ABSTRACT
Background
We investigated the long-term safety and effectiveness of linagliptin in Japanese type 2 diabetes (T2D) patients starting linagliptin add-on therapy in routine clinical practice.
Research design and methods
This 3-year prospective, observational, post-marketing surveillance (PMS) was conducted in Japanese patients starting linagliptin add-on therapy. The primary outcome was the incidence of adverse drug reactions (ADRs). The secondary outcome was the change from baseline in HbA1c.
Results
The safety analysis set comprised of 3,372 patients. Mean ± standard deviation (SD) age was 66.5 ± 12.4 years. Most patients (63.2%) received linagliptin in combination with another antidiabetic drug, most commonly a sulfonylurea (38.6%). The incidence of ADRs was 11.39%; the most common ADRs according to MedDRA preferred terms were diabetes mellitus (1.25%), hypertension (0.83%), and hypoglycemia (0.80%). In the effectiveness analysis set (n = 3,029), mean ± SD HbA1c was 7.76 ± 1.37% at baseline and 7.26 ± 1.19% at last observation; mean change from baseline to last observation was – 0.49 ± 1.33%; sustained reductions in HbA1c were observed. These results were consistent across patient subgroups.
Conclusions
In this PMS, linagliptin add-on therapy for Japanese T2D patients had a safety profile consistent with its known profile and HbA1c reductions over 3 years were observed.
ClinicalTrials.gov
NCT01904383
1. Introduction
The estimated number of patients with type 2 diabetes (T2D) worldwide in 2017 was approximately 450 million [Citation1]. According to the 2016 National Health and Nutrition Survey by Japanese Ministry of Health, Labour and Welfare (MHLW), about 10 million people in Japan were strongly suspected to have diabetes [Citation2]. A 2017 patient survey by the MHLW estimated that 71% of Japanese patients with diabetes were aged ≥65 years and 38% were ≥75 years [Citation3]. As the risk of hypoglycemia increases with age [Citation4,Citation5], the Japan Diabetes Society not only recommends glycated hemoglobin (HbA1c) target levels for older patients with T2D but also defines a lower HbA1c limit for this patient age group who are at risk of severe hypoglycemia, such as patients using drugs potentially associated with hypoglycemia (e.g. insulin, sulfonylureas, glinides) [Citation6]. Therefore, it is particularly important for elderly patients to have antidiabetic drugs that can provide effective and safe glycemic control with minimal hypoglycemia.
Dipeptidyl peptidase-4 inhibitors (DPP-4is) are one class of antidiabetic drug available for the treatment of T2D in Japan. More than 70% of Japanese patients with T2D who are treated with oral antidiabetic drugs (OADs) receive DPP-4is [Citation7] either as monotherapy or as add-on therapy to other antidiabetic drugs, such as sulfonylureas (SUs), biguanides (BGs), or alpha-glucosidase inhibitors (AGIs) [Citation7,Citation8]. Linagliptin is a DPP-4i with a xanthine-based structure and is orally administered once-daily [Citation9,Citation10]. Linagliptin inhibits the proteolytic activity of the DPP4 enzyme, thereby increasing plasma levels of active glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide after a meal, which results in decreased plasma glucose levels via enhanced insulin secretion from beta cells and decreased glucagon secretion from alpha cells [Citation11].
The safety, including the incidence of hypoglycemia, and efficacy of linagliptin in patients with T2D have been confirmed in several previous Phase 3 clinical trials [Citation12–20], including a 52-week Phase 3 trial that evaluated the use of linagliptin as add-on therapy to other OADs in Japanese patients with T2D [Citation21]. In addition, the long-term cardiovascular and renal safety of linagliptin has been demonstrated in a broad range of patients with T2D in the CARMELINA® trial (median follow-up 2.2 years) [Citation22] and CAROLINA® trial (median follow-up 6.3 years) [Citation23], in which 8.0% and 15.5% of patients, respectively, were from Asia (including Japan) [Citation24,Citation25]. However, these trials had several limitations. For example, in the Phase 3 clinical trial in Japanese patients with T2D, only about 30% of patients were aged ≥65 years and about 8% aged ≥75 years, and the observation period was 52 weeks [Citation21]. Therefore, data on the long-term safety and effectiveness of linagliptin in a larger population of Japanese patients in routine clinical practice are needed. A post-marketing surveillance (PMS) study in a population with an age distribution similar to that encountered in routine clinical practice would provide valuable information on the long-term safety and effectiveness of linagliptin in Japanese patients with T2D.
