the contribution of specific white matter tracts in the internal capsule.
There is considerable heterogeneity in TRD as a syndrome and this is not well understood within DBS cohorts. Determining the relationship between different white matter tracts and symptoms, such as low mood, apathy, anhedonia, mania, anxiety, and impulsivity, may enable tailored stimulation for the management of specific symptoms. If specific white matter tracts are identified, it may be possible to modulate multiple tracts, and thus symptoms, with the same DBS contact or to use different DBS contacts to modulate different tracts.
Heterogeneity may also be addressed using electrophysiology. Closed-loop DBS systems guided by electrophysiological symptom biomarkers are being developed for use in movement disorders [Citation125] and responsive neurostimulation is used to treat drug-resistant focal epilepsy by delivering stimulation when epileptogenic activity is detected by continuously recording electrodes [Citation126]. Accurate DBS targeting for TRD, facilitated by tractography, may help identify similar accurate and effective electrophysiological biomarkers for depression. For example, a recent study using tractography-guided SCC DBS found SCC local field potentials that may indicate a patient’s clinical state and may help optimize stimulation [Citation127]. Overall, the development of tractography alongside DBS for TRD has the potential to facilitate precision neuromodulation in clinical practice.
Further investigation to establish effective targets for DBS for TRD using tractography will ultimately culminate in the standardization of methods for tractography-guided DBS. Optimal tractography methods must have the ability to identify white matter tracts of interest with sufficient accuracy and be compatible with readily available MRI technology. Perhaps most importantly, tractography must be feasible in clinical practice. Tractography-guided DBS is likely integral to the future of DBS for TRD and the standardization of approaches for its targets will be essential to transforming it into an established therapy for TRD.
Declaration of interest
JL Chan was supported by a Parkinson’s Foundation Institutional Movement Disorders Fellowship. EH Middlebrooks has received research support from Varian Medical Systems and Vigil Neuroscience and is a consultant and advisory board member for Boston Scientific, Varian Medical Systems, and Cortechs.ai. MS Okun serves as Medical Advisor for the Parkinson’s Foundation and has received research grants from the NIH, Parkinson’s Foundation, Michael J. Fox Foundation, the Parkinson Alliance, the Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. He is an associate editor for New England Journal of Medicine Journal Watch Neurology and JAMA Neurology. MS Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford, and Cambridge. He has participated in continuing medical education and educational activities on movement disorders sponsored by WebMD/Medscape, RMEI Medical Education, the American Academy of Neurology, the International Parkinson and Movement Disorder Society, Mediflix, and Vanderbilt University. The institution and not MS Okun receives grants from industry. He has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years, but has not received honoraria. JK Wong has received research support from the NIH. Finally, Medtronic and NeuroPace have donated devices for several NIH studies at the University of Florida. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9
- Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9(1):90.
- Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346.
- Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2(1):16065.
- Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917.
- Voineskos D, Daskalakis ZJ, Blumberger DM. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat. 2020;16: 221–234. 10.2147/NDT.S198774
- Marwaha S, Palmer E, Suppes T, et al. Novel and emerging treatments for major depression. Lancet. 2022;401(10371):141–153.
- Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15(3):148–160. doi: 10.1038/s41582-018-0128-2
- Moniz E. Prefrontal leucotomy in the treatment of mental disorders. Am J Psychiatry. 1937;93(6):1379–1385. doi: 10.1176/ajp.93.6.1379
- Hoffman JL. Clinical observations concerning schizophrenic patients treated by prefrontal leukotomy. N Engl J Med. 1949;241(6):233–236. doi: 10.1056/NEJM194908112410604
- Caruso JP, Sheehan JP. Psychosurgery, ethics, and media: a history of Walter Freeman and the lobotomy. Neurosurg Focus. 2017;43(3):E6. doi: 10.3171/2017.6.FOCUS17257
- Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. 2010;29(2):E1. doi: 10.3171/2010.4.FOCUS10106
- Bechtereva NP, Bondartchuk AN, Smirnov VM, et al. Method of electrostimulation of the deep brain structures in treatment of some chronic diseases. Confin Neurol. 1975;37(1–3):136–140.
