ABSTRACT
Introduction: Treatment with immune checkpoint inhibitors in melanoma patients can cause immune-related adverse effects, such as vitiligo. In vitiligo, specific autoimmunity against melanocytes results in depigmentation of the skin. Melanoma-associated vitiligo occurring in melanoma patients treated with immune checkpoint inhibitors can be seen as a good prognostic sign as higher survival rates in melanoma-associated vitiligo cases have been reported.
Areas covered: This review gives an insight into the pathophysiology, clinical presentation, and management of melanoma-associated vitiligo caused by immune checkpoint inhibitors.
Expert opinion: Development of melanoma-associated vitiligo induced by immune checkpoint inhibitors could be a good clinical marker for response and overall survival. Induction of vitiligo in these patients could also potentially lead to better response and survival rates. Further research should focus on several aspects of melanoma-associated vitiligo, such as better screening and registration, more understanding of pathophysiology of the type of immune response and the predictive value of melanoma-associated in patients treated with immune checkpoint inhibitors.
1. Introduction
Vitiligo can occur in advanced melanoma patients either spontaneously or as an immune-related adverse effect upon treatment with immune checkpoint inhibitors (ICI). Vitiligo occurring in melanoma patients, with or without treatment, is called melanoma-associated vitiligo. Furthermore, also other melanoma treatments, such as targeted therapy (e.g. BRAF and MEK inhibitors), can lead to melanoma-associated vitiligo [Citation1]. Vitiligo is characterized by depigmentation of the skin, which is caused by local melanocyte loss of the skin caused by specific auto-immunity responses against cutaneous melanocytes. Vitiligo induced by ICI is a favorable clinical sign in advanced melanoma.
2. Immune checkpoint inhibitors
Immune checkpoint pathways have an inhibitory effect on T-cell immunity through negative feedback loops and therefore play an important role in keeping the immune system in balance [Citation2]. Tumor cells escape normal immunosurveillance by expressing ligands of immune checkpoint pathways, subsequently leading to inactivation of antitumoral immune responses. ICI prolong the activation of these antitumor immune responses by blockage of immune checkpoint pathways. ICIs are monoclonal antibodies which mainly target two immune checkpoint pathways: 1) the cytotoxic T-lymphocyte antigen-4 (CTLA-4) pathway that acts centrally in lymphoid organs during T-cell priming by antigen-presenting cells (APC) and 2) programmed cell death-1 protein (PD-1) pathway that acts both centrally and in the periphery during tumor cell recognition by T-cells. Blockage of the CTLA-4 pathway leads to upregulation of the antitumor immunity response by prolonged T-cell activation and infiltration of T-cells in tumor cells. Targeting the PD-1 pathway will lead to prolonged T-cell activation by either direct blockage of the PD-1 receptor or by blockage of the programmed cell death-1 ligand (PDL1) or programmed cell death-2 ligand (anti-PD-L2).
The first ICI were approved for treatment in metastatic melanoma. However, in the past few years, several ICI have been approved for other advanced cancer treatments, for example, non-small cell lung carcinoma, renal cell carcinoma, Hodgkin lymphoma, head and neck squamous cell carcinoma, and urothelial carcinoma [Citation3]. The development of ICI has been a major breakthrough in the treatment of melanoma and other advanced cancers. ICI therapies have significant higher response rates than standard chemotherapies and have resulted in significantly improved survival rates in metastasized malignant melanoma [Citation4–6].
Prolonged T-cell activation by immune checkpoint inhibition leads to favorable antitumor immunity responses. However, this activation of T-cells can also lead to several immune-related adverse effects. More than 60% of the treated patients develop these immune-related adverse effects, which can involve many body organs: thyroiditis, dermatitis, pneumonitis, colitis, hepatitis, hypophysitis, uveitis, polyneuritis, pancreatitis, etc. [Citation7]. Adverse effects of the skin occur in 30–50% of the patients treated with ICIs, which makes these cutaneous adverse effects the most common [Citation8,Citation9].
3. Vitiligo
Vitiligo is an acquired depigmenting disfiguring asymptomatic skin disease and affects approximately 0.5–1% of the world’s population with no differences between sexes, ages, or skin types [Citation10]. Vitiligo is characterized by white, well demarcated, uniform patches surrounded by normal skin. The diagnosis of vitiligo is usually made clinically with the use of a Wood’s lamp (handheld ultraviolet-A irradiation device) [Citation11,Citation12].
The depigmentations are caused by selective destruction of melanocytes, which are the melanin-producing cells in the skin. The most common type of vitiligo, e.g., non-segmental vitiligo, is an autoimmune disease in which intrinsic defects within melanocytes and autoimmunity targeting the melanocytes contribute to the development of vitiligo [Citation13]. The main effectors of this autoimmune response are the cytotoxic CD8+ T-cells that recognize melanocyte differentiation antigens, such as gp100, MART1, tyrosinase, or tyrosinase-related proteins [Citation14–16]. Furthermore, the IFN-γ induced chemokine CXCL10 and its receptor CXCR3 are involved in the T-cell recruitment in vitiligo and therefore play an essential role in driving the autoimmunity [Citation13,Citation17–19]. Recent genome-wide association studies have identified 50 genetic loci that contribute to the risk of developing vitiligo [Citation20]. The majority of loci are involved in immune regulation and others regulate functions of melanocytes, which confirms the critical role of the immune system in vitiligo pathogenesis [Citation13]. The autoimmune hypothesis in non-segmental vitiligo is also supported by the high prevalence of other autoimmune diseases in vitiligo, predominantly including autoimmune thyroid disease [Citation21,Citation22].