Linagliptin was first launched in Japan on 1 July 2011 as monotherapy for patients with T2D inadequately controlled with medical diet and physical exercise. The results of a 3-year PMS which evaluated the long-term safety and effectiveness of linagliptin monotherapy in Japanese patients with T2D in routine clinical practice have been previously reported [Citation26]. On 25 March 2013, the indication for linagliptin was extended to include add-on therapy for patients with T2D who had inadequate glycemic control during treatment with other OADs and/or insulin. A 3-year PMS that evaluated the long-term safety and effectiveness of linagliptin as add-on therapy in Japanese patients with T2D in routine clinical practice was conducted from July 2013 and an interim analysis was previously published [Citation27]. Here we report the final results of this PMS, and describe our analysis of the safety and effectiveness of linagliptin in patient subgroups based on their concomitant antidiabetic drugs and age at baseline.
2. Patients and methods
2.1. Study design
This was a prospective, observational PMS conducted between July 2013 (the administration start date of the first patient) and October 2018 (the observation date of the last patient) in Japan in patients with T2D who started linagliptin as add-on therapy. The aim of the PMS was to investigate the long-term safety and effectiveness of linagliptin in routine clinical practice and meet regulatory obligations based on the Japanese Pharmaceutical Affairs Law. The protocol for this PMS was approved by the Japanese MHLW, and this PMS was conducted in full compliance with Japanese Good Post-marketing Study Practice regulations. This PMS was registered on ClinicalTrials.gov (NCT01904383).
2.2. Patients and treatment
Patients were eligible for inclusion if they had T2D, were receiving treatment with any other antidiabetic drugs, and were initiating treatment with linagliptin for the first time. No other inclusion or exclusion criteria were applied.
All treatment decisions were made at investigator’s discretion because this survey aimed to investigate the safety and effectiveness of linagliptin in routine clinical practice. This included all decisions around starting linagliptin therapy, the choice of concomitant antidiabetic drugs, and any changes to dosages (escalation or reduction).
2.3. Data collection
Patient registration and data collection were performed using an electronic data capture system. Investigators were asked to enter data on each patient’s clinical and demographic characteristics at baseline, and data on body weight, laboratory tests (HbA1c, fasting plasma glucose [FPG] and estimated glomerular filtration rate [eGFR]), vital signs (systolic and diastolic blood pressure [SBP and DBP]) and the dose of concomitant antidiabetic drugs at each visit. Laboratory tests were performed with the routine assays used at each investigator’s site. Observation time points were defined as Week 0 (baseline), 12, 26, 40, 52, 64, 78, 104, 130, and 156 weeks after starting linagliptin therapy, and the time of linagliptin discontinuation. However, because data collection time points were dependent on patient visits to their physicians according to their usual clinical care, data were collected at the visits closest to the time points defined above.
2.4. Endpoints
The primary safety endpoint of this PMS was the incidence of adverse drug reactions (ADRs). ADRs were defined as adverse events (AEs) whose causal relationships to linagliptin were assessed by the investigator and/or sponsor as ‘definite,’ ‘probably definite,’ or ‘cannot be denied.’ AEs were coded using lowest level terms (LLTs) of the Medical Dictionary for Regulatory Activities (MedDRA) version 21.1. The frequency of ADRs was tabulated by preferred terms (PTs) of MedDRA. In this PMS, ‘worsening of the underlying disease’ or ‘worsening of complications’ were recorded as AEs according to the protocol. For example, if investigators reported ‘worsening control of type 2 diabetes,’ it was coded using the LLT of ‘diabetes mellitus aggravated’ and categorized in the PT category of ‘diabetes mellitus.’ If investigators reported ‘worsening control of blood sugar,’ it was coded as the LLT of ‘loss of control of blood sugar,’ and categorized in the PT category of ‘diabetes mellitus inadequate control.’ ADRs of special interest in this study were ‘hypoglycemia,’ ‘hepatic dysfunction,’ ‘cardiac failure,’ ‘hypersensitivity,’ ‘worsening of renal function,’ ‘pancreatic cancer,’ ‘pemphigoid,’ ‘intestinal obstruction,’ ‘pancreatitis,’ ‘skin lesions,’ ‘infections,’ and ‘Interstitial lung disease.’ ADRs of special interest were categorized according to the standardized MedDRA queries (SMQs), MedDRA SOCs (System Organ Classes), or Boehringer Ingelheim-customized MedDRA queries (BIcMQs).