- Cooper IS, Upton AR, Amin I. Reversibility of chronic neurologic deficits. Some effects of electrical stimulation of the thalamus and internal capsule in man. Appl Neurophysiol. 1980;43(3–5):244–258. doi: 10.1159/000102263
- Andy OJ. Thalamic stimulation for control of movement disorders. Appl Neurophysiol. 1983;46(1–4):107–111. doi: 10.1159/000101248
- Benabid AL, Pollak P, Louveau A, et al. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50(1–6):344–346.
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. 2013.
- Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377.
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15(7):1137–1140. doi: 10.1097/00001756-200405190-00011
- Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682.
- Coenen VA, Schlaepfer TE, Sajonz B, et al. Tractographic description of major subcortical projection pathways passing the anterior limb of the internal capsule. Corticopetal organization of networks relevant for psychiatric disorders. NeuroImage Clin. 2020;25:102165. doi: 10.1016/j.nicl.2020.102165
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11(7):503–513. doi: 10.1038/nrn2866
- Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci. 2020;21(5):277–295. doi: 10.1038/s41583-020-0292-4
- Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41(1):126–141. doi: 10.1038/npp.2015.185
- Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21(4):450–463. doi: 10.1038/mp.2016.1
- Velasco F, Velasco M, Jiménez F, et al. Neurobiological background for performing surgical intervention in the inferior thalamic peduncle for treatment of major depression disorders. Neurosurgery. 2005;57(3):439–448. doi: 10.1227/01.NEU.0000172172.51818.51
- Dandekar MP, Fenoy AJ, Carvalho AF, et al. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. 2018;23(5):1094–1112.
- Choi KS, Riva-Posse P, Gross RE, et al. Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72(11):1252–1260.
- Malone DA Jr., Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267–275.
- Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204–1212.
- Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839–49. doi: 10.1016/S2215-0366(17)30371-1
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. (NY): Oxford University Press; 2006.
- Hagmann P From diffusion MRI to brain connectomics [ dissertation]. Lausanne: École Polytechnique Fédérale de Lausanne; 2005.
- Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1(4):e42. doi: 10.1371/journal.pcbi.0010042
- Henderson JM. “Connectomic surgery”: diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Front Integr Neurosci. 2012;6:15. doi: 10.3389/fnint.2012.00015
- Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407.
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119
- Johansen-Berg H, Rushworth MFS. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32(1):75–94. doi: 10.1146/annurev.neuro.051508.135735
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782
- Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1
- Basser PJ, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037
- Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269.
- Behrens TEJ, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088.
- Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):893–902. doi: 10.1098/rstb.2005.1639
- Calabrese E. Diffusion tractography in deep brain stimulation surgery: a review. Front Neuroanat. 2016;10:45. doi: 10.3389/fnana.2016.00045
- Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029
- Essayed WI, Zhang F, Unadkat P, et al. White matter tractography for neurosurgical planning: a topography-based review of the current state of the art. NeuroImage Clin. 2017;15:659–672. doi: 10.1016/j.nicl.2017.06.011
- Maier-Hein KH, Neher PF, Houde J, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat Commun. 2017;8(1):1349.
- Schilling KG, Petit L, Rheault F, et al. Brain connections derived from diffusion MRI tractography can be highly anatomically accurate – if we know where white matter pathways start, where they end, and where they do not go. Brain Struct Funct. 2020;255(8):2387–2402.
- Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18(6):1374–1383.
- Gutman DA, Holtzheimer PE, Behrens TEJ, et al. A tractography analysis of two deep brain stimulation white matter targets for depression. Biological Psychiatry. 2009;65(4):276–282.
- Bhatia KD, Henderson L, Ramsey-Stewart G, et al. Diffusion tensor imaging to aid subgenual cingulum target selection for deep brain stimulation in depression. Stereotact Funct Neurosurg. 2012;90(4):225–232. doi: 10.1159/000338083
- Hamani C, Mayberg H, Snyder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209–1215.
- Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64(6):461–467.
- Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502–510.
- Merkl A, Schneider GH, Schönecker T, et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp Neurol. 2013;249:160–168. doi: 10.1016/j.expneurol.2013.08.017
- Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69(2):150–8.
- Puigdemont D, Portella M, Pérez-Egea R, et al. A randomized double-blind crossover trial of deep brain stimulation of the subcallosal cingulate gyrus in patients with treatment-resistant depression: a pilot study of relapse prevention. J Psychiatry Neurosci. 2015;40(4):224–231.
- Aibar-Durán J, Rodríguez Rodríguez R, de Diego Adeliño FJ, et al. Long-term results of deep brain stimulation for treatment-resistant depression: outcome analysis and correlation with lead position and electrical parameters. Neurosurgery. 2022;90(1):72–80.
- Riva-Posse P, Choi KS, Holtzheimer PE, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2018;23(4):843–849. doi: 10.1038/mp.2017.59
- Tsolaki E, Sheth SA, Pouratian N. Variability of white matter anatomy in the subcallosal cingulate area. Hum Brain Mapp. 2021;42(7):2005–17. doi: 10.1002/hbm.25341
- Clark DL, Johnson KA, Butson CR, et al. Tract-based analysis of target engagement by subcallosal cingulate deep brain stimulation for treatment resistant depression. Brain Stimulation. 2020;13(4):1094–1101. doi: 10.1016/j.brs.2020.03.006
- Haber SN, Yendiki A, Jbabdi S. Four deep brain stimulation targets for obsessive-compulsive disorder: are they different? Biol Psychiatry. 2021;90(10):667–677. doi: 10.1016/j.biopsych.2020.06.031
- Coenen VA, Döbrössy MD, Teo SJ, et al. Diverging prefrontal cortex fiber connection routes to the subthalamic nucleus and the mesencephalic ventral tegmentum investigated with long range (normative) and short range (ex-vivo high resolution) 7T DTI. Brain Struct Funct. 2022;227(1):23–47.
- Coenen VA, Panksepp J, Hurwitz TA, et al. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J Neuropsychiatry Clin Neurosci. 2012;24(2):223–236.
- Coenen VA, Sajonz B, Reisert M, et al. Tractography-assisted deep brain stimulation of the superolateral branch of the medial forebrain bundle (slMFB DBS) in major depression. NeuroImage Clin. 2018;20:580–593. doi: 10.1016/j.nicl.2018.08.020
- Bewernick BH, Kayser S, Gippert SM, et al. Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimul. 2017;10(3):664–671.
- Fenoy AJ, Schulz P, Selvaraj S, et al. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143–151. doi: 10.1016/j.jad.2016.05.064
- Fenoy AJ, Schulz PE, Sanches M, et al. Deep brain stimulation of the “medial forebrain bundle”: sustained efficacy of antidepressant effect over years. Mol Psychiatry. 2022;27(5):2546–2553.
- Coenen VA, Bewernick BH, Kayser S, et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;44(7):1224–32.
- Davidson B, Giacobbe P, Mithani K, et al. Lack of clinical response to deep brain stimulation of the medial forebrain bundle in depression. Brain Stimul. 2020;13(5):1268–1270.
- Salgado S, Kaplitt MG. The nucleus accumbens: A comprehensive review. Stereotact Funct Neurosurg. 2015;93(2):75–93. doi: 10.1159/000368279
- Abelson JL, Curtis GC, Sagher O, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biological Psychiatry. 2005;57(5):510–516.
- Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive–compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384–2393.
- Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–116.
- Bewernick BH, Kayser S, Sturm V, et al. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37(9):1975–1985. doi: 10.1038/npp.2012.44
- Millet B, Jaafari N, Polosan M, et al. Limbic versus cognitive target for deep brain stimulation in treatment-resistant depression: accumbens more promising than caudate. Eur Neuropsychopharmacol. 2014;24(8):1229–1239.
- Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240–248.
- Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456–64.
- Lujan JL, Chaturvedi A, Malone DA, et al. Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Hum Brain Map. 2012;33(4):958–968. doi: 10.1002/hbm.21262
- Liebrand LC, Natarajan SJ, Caan MWA, et al. Distance to white matter trajectories is associated with treatment response to internal capsule deep brain stimulation in treatment-refractory depression. NeuroImage Clin. 2020;28:102363. doi: 10.1016/j.nicl.2020.102363
- Lai Y, Dai L, Wang T, et al. Structural and functional correlates of the response to deep brain stimulation at ventral capsule/ventral striatum region for treatment-related depression. J Neurol Neurosurg Psychiatry. 2023;94(5):379–388.
- Lawson RP, Drevets WC, Roiser JP. Defining the habenula in human neuroimaging studies. Neuroimage. 2013;64:722–727. doi: 10.1016/j.neuroimage.2012.08.076
- Sartorius A, Kiening KL, Kirsch P, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67(2):e9–e11.
- Kiening K, Sartorius A. A new translational target for deep brain stimulation to treat depression. EMBO Mol Med. 2013;5(8):1151–1153. doi: 10.1002/emmm.201302947
- Wang Z, Cai X, Qiu R, et al. Case report: Lateral habenula deep brain stimulation for treatment-resistant depression. Front Psychiatry. 2021;11:616501. doi: 10.3389/fpsyt.2020.616501
- Zhang C, Zhang Y, Luo H, et al. Bilateral Habenula deep brain stimulation for treatment-resistant depression: clinical findings and electrophysiological features. Transl Psychiatry. 2022;12(1):52.
- He N, Sethi SK, Zhang C, et al. Visualizing the lateral habenula using susceptibility weighted imaging and quantitative susceptibility mapping. Magn Reson Imaging. 2020;65:55–61. doi: 10.1016/j.mri.2019.09.005
- Krüger OS, Kreifelts B, Scheffler K, et al. Three distinct fiber pathways of the bed nucleus of the stria terminalis to the amygdala and prefrontal cortex. Cortex. 2015;66:60–68. doi: 10.1016/j.cortex.2015.02.007
- Greenberg BD, Gabriels LA, Malone DA, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64–79.
- Luyten L, Hendrickx S, Raymaekers S, et al. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21(9):1272–1280.
- Raymaekers S, Luyten L, Bervoets C, et al. Deep brain stimulation for treatment-resistant major depressive disorder: a comparison of two targets and long-term follow-up. Transl Psychiatry. 2017;7(10):e1251.
- Blomstedt P, Naesström M, Bodlund O. Deep brain stimulation in the bed nucleus of the stria terminalis and medial forebrain bundle in a patient with major depressive disorder and anorexia nervosa. Clin Case Rep. 2017;5(5):679–684. doi: 10.1002/ccr3.856
- Cassimjee N, van Coller R, Slabbert P, et al. Longitudinal neuropsychological outcomes in treatment-resistant depression following bed nucleus of the stria terminalis-area deep brain stimulation: a case review. Neurocase. 2018;24(5–6):231–237. doi: 10.1080/13554794.2018.1549680
- Fitzgerald PB, Segrave R, Richardson KE, et al. A pilot study of bed nucleus of the stria terminalis deep brain stimulation in treatment-resistant depression. Brain Stimul. 2018;11(4):921–928.
- Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585–593.
- Jiménez F, Nicolini H, Lozano AM, et al. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80(3–4):S30.e17–25. doi: 10.1016/j.wneu.2012.07.010
- Choi S, Cunningham DT, Aguila F, et al. DTI at 7 and 3 T: systematic comparison of SNR and its influence on quantitative metrics. Magn Reson Imaging. 2011;29(6):739–751.
- Polders DL, Leemans A, Hendrikse J, et al. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 Tesla. J Magn Reson Imaging. 2011;33(6):1456–1463.