4. Vitiligo induced by ICI
Vitiligo can occur as an immune-related adverse effect in patients treated with ICI and is mainly described in melanoma patients. Depigmentation occurring in melanoma, with or without ICI-therapy, is called melanoma-associated vitiligo and can occur in up to 2–25% of melanoma patients receiving ICI therapy, which is higher than the normal prevalence of vitiligo. [Citation7,Citation23–25] In a recent meta-analysis, 7.5% and 8.5% of the patients treated with nivolumab and pembrolizumab, respectively, were diagnosed with melanoma-associated vitiligo during or after treatment [Citation26]. Depigmentation can also occur in patients with melanoma without ICI therapy. The estimated prevalence of melanoma-associated vitiligo is 0.15% of all vitiligo cases and in 2.8% of melanoma patients melanoma-associated vitiligo is present. [Citation24,Citation27]
4.1. Pathophysiology
It is hypothesized that melanoma-associated vitiligo is caused by anti-melanoma immunity targeting healthy and malignant melanocytes, due to shared expression of melanocyte differentiation antigens (MART-1, gp100, tyrosinase-related proteins 1 and 2) on melanoma cells and normal melanocytes [Citation23,Citation28]. CD8+ T-cells are thought to be the main effector cells that recognize these shared melanocyte antigens, which were found to infiltrate both tumor and vitiligo tissue [Citation29]. However, it is still unclear whether the specific immune responses of non-segmental vitiligo and melanoma-associated vitiligo are identical or that other pathomechanisms are involved [Citation30,Citation31]. Although immunity against melanocyte differentiation antigens is frequently found in melanoma patients, immune responses to mutation-derived neoantigens were shown to be potent tumor rejection antigens during cancer immunotherapy. [Citation32] However, recent research [Citation33] has shown the relevance of immunity against melanocyte differentiation antigens in rescuing suboptimal immune activation in melanoma patients with neoantigen-low tumors [Citation32]. Responding patients to the ICI therapy, despite low tumor neoantigen load, showed an expansion of CD8+ T cell responses against melanocyte differentiation antigens, indicating that immunity to self-antigens is involved in a successful ICI therapy response in these patients. As this immunity can also attack normal melanocytes, vitiligo may occur as a side-effect, indicative of the autoreactive nature of the immune response induced. Conversely, responding patients without vitiligo may have sufficiently effective activation of neoantigen-reactive immunity, which does not target normal melanocytes.
4.2. Clinical presentation
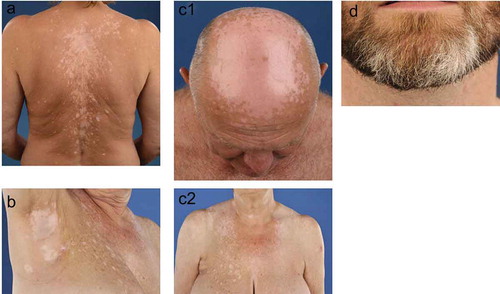
Patients with melanoma-associated vitiligo have a higher age at the onset of vitiligo and mostly have highly progressive skin depigmentations, which results in confetti-like depigmentation and hypochromic lesions ()) [Citation24,Citation27,Citation34]. Depigmentations can occur on the same predilections sites as non-segmental vitiligo ()) and on sun-exposed areas ()), such as the face, chest, and hands [Citation31]. Furthermore, also leucotrichia (whitening of the hairs, )) and halo nevi (depigmentations around benign melanocytic nevi) can be seen in melanoma-associated vitiligo () [Citation24]. In rare cases, melanoma-associated vitiligo can be a symptom of Vogt–Koyanagi–Harada disease, i.e., autoimmune bilateral diffuse granulomatous uveitis associated with vitiligo, during or after ICI therapy [Citation35]. Manifestation of vitiligo during ICI therapy occurs after a mean time of 9 months [Citation31,Citation36]. Similar to non-segmental vitiligo, depigmentations induced by ICI also show bilateral distributions. In some cases, depigmentations occur around the primary melanoma site or around cutaneous metastasis [Citation37]. Depigmentations usually reside after discontinuation of ICI, although repigmentation can occur. Discrimination between melanoma-associated vitiligo and non-segmental vitiligo is challenging due to the lack of discriminative clinical and histological features [Citation24,Citation34]. Melanoma-associated vitiligo can also occur in melanoma patients before detection of melanoma and without treatment with ICI [Citation25]. Since discrimination between the two types of vitiligo is difficult, a small percentage of these melanoma patients could be misdiagnosed as having normal vitiligo and later develop melanoma metastases due to late detection of the primary melanoma.