The secondary effectiveness endpoint was the change from baseline in HbA1c at the last observation. Other effectiveness endpoints included changes from baseline in HbA1c and FPG over time during the observation period. Other endpoints included body weight, eGFR, SBP, and DBP.
2.5. Statistical analysis
We estimated that a sample size of 3,000 patients would have 95% probability of being able to detect ≥1 ADR with an incidence 0.1%, and 99% probability of being able to detect an ADR with an incidence of 0.16%.
The safety evaluation was conducted in the safety analysis set, which included all patients who received linagliptin as add-on therapy and had ≥1 post-baseline visit. The effectiveness evaluation was conducted in the effectiveness analysis set, which consisted of patients included in the safety analysis set, except those who had no HbA1c values at baseline or after study entry, did not have T2D, or started linagliptin concomitantly with another OAD but had not been taking any antidiabetic medications before starting linagliptin.
Because this was an observational study without a comparator arm, most analyses were descriptive using frequencies and proportions for categorical variables, and mean and standard error or standard deviation (SD), median and range for continuous variables, such as HbA1c and FPG, except for 95% confidence intervals (CI) for the change from baseline in HbA1c and FPG at the last observation. Descriptive statistics were calculated for change from baseline in HbA1c, FPG, body weight, eGFR, SBP, and DBP. For changes from baseline in HbA1c and FPG over time, a mixed model for repeated measures was used.
Subgroup analyses for primary endpoints, secondary endpoints, and change from baseline in HbA1c over time were conducted according to baseline concomitant antidiabetic drug and baseline age (<65 years, 65–<75 years, ≥75 years). Patients who had been prescribed linagliptin in combination with ≥2 other antidiabetic drugs at baseline were included in each concomitant antidiabetic drug subgroup. In addition, change from baseline in HbA1c over time was also analyzed in a subgroup of patients who started linagliptin in combination with another antidiabetic drug and had no change in concomitant antidiabetic drug regimen and dose during the observation period. Patients who were treated with fixed-dose combination drugs at baseline were excluded from the subgroup analyses by concomitant antidiabetic drug because it was impossible to determine which ingredients in the fixed-dose combination were responsible for the effect. Similarly, no subgroup analysis for effectiveness was conducted in patients who were treated with GLP-1 receptor agonists (GLP-1RAs) or DPP-4is at baseline because these agents had the same antidiabetic mechanisms as linagliptin.
All statistical analyses were conducted using SAS version 9.4 (SAS institute, Cary, NC, USA).
3. Results
3.1. Disposition of patients
A total of 4,057 patients were registered at 658 sites, and case report forms were collected from 3,940 patients (). The safety analysis set included 3,372 patients; 568 patients were excluded because they did not start linagliptin as add-on therapy at baseline or did not return for a patient visit after registration. Of the 3,372 patients included in the safety analysis set, 343 patients were excluded because they did not have T2D, they started linagliptin concomitantly with another OAD but had not been taking any antidiabetic medications before starting linagliptin, or they did not have baseline or post-baseline HbA1c values. Therefore, 3,029 patients were included in the effectiveness analysis set.
Figure 1. Patient disposition. *Patient could have ≥1 reason for exclusion. CRF, case report form; HbA1c, glycated hemoglobin based on the National Glycohemoglobin Standardization Program (NGSP); OAD, oral antidiabetic drug
Of the 3,372 patients in the safety analysis set, 61.2% were male (). The mean ± SD age was 66.5 ± 12.4 years; 61.1% of patients were aged ≥65 years and 28.4% were aged ≥75 years. The mean ± SD body mass index (BMI) was 25.3 ± 4.6 kg/m2 and body weight was 65.3 ± 14.7 kg. Other patient characteristics are shown in .
Table 1. Baseline characteristics and duration of linagliptin use of patients in the safety analysis set
Most patients (n = 2,130; 63.2%) received linagliptin in combination with one other antidiabetic drug, and 1,242 (36.8%) received linagliptin in combination with ≥2 other antidiabetic drugs. The most common combination at baseline was with SUs (n = 1,301, 38.6%), followed by BGs (n = 1,201, 35.6%), AGIs (n = 732, 21.7%), and insulin (n = 714, 21.2%). Baseline patient characteristics by concomitant antidiabetic drugs are shown in Supplementary Table S1, and by age in Supplementary Table S2.
Patients received linagliptin for a mean ± SD 113.8 ± 56.1 weeks, and 57.9% of patients received linagliptin for >130 weeks. Among patients who discontinued linagliptin, the main reasons were lack of efficacy (n = 370, 9.4%), loss to follow-up after changing hospital (n = 330, 8.4%), loss to follow-up due to unknown reason (n = 288, 7.3%), AEs (n = 271, 6.9%) and improvement (n = 141, 3.6%); 3.23% of patients (n = 109) discontinued linagliptin because of an ADR.
3.2. Primary endpoint: ADRs
In the safety analysis set, the incidence of any ADRs was 11.39% (n = 384) (). The most common ADRs according to MedDRA PTs were ‘diabetes mellitus’ (n = 42, 1.25%), ‘hypertension’ (n = 28, 0.83%), ‘hypoglycemia’ (n = 27, 0.80%), ‘constipation’ (n = 20, 0.59%), ‘hyperuricemia,’ and ‘glycosylated hemoglobin increased’ (each n = 18, 0.53%).
Table 2. Adverse drug reactions by concomitant antidiabetic drug class
There was no major difference in the incidence of ADRs in subgroups of patients based on concomitant antidiabetic drug class; the incidence of ADRs in each subgroup is shown in .
The incidence of ADRs was also similar across age groups; Supplementary Table S3 shows the incidence in each age subgroup.
Overall, 2.49% of patients (n = 84) developed a serious ADRs (). The most common serious ADRs were ‘hypoglycemia’ (n = 6, 0.18%), ‘cardiac failure congestive’ (n = 4, 0.12%) and ‘diabetic nephropathy’ (n = 4, 0.12%). The serious ‘hypoglycemia’ ADRs developed in 0.08% of patients (n = 1) in the BG subgroup, 0.28% (n = 1) in the TZD subgroup, 0.14% (n = 1) in the AGI subgroup and 0.70% (n = 5) in the insulin subgroup. Fatal ADRs occurred in 0.39% of patients (n = 13); fatal ADRs that occurred in more than one patient were death (n = 4) including sudden death and cardiorespiratory arrest (n = 2).
Table 3. Serious ADRs that occurred in ≥2 patients according to the preferred term
The incidences of ADRs of special interest were low and no patient developed interstitial lung disease (). The most common ADR of special interest was ‘hypoglycemia’ (defined by the SMQ), which developed in 28 patients (0.83%), including the 27 patients with ‘hypoglycemia’ (MedDRA PT) and one patient with ‘hypoglycemic coma’ (MedDRA PT). The ‘hypoglycemia’ as an ADR of special interest developed in 0.54% of patients (n = 7) in the SU subgroup, 0.67% (n = 8) in the BG subgroup, 0.96% (n= 7) in the AGI subgroup, and 2.38% (n = 17) in the insulin subgroup. In the age subgroups, ‘hypoglycemia’ as an ADR of special interest developed in 0.61% of patients aged <65 years (n = 8), 1.00% of those aged 65–<75 years (n = 11), and 0.94% of those aged ≥75 years (n = 9).
Table 4. ADRs of special interest that occurred in the safety analysis set
3.3. Effectiveness endpoints: HbA1c and FPG
In the 3,029 patients in the effectiveness analysis set, mean ± SD HbA1c decreased from 7.76 ± 1.37% at baseline to 7.26 ± 1.19% at the last observation. The mean ± SD change in HbA1c from baseline at the last observation was – 0.49 ± 1.33% (95% CI: – 0.54 to – 0.44%; ). A reduction in HbA1c was noted at 12 weeks and the reduction was sustained throughout the observation period, with the mean change in HbA1c ranging from – 0.57% to – 0.46% (). Of patients in the safety analysis set who started linagliptin in combination with one concomitant antidiabetic drug (n = 2,130), 61.9% (n = 1,319) of patients had no change in their concomitant antidiabetic drug regimen and dose during the observation period. In this group, 1,168 were included in the effectiveness analysis set and a sustained reduction in HbA1c was also observed throughout the observation period, with the mean change in HbA1c ranging from – 0.59% to – 0.46% (Supplementary Figure S1). In subgroups based on the type of concomitant medication, the mean change in HbA1c from baseline to last observation ranged from −0.29% in the AGI subgroup to −0.55% in the insulin subgroup (). A reduction in HbA1c was noted at 12 weeks and was sustained throughout the observation period across all concomitant antidiabetic drug groups (Supplementary Figure S2). This pattern was also noted in all age subgroups (Supplementary Figure S3).
Figure 2. Adjusted mean ± standard error levels of (a) HbA1c and (b) FPG over time in the effectiveness analysis set (determined via mixed model for repeated measures analysis). FPG, fasting plasma glucose; HbA1c, glycated hemoglobin based on the National Glycohemoglobin Standardization Program (NGSP)
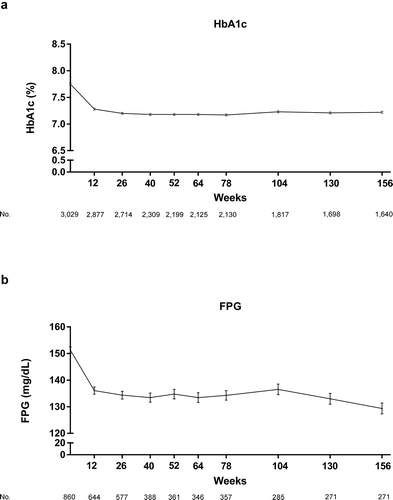
Table 5. Change from baseline in HbA1c at the last observation (secondary endpoint) in the effectiveness analysis set and subgroups according to concomitant antidiabetic drug class
Of the 3,029 patients in the effectiveness analysis set, 860 patients had baseline FPG values. The mean ± SD FPG decreased from 151.68 ± 52.20 mg/dL at baseline to 136.72 ± 44.27 mg/dL at the last observation, a change of – 15.27 ± 59.96 mg/dL (95% CI: – 19.54 to – 11.00 mg/dL).
The pattern of FPG change over time was similar to the pattern of change in HbA1c ().
3.4. Other endpoints
The mean ± SD change from baseline to the last observation was – 0.8 ± 4.3 kg for bodyweight, – 1.8 ± 31.4 mL/min/1.73 m2 for eGFR, – 1.1 ± 18.3 mmHg for SBP, and – 1.8 ± 11.7 mm Hg for DBP. Similar changes in these parameters were seen in all subgroups according to concomitant antidiabetic drug class (data not shown).