- Pipe J. Pulse sequences for diffusion-weighted MRI. In: Johansen-Berg H Behrens T, editors Diffusion MRI: from quantitative measurement to in vivo neuroanatomy. London: Elsevier; 2009. p. 11–35.
- Tuch DS, Reese TG, Wiegell MR, et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–582.
- Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52(6):1358–1372. doi: 10.1002/mrm.20279
- Wedeen VJ, Hagmann P, Tseng WI, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54(6):1377–1386.
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. Neuroimage. 2005;27(1):48–58. doi: 10.1016/j.neuroimage.2005.03.042
- Zhang F, Daducci A, He Y, et al. Quantitative mapping of the brain’s structural connectivity using diffusion MRI tractography: a review. Neuroimage. 2022;249:118870. doi: 10.1016/j.neuroimage.2021.118870
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallicies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081
- Bürgel U, Amunts K, Hoemke L, et al. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29(4):1092–1105.
- Lawes IN, Barrick TR, Murugam V, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39(1):62–79.
- Van Essen DC, Smith SM, Barch DM, et al. The WU-Minn human connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041
- Lujan JL, Chaturvedi A, Choi KS, et al. Tractography-activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimul. 2013;6(5):737–739.
- Riva-Posse P, Crowell AL, Wright K, et al. Rapid antidepressant effects of deep brain stimulation and their relation to surgical protocol. Biol Psychiatry. 2020;88(8):e37–e39.
- Guye M, Parker GJM, Symms M, et al. Combined functional MRI and tractography to demonstrate the connectivity of the human primary motor cortex in vivo. Neuroimage. 2003;19(4):1349–1360.
- Staempfli P, Reischauer C, Jaermann T, et al. Combining fMRI and DTI: a framework for exploring the limits of fMRI-guided DTI fiber tracking and for verifying DTI-based fiber tractography results. Neuroimage. 2008;39(1):119–126.
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004
- Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165.
- Sarica C, Yamamoto K, Loh A, et al. Blood oxygen level-dependent (BOLD) response patterns with thalamic deep brain stimulation in patients with medically refractory epilepsy. Epilepsy Behav. 2021;122:108153. doi: 10.1016/j.yebeh.2021.108153
- Middlebrooks EH, Jain A, Okromelidze L, et al. Acute brain activation patterns of high- versus low-frequency stimulation of the anterior nucleus of the thalamus during deep brain stimulation for epilepsy. Neurosurgery. 2021;89(5):901–908.
- Senova S, Clair A, Palfi S, et al. Deep brain stimulation for refractory obsessive-compulsive disorder: Towards an individualized approach. Front Psychiatry. 2019;10:905. doi: 10.3389/fpsyt.2019.00905
- Sheth SA, Mayberg HS. Deep brain stimulation for obsessive-compulsive disorder and depression. Annu Rev Neurosci. 2023;46(1):341–358. doi: 10.1146/annurev-neuro-110122-110434
- Hsu TI, Nguyen A, Gupta N, et al. Effectiveness of deep brain stimulation in treatment of anorexia nervosa and obesity: a systematic review. World Neurosurg. 2022;168:179–189. doi: 10.1016/j.wneu.2022.09.114
- Yuen J, Kouzani AZ, Berk M, et al. Deep brain stimulation for addictive disorders – where are we now? Neurotherapeutics. 2022;19(4):1193–1215.
- Nanda P, Banks GP, Pathak YJ, et al. Connectivity-based parcellation of the anterior limb of the internal capsule. Hum Brain Mapp. 2017;38(12):6107–6117. doi: 10.1002/hbm.23815
- Neumann W, Gilron R, Little S, et al. Adaptive deep brain stimulation: From experimental evidence toward practical implementation. Mov Disord. 2023;38(6):937–948.
- Nair DR, Laxer KD, Weber PB, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):e1244–1256.
- Alagapan S, Choi KS, Heisig S, et al. Cingulate dynamics track depression recovery with deep brain stimulation. Nature. 2023;622(7981):130–138.