Figure 1. Clinical presentation of melanoma-associated vitiligo
4.3. Good prognostic sign
Vitiligo occurring in melanoma patients treated with ICI is seen as a good prognostic sign. Melanoma-associated vitiligo in patients treated with immunotherapy (e.g. ICI, vaccines, adoptive cell therapy, antibodies) is significantly associated with higher progression-free and overall survival, resulting in two to four times, respectively, lower risk of disease progression and death, as compared to patients without vitiligo [Citation23]. The occurrence of vitiligo in melanoma patients treated with anti-PD-1 agents is independently associated with better response rates and overall survival. [Citation37,Citation38] Other studies reporting on the development of several skin reactions, including vitiligo, show a 40–50% less risk of disease progression or death in melanoma patients developing skin reactions and receiving PD-1 therapy [Citation36,Citation39]. Despite the promising results with ICI, the overall response rates of advanced melanoma patients are still low or moderate. Less than 20% of advanced melanoma patients experience a long-term response to ipilimumab. PD-1 ICI are effective in more patients; however, durable responses to these therapies are limited to 30–40% of the patients, or up to 60% for a combination of these drugs. This means that durable responses are still not seen in 40–60% of the patients.
4.4. Management
Upon vitiligo development, ICI treatment can be continued as melanoma-associated vitiligo is associated with better survival and response rates [Citation23,Citation31,Citation37]. In non-segmental vitiligo patients, lesions are normally treated with immunosuppressive ointments, such as topical corticosteroids and topical calcineurin-inhibitors [Citation10]. These topical treatments can be combined with UV-therapy and systemic corticosteroids in highly active or extensive cases. However, these treatments suppress the anti-melanocyte immunity against healthy melanocyte and can hypothetically also suppress the anti-melanocyte immunity against malignant melanocyte and melanoma cells. Management of melanoma-associated vitiligo with local or systemic immunosuppressive treatments is not recommended, as this hypothetically may decrease ICI therapy response. Camouflage therapy can be a useful advice in cosmetically disfiguring cases [Citation40]. In some cases, when melanoma-associated vitiligo persists after long discontinuation of ICI therapy, local immunosuppressive treatment can be considered.
5. Conclusion
Vitiligo induced by ICI is a good prognostic sign in melanoma patients. Depigmentations are caused by autoimmunity against shared antigens in melanocytes in healthy tissue and melanoma tissue. Clinical presentation of melanoma-associated vitiligo is similar to active vitiligo and depigmentation occurs frequently on sun-exposed areas. Treatment of these depigmentations is not recommended, as immune suppressive treatment of vitiligo may also suppress antimelanoma immunity and ICI therapy response and survival rates.
6. Expert opinion
Overall response rates of melanoma patients to ICI therapy are still low to moderate. Therefore, there is a high need for biomarkers that can predict whether and how melanoma patients respond to ICI treatment. Development of vitiligo lesions in melanoma patients treated with ICI is associated with improved survival and response rates. Hypothetically, susceptibility to vitiligo could be a good predictive marker for the responsiveness of ICI therapy in advanced melanoma patients. As discussed in this article, genome-wide association studies have already identified 50 genetic loci, mainly involved in immune regulation, that contribute to the risk of developing vitiligo. Vitiligo susceptibility, in patients and/or their relatives, could be a potential biomarker for responsiveness of ICI therapy in advanced melanoma patients, which is the subject of an ongoing large international study.
Acknowledgment of vitiligo as a clinical biomarker for better outcomes after ICI is required. It is important that clinicians and oncologists, who treat melanoma patients with ICI, acknowledge the importance of and recognition of the development of vitiligo. The use of a Wood’s lamp is recommended to ensure the diagnosis in these patients, especially in patients with a fair skin type. Furthermore, screening and registration (e.g. in electronic health records and melanoma registries) of the development of vitiligo during or after ICI is necessary. Better screening and registration of vitiligo will potentially lead to a better understanding of the phenomenon. As mentioned previously in this article, melanoma-associated vitiligo can also occur without treatment with ICI. It is recommended that patients are screened for melanoma-associated vitiligo before initiation of treatment with ICI, as this could be a good clinical biomarker for later response of the ICI. Moreover, vitiligo can also occur prior to detection of the primary melanoma, which bears the risk that the vitiligo is treated by immune suppressive therapy, and thereby unintendedly increasing the potential risk of melanoma progression. Therefore, it is recommended that dermatologists perform a total body inspection on suspected melanocytic lesions in vitiligo patients, especially in patients with a higher age at the onset of their vitiligo.
Although vitiligo is seen as a good prognostic sign in melanoma patients treated with ICI, further research on the predictive value of vitiligo development in these patients is needed [Citation37,Citation38]. ICI are high-cost therapies and 40–60% of these patients do not respond, e.g., high treatment costs without clinical benefit, which results in an unfavorