1. Introduction and definition
Status epilepticus (SE) is a medical emergency that can lead to permanent neurological damage if not adequately managed [Citation1]. The incidence in the pediatric population is approximately 20 cases per 100,000 children per year, with a peak in children under the age of 2 years. The mortality rate is about 3%, but it can overcome 10% in children admitted to the intensive care unit (ICU) for refractory SE [Citation2].
Over the past decades, it has become clear that the most efficient way to prevent SE is to stop the seizure as fast as possible, and early treatment of prolonged convulsive seizures has become an integral part of the overall treatment strategy in epilepsy. Additionally, the terms ‘early’ or ‘impending’ SE have been defined as continuous or intermittent seizures lasting longer than 5 minutes without full recovery of consciousness between seizures [Citation3].
Currently, the appropriate treatment of SE is hindered by numerous factors, including the underdosed use of ‘rescue medications’ by caregivers and pre-hospital healthcare providers and the inadequate application of defined (also in timing) treatment protocols upon arrival in the emergency department (ED) [Citation4,Citation5]. In general, 10% of epilepsy patients have a prescription for rescue medications, most of whom are of pediatric age, especially patients with febrile convulsions. The pre-hospital availability of rescue medications and adequate training of operators and caregivers in managing the episode are capable of reducing rescue times and the risk of prolonged seizures evolving into SE, consequently improving the outcome. Specifically, caregivers should be able to recognize seizures, know which medications to administer (correct route and dosage), and under which circumstances to seek help from healthcare professionals [Citation6].
While some therapeutic strategies are supported by Level I evidence, such as the use of benzodiazepines as first-line drugs, others are based on limited data, especially in pediatric settings. This variability is due to the scarcity of studies aimed at comparing the effectiveness of individual second and third-line drugs on an appropriate population (heterogeneous case studies and low sample size). For purely practical purposes, this editorial divides the recommended treatment into phases ().
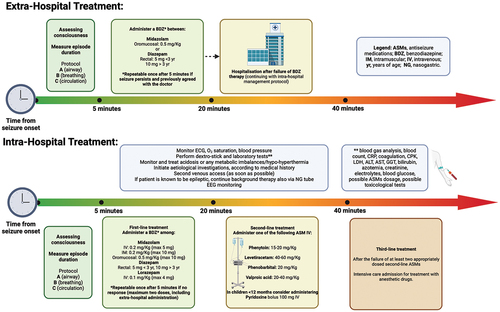
2. Treatment protocol
2.1. PHASE I. Stabilization and pre-hospital treatment
2.1.1. General measures
Evaluate the level of consciousness.
Measure the duration of the seizure episode.
Initiate pre-hospital treatment and call emergency services (911 or equivalent).
Assess and stabilize vital functions following the ABC sequence of Basic Life Support (Airway, Breathing, Circulation).
2.1.2. Pharmacological treatment
The ideal drug in the early phases of the seizure should: 1) have a rapid and sustained action, 2) be easy to administer, 3) ensure an acceptable safety profile with limited adverse events (AEs), especially in terms of respiratory depression and hemodynamic stability, 4) be socially acceptable, easy to store, transport, and use. In general, the early and adequate use of benzodiazepines is the preferred treatment [Citation7,Citation8]. This therapy can be repeated a second time after about 5 minutes if the seizure persists [Citation8]. In the absence of intravenous access, buccal midazolam and rectal diazepam are the first-line treatments for prolonged seizures and convulsive SE [Citation8]. Midazolam owns pharmacokinetic properties that ensure rapid action and a short half-life, supporting its use with various administration routes (intravenous, intramuscular, buccal, intranasal). Diazepam has rapid action, but rectal absorption is variable and not always predictable. Additionally, the use of diazepam is associated with an increased risk of recurrence and respiratory depression [Citation9]. However, in our experience, we prefer rectal administration for children younger than 3 years of age ().
Table 1. Extra-hospital pharmacological treatment of prolonged convulsive seizures.
2.2. PHASE II. Intra-hospital treatment at the first and second levels
Intra-hospital treatment should be based on the application of institutional protocols/algorithms that define the different phases of treatment and their respective medications (administration route, dosage, timing). The main published algorithms are divided into the following phases:
STABILIZATION (0–5 minutes): Initial phase where general supportive measures are applied.
FIRST-LINE TREATMENT (5–20 minutes): Administration of benzodiazepines.
SECOND-LINE TREATMENT (20–40 minutes): Lack of response to benzodiazepines indicates the administration of antiseizure medications (ASMs) belonging to different pharmacological classes.
THIRD-LINE TREATMENT (40–60 minutes): Refractoriness to at least two adequately dosed second-line ASMs leads to admission to the ICU for treatment with anesthetic drugs [Citation8,Citation10].
2.2.1. Pharmacological treatment at the first and second levels
In addition to its use in pre-hospital treatment, the effectiveness of benzodiazepines has also been demonstrated in the ED in 40–80% of cases [Citation7,Citation8]. The intravenous route should be preferred if venous access is present. However, no more than two doses of intravenous benzodiazepines should be administered (including any pre-hospital dose) as the effectiveness of a third dose would be limited and increase the risk of respiratory depression [Citation11].
lists the benzodiazepines commonly used in a hospital setting.
Table 2. Intra-hospital pharmacological treatment of status epilepticus.
If the seizure persists (more than 5 minutes after the last administration), the administration of a second ASM not belonging to the benzodiazepine class is indicated. Phenytoin and phenobarbital have specific indications and are present in most of the protocols [Citation8]. However, in recent years, other drugs such as levetiracetam, valproic acid, lacosamide, and brivaracetam have also become available, albeit ‘off-label’ [Citation9]. Large randomized controlled studies in pediatric patients published in recent years have shown that phenytoin, levetiracetam, and valproic acid have comparable efficacy, but the following considerations should be made: ease of administration, pharmacokinetic characteristics, and safety profile make levetiracetam a valid alternative to phenytoin; the use of valproic acid is limited due to its contraindications (metabolic diseases, mitochondrial disorders, liver disease), particularly in young children where these conditions cannot be ruled out at the onset [Citation9,Citation12,Citation13]. The dosage and administration methods of second-line drugs are summarized in .
In patients under 12 months old, the administration of pyridoxine as a bolus can be considered under EEG monitoring: 100 mg IV (for pyridoxine-dependent epilepsy) [Citation14].
If the seizures persist after 5 minutes from the administration of the first second-line drug, another second-line drug with a different mechanism of action should be administered, paying attention to pharmacological interactions (e.g. phenytoin and valproic acid compete for the protein binding site). If the seizure continues beyond this point, further treatments should be avoided as they are ineffective in over 85% of cases and may delay the appropriate management in intensive care [Citation1,Citation8].
2.3. PHASE III. Intensive care management and third-level treatment
Intensive care treatment of SE is indicated in case of no response to first and second-line drugs (refractory SE) or in the presence of systemic complications such as hemodynamic instability and/or respiratory failure. In this phase, continuous EEG monitoring is mandatory for diagnostic purposes (identification of non-convulsive SE) and for monitoring the treatment with continuous infusion anesthetic drugs.
2.3.1. Pharmacological therapy
The commonly used medications in children and adults, with induction and continuous infusion doses, are midazolam, thiopental, ketamine (). Propofol is not recommended in pediatric patients due to the potentially fatal risk of propofol infusion syndrome (rhabdomyolysis, acidosis, cardiac arrhythmia) for infusions at doses greater than 4 mg/kg/h for more than 24–48 hours. Other pharmacological options include topiramate, isoflurane, lidocaine, valproic acid, high-dose phenobarbital, lacosamide, perampanel, zonisamide, and brivaracetam, and adjuvant therapies can be applied, such as ketogenic diet, hypothermia and vagus nerve stimulation.
There are no randomized clinical trials supporting the choice of a particular pharmacological strategy. The EEG target of the pharmacological treatment is not well defined and could be represented by either suppression of paroxysmal activity or burst suppression for at least 24–48 hours.