4. Discussion
To our knowledge, this is the first report to describe the 3-year safety and effectiveness of linagliptin as add-on therapy in patients who started this treatment during routine clinical practice. In this prospective, observational PMS, add-on linagliptin therapy for Japanese patients with T2D was well tolerated, and long-term sustained reductions in HbA1c were observed, irrespective of baseline concomitant antidiabetic drugs and age. The mean age of patients in the safety analysis of this PMS was 66.5 ± 12.4 years, with the proportions of patients aged 65–<75 and ≥75 years being 32.7% and 28.4%, respectively. This age distribution is similar to that in the 2017 patient survey of patients with diabetes by the Japanese MHLW [Citation3]. In addition, the mean age and BMI of patients in this PMS were also similar to those in the 55,226 patients enrolled in the Japan Diabetes Clinical Data Management Study Group [Citation28]. Also, there was no major difference in the percentages of patients who were taking each concomitant antidiabetic medication at baseline among the patients starting DPP-4 inhibitor add-on therapy between this PMS and other PMS studies with other DPP-4is [Citation29,Citation30]. Therefore, the characteristics of patients in this PMS cohort appear to be consistent with the typical population of patients with T2D seen throughout Japan.
The incidence of any ADR in this 3-year PMS was 11.39%, which is similar to the incidence reported (10.7%) in a previous 3-year PMS that evaluated the safety and effectiveness of linagliptin as monotherapy in routine clinical practice [Citation26]. The most common ADRs by MedDRA preferred terms in the 3-year monotherapy PMS were ‘diabetes mellitus’ (1.6%), ‘constipation’ (0.9%), ‘diabetes mellitus inadequate control’ (0.6%), ‘hypertension’ (0.6%), and ‘hyperuricemia’ (0.5%) [Citation26], which are similar to the most common ADRs in this PMS. Of note, in the 52-week clinical trial of linagliptin as add-on therapy in Japanese patients with T2D, the incidence of any ADR was 10.7% [Citation21]. The incidences of any ADR did not differ between groups stratified by concomitant antidiabetic drug, which was also consistent with the results of the previous 52-week clinical trial [Citation21]. Although the number of patients who started linagliptin as add-on to SGLT2is was relatively small in this PMS (n = 73) and further data in a real-world setting are needed, a previous clinical trial has demonstrated the safety of linagliptin as add-on therapy to empagliflozin, an SGLT2i, in Japanese patients with T2D [Citation31]. The subgroup analysis by age group of this PMS found a comparable incidence of any ADR between age groups.
Hypoglycemia is described as one of the clinically significant ADRs in the precautions section of the Japanese product package insert for linagliptin and is defined as one of the important identified risks of linagliptin in the Japanese risk management plan. Hypoglycemic AEs occurred in 5.8% of Japanese patients with T2D in the previous clinical trial of linagliptin as add-on therapy [Citation21], while the incidence of ‘hypoglycemia’ as an ADR of special interest in this PMS was 0.83%. In the subgroup analysis by baseline concomitant antidiabetic drug, the incidence of ‘hypoglycemia’ was comparable between most drug class subgroups, except the group receiving concomitant insulin. This group had an incidence of ‘hypoglycemia’ of 2.38% as an ADR of special interest, which is similar to the incidence in patients receiving DPP-4is and insulin concomitantly in a previous PMS assessing the safety and efficacy of basal supported oral therapy with insulin [Citation32]. Moreover, it is noteworthy that the incidence of investigator-identified hypoglycemic AEs was similar in patients receiving linagliptin or placebo in addition to insulin in a pooled analysis of randomized studies in patients with T2D [Citation33]. In the subgroup analysis by age, the incidence of ‘hypoglycemia’ as an ADR of special interest was numerically higher in the older age groups: 0.61% in those aged <65 years versus 1.00% and 0.94%, respectively, in those aged 65–<75 and ≥75 years. Although a similar trend has also been noted in previous studies including a subgroup analysis of CARMELINA® trial [Citation34] and a comprehensive pooled analysis of 22 placebo-controlled studies of linagliptin [Citation33], it is important to note that in both these analyses the incidence of hypoglycemia with linagliptin was comparable to that with placebo [Citation33,Citation34].
In the effectiveness analysis set, a reduction in HbA1c was seen after 12 weeks of linagliptin treatment, and was sustained throughout the observation period. In addition, a similar pattern was seen in patients who had no change in concomitant antidiabetic drug regimen and dose during the observation period, which suggests that add-on linagliptin treatment may lead to sustained reductions in HbA1c in real-world practice in Japan. This sustained reduction in HbA1c was also observed in patients who started linagliptin monotherapy in the previous PMS [Citation26]. In a subgroup analysis by concomitant antidiabetic drug, the sustained reductions were also observed across the concomitant antidiabetic drug groups and the changes from baseline in HbA1c were comparable between groups. These patterns are consistent with previous clinical trials of linagliptin as add-on therapy in Japanese patients [Citation21,Citation31], although these earlier studies had shorter follow-up durations (24–52 weeks) than this PMS.