SE persisting at least 24 hours after onset of anesthesia, either without interruption despite appropriate treatment with anesthesia, or recurring while on appropriate anesthetic treatment, or recurring after withdrawal of anesthesia is defined as super refractory SE. In cases of super-refractory SE caused by autoimmune encephalitis (confirmed or suspected diagnosis), immunomodulating therapy includes first-line treatment (steroids, intravenous immunoglobulins, plasmapheresis) and second-line drugs if indicated (rituximab, anakinra, bortezomib, tocilizumab, cyclophosphamide) [Citation15].
3. Conclusions
Status epilepticus is a neurological emergency that must be recognized and treated promptly to prevent progression to refractory SE and secondary neurological damage. Institutional protocols and structured algorithms from the first to the third level (timings, medications, doses) are essential to implement appropriate and timely treatment strategies and ensure the best prognosis.
4. Expert opinion
The purpose of this editorial is to share a therapeutic algorithm that can be helpful in clinical practice, especially for first and second-line treatment of SE. In fact, involvement of specialized neurologists often occurs only at a later stage, starting from the second or third-line treatments.
Additionally, recent advancements in epilepsy pharmacology imply a continuous need for updates, and the complex dynamics of drug authorization by regulatory agencies for the specific region become more and more relevant. For example, oromucosal midazolam in its pre-dosed form is currently approved by the European Medical Agency but not yet by the Food and Drug Administration. On the other hand, the use of intranasal benzodiazepines (midazolam or diazepam) is not yet available in some Countries. Consequently, protocols primarily reflect the current approvals from the drug regulatory agencies of the Countries where they are applied.
An interesting point of development for the coming years is the placement of new-generation ASMs in future protocols, ‘gaining positions’ as therapeutic options after the failure of benzodiazepines. Moreover, ketamine, commonly used in anesthesia and in the ED for short procedures, presents antiepileptic and neuroprotective properties and has been recently proposed as second-line agent for its unique pharmacological and safety profiles [Citation16].
As shown in our protocol, in choosing a second-line drug, it is essential to consider the patient’s comorbidities and, if known, the cause of epilepsy. Therefore, the use of a sodium blocker as phenytoin should be avoided in the case of specific channelopathies such as Dravet syndrome [Citation17], such as the use of valproic acid if an underlying metabolic disorder is even just suspected.
Not least important for the prevention and management of SE is caregiver education, in the case of known epileptic patients. Caregivers must be able to recognize and promptly handle all ‘urgency/emergency’ situations that require access to the emergency room or call to 911: typically a prolonged seizure lasting more than 5 minutes and/or a seizure cluster without recovery to the baseline neurological state between seizures (for which we suggest management according to the ‘SE protocol’), the presence of respiratory impairment, or failure to regain consciousness at the end of the seizure.
Seizure clusters, also called acute repetitive seizures, are defined as a series of grouped seizures that have short interictal periods. The exact definition is still controversial, but it refers to a condition of a significant increase in the frequency of epileptic seizures, which puts the patient at risk of developing SE [Citation18]. A seizure cluster, unlike SE, implies a complete recovery to the baseline neurological state between episodes.
Epileptic patients at risk of developing acute repetitive seizures should have an extra-hospital plan, often including the administration of benzodiazepines, in an attempt to prevent the cluster from progressing.
A discussion on the different treatment options for repetitive acute seizures, as well as the treatment of neonatal seizures, is beyond the scope of this editorial.
Declaration of interest
IRCCS ‘G. Gaslini’ is a member of ERN-Epicare. A Riva has received honoraria from Kolfarma, Proveca Pharma, PTC Therapeutics, Jazz Pharmaceuticals, and UCB Pharma; E Russo has received speaker fees or funding from, and has participated in advisory boards for, Angelini Pharma, Eisai, Pfizer, GW Pharmaceuticals, Jazz pharmaceuticals, UCB and Kolfarma; P Striano has received speaking grants from Neuraxpharma, Jazz pharma, BioMarin, Proveca, UCB Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We would like Dr. Luigi Francesco Iannone for the creation of the figure.
Additional information
Funding
References
- Gaínza-Lein M, Sánchez Fernández I, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 2018;75(4):410. doi: 10.1001/jamaneurol.2017.4382
- Gurcharran K, Grinspan ZM. The burden of pediatric status epilepticus: epidemiology, morbidity, mortality, and costs. Seizure. 2019;68:3–8. doi: 10.1016/j.seizure.2018.08.021
- McKenzie KC, Hahn CD, Friedman JN. Emergency management of the paediatric patient with convulsive status epilepticus. Paediatr Child Health. 2021 Jan 21;26(1):50–66. doi: 10.1093/pch/pxaa127
- Sathe AG, Underwood E, Coles LD, et al. Patterns of benzodiazepine underdosing in the established status epilepticus treatment trial. Epilepsia. 2021 Mar;62(3):795–806.
- Sánchez Fernández I, Abend NS, Agadi S, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015 Jun 9;84(23):2304–11. doi: 10.1212/WNL.0000000000001673
- Fesler JR, Belcher AE, Moosa AN, et al. The efficacy and use of a pocket card algorithm in status epilepticus treatment. Neurol Clin Pract. 2021;11(5):406–412. doi: 10.1212/CPJ.0000000000000922
- Mctague A, Martland T, Appleton R. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev. 2018;2018(1). doi: 10.1002/14651858.CD001905.pub3
- Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American epilepsy society. Epilepsy Curr. 2016;16(1):48–61. doi: 10.5698/1535-7597-16.1.48
- Lawton B, Davis T, Goldstein H, et al. An update in the initial management of paediatric status epilepticus. Curr Opin Pediatr. 2018;30(3):359–363. doi: 10.1097/MOP.0000000000000616
- Vasquez A, Gaínza-Lein M, Sánchez Fernández I, et al. Hospital emergency treatment of convulsive status epilepticus: comparison of pathways from Ten pediatric research centers. Pediatr Neurol. 2018;86:33–41. doi: 10.1016/j.pediatrneurol.2018.06.004
- Hill CE, Parikh AO, Ellis C, et al. Timing is everything: where status epilepticus treatment fails. Ann Neurol. 2017;82(2):155–165. doi: 10.1002/ana.24986
- Chamberlain JM, Kapur J, Shinnar S, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395(10231):1217–1224. doi: 10.1016/S0140-6736(20)30611-5
- D’Onofrio G, Riva A, Amadori E, et al. Pharmacokinetic considerations surrounding the use of levetiracetam for seizure prophylaxis in neurocritical care - an overview. Expert Opin Drug Metab Toxicol. 2022 Sep;18(9):575–585.
- Gospe SM Jr. Pyridoxine-Dependent Epilepsy – ALDH7A1. In: Adam M, Feldman J Mirzaa G, et al. Eds. GeneReviews® Internet. Seattle (WA): University of Washington, Seattle; 1993-2024 2001 Dec 7 [cited 2022 Sep 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1486/
- Wickstrom R, Taraschenko O, Dilena R, et al. International consensus recommendations for management of new onset refractory status epilepticus (NORSE) including febrile infection-related epilepsy syndrome (FIRES): summary and clinical tools. Epilepsia. 2022 Aug 11;63(11):2827–2839. doi: 10.1111/epi.17391
- Buratti S, Giacheri E, Palmieri A, et al. Ketamine as advanced second-line treatment in benzodiazepine-refractory convulsive status epilepticus in children. Epilepsia. 2023 Apr;64(4):797–810.
- Riva A, D’Onofrio G, Amadori E, et al. Current and promising therapeutic options for Dravet syndrome. Expert Opin Pharmacother. 2022 Oct;23(15):1727–1736.
- Haut SR, Nabbout R. Recognizing seizure clusters in the community: the path to uniformity and individualization in nomenclature and definition. Epilepsia. 2022 Sep;63(Suppl 1):S6–S13. doi: 10.1111/epi.17346