This PMS had several limitations. First, there was no control group against which to compare the safety and effectiveness of linagliptin. Second, all treatment decisions regarding the choice and dosage of concomitant antidiabetic therapy were made at the investigator’s discretion, including any changes in dose, and not dependent on whether the patient had achieved treatment targets. However, allowing the investigators to determine all treatment decisions reflects routine clinical practice. Finally, no formal statistical analysis was performed, other than descriptive statistics. Therefore, the number of patients in each subgroup based on baseline concomitant antidiabetic drug class or age was dependent on patient registration and not calculated statistically.
Despite these limitations, the characteristics of patients in this study are consistent with previous reports of patients with T2D all over Japan [Citation3,Citation28], particularly in terms of age distribution and BMI. Moreover, this study shows the long-term safety and effectiveness of linagliptin as add-on therapy in a real-world Japanese setting and provides clinically important information on the long-term durability of linagliptin as add-on therapy during routine clinical practice.
5. Conclusion
In this 3-year PMS, the safety profile of linagliptin in Japanese patients with T2D was consistent with previous reports, and no new safety concerns were observed. Reductions in HbA1c were observed over the course of the 3-year period in these patients who had started linagliptin as add-on therapy to other antidiabetic drugs. The safety and effectiveness of add-on linagliptin was consistent across subgroups of patients based on baseline concomitant antidiabetic drug class or age.
Author contributions
Naoki Shimmoto and Kaori Ochiai contributed to conception and design of the study. Naoki Shimmoto contributed to data acquisition and analysis. Kaori Ochiai performed statistical analysis. All authors were involved in the interpretation of the data, drafting and revising the manuscript and approving the final draft to be published.
Declaration of interest
T Ito, Y Naito, N Shimmoto, N Hayashi and T Okamura are employees of Nippon Boehringer Ingelheim Co., Ltd. K Ochiai is an employee of EPS Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have received lecture fees from Boehringer Ingelheim and Eli Lilly. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the ICMJE criteria.
Furthermore, clinical study documents (e.g. study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/
Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants.
Clinical Study Reports and Related Clinical Documents can also be requested via the link https://trials.boehringer-ingelheim.com/
All requests will be governed by a Document Sharing Agreement.
Bona fide, qualified scientific and medical researchers may request access to de-identified, analyzable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request.
Researchers should use the https://trials.boehringer-ingelheim.com/link to request access to study data.
Linagliptin_PMS_supplementary_figures_1-3.docx
Download MS Word (312 KB)Linagliptin_PMS_supplementary_tables1-3.docx
Download MS Word (33 KB)Acknowledgments
We would like to thank Catherine Rees of Springer Healthcare Communications who wrote the first draft of this article. This medical writing assistance was funded by Boehringer Ingelheim. We would like also to thank Rie Ikeda who contributed to conception and design of the study.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281.
- Ministry of Health Labour and Welfare. The National Health and Nutrition Survey in Japan 2016 [cited 2020 Mar 30]. Available from: https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h28-houkoku-03.pdf (Japanese).
- Ministry of Health Labour and Welfare. Patient Survey 2017 [cited 2020 Mar 30]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/17/dl/toukei.pdf (Japanese).
- Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664.
- Ikeda Y, Kubo T, Oda E, et al. Incidence rate and patient characteristics of severe hypoglycemia in treated type 2 diabetes mellitus patients in Japan: retrospective Diagnosis Procedure Combination database analysis. J Diabetes Investig. 2018;9:925–936.
- Haneda M, Noda M, Origasa H, et al. Japanese Clinical Practice Guideline for Diabetes 2016. J Diabetes Investig. 2018;657–697.
- Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109.
- Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9:e025806.
- Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2010;19:133–140.
- Heise T, Graefe-Mody EU, Huttner S, et al. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diab Obes Metab. 2009;11(8):786–794.
- McKeage K. Linagliptin: an update of its use in patients with type 2 diabetes mellitus. Drugs. 2014;74:1927–1946.
- Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diab Obes Metab. 2011;13:258–267.
- Gomis R, Espadero RM, Jones R, et al. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diab Obes Metab. 2011;13:653–661.
- Owens DR, Swallow R, Dugi KA, et al. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28:1352–1361.
- Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diab Obes Metab. 2011;13:65–74.
- Barnett AH, Patel S, Harper R, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diab Obes Metab. 2012;14:1145–1154.
- Kawamori R, Inagaki N, Araki E, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diab Obes Metab. 2012;14:348–357.
- Araki E, Kawamori R, Inagaki N, et al. Long-term safety of linagliptin monotherapy in Japanese patients with type 2 diabetes. Diab Obes Metab. 2013;15:364–371.
- Barnett AH, Huisman H, Jones R, et al. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1413–1423.
- Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–3881.
- Inagaki N, Watada H, Murai M, et al. Linagliptin provides effective, well-tolerated add-on therapy to pre-existing oral antidiabetic therapy over 1 year in Japanese patients with type 2 diabetes. Diab Obes Metab. 2013;15:833–843.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79.
- Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019 1155–1166 doi:10.1001/jama.2019.13772
- Inagaki N, Yang W, Watada H, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA® trial. Diabetol Int. 2020;11:129–141.
- Kadowaki T, Wang G, Rosenstock J, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA® trial. Diabetol Int [Internet]. 2020 2020 Jun 27. Available from: https://doi.org/10.1007/s13340-020-00447-5.
- Yamamoto F, Unno Y, Okamura T, et al. Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes mellitus: a 3-year post-marketing surveillance study. Diabetes Ther. 2020;11:107–117.
- Unno Y, Ikeda R, Ochiai K, et al. Safety and efficacy of long-term combination therapy with linagliptin (Trazenta Tablet 5mg), a DPP-4 inhibitor, in patients with type 2 diabetes mellitus – interim report from a special drug use-results survey (in Japanese). J New Rem Clin. 2018;67:799–822.
- Japan Diabetes Clinical Data Management Study Group. Japan diabetes clinical data management study 2018 [cited 2020 Mar 30]. Available from: http://jddm.jp/data/index-2018/(Japanese).
- Kadowaki T, Haneda M, Ito H, et al. Long-Term, Real-World Safety and Efficacy of Teneligliptin: A Post-Marketing Surveillance of More Than 10,000 Patients with Type 2 Diabetes in Japan. Adv Ther. 2020;37:1065–1086.
- Hayashi T, Murayama H, Shinfuku Y, et al. Safety and efficacy of vildagliptin: 52-week post-marketing surveillance of Japanese patients with type 2 diabetes in combination with other oral antidiabetics and insulin. Expert Opin Pharmacother. 2020;21:121–130.
- Kaku K, Haneda M, Tanaka Y, et al. Linagliptin as add-on to empagliflozin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a two-part, randomized, placebo-controlled trial. Diab Obes Metab. 2019;21:136–145.
- Tsukube S, Kadowaki T, Odawara M. Efficacy and safety assessment of basal supported oral therapy (BOT) with insulin glargine in a real-life clinical setting, stratified by concomitant orally administered antidiabetic agent (OAD) regimens including dipeptidyl peptidase-4 inhibitor (DPP-4i): subanalysis of the ALOHA2 study, drug-use surveillance in Japan. Diabetol Int. 2016;7:299–307.
- Lehrke M, Marx N, Patel S, et al. Safety and tolerability of linagliptin in patients with type 2 diabetes: a comprehensive pooled analysis of 22 placebo-controlled studies. Clin Ther. 2014;36:1130–1146.
- Cooper ME, Rosenstock J, Kadowaki T, et al. Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: a prespecified subgroup analysis of the randomized, placebo-controlled CARMELINA® trial. Diab Obes Metab. 2020;22:1062–1073.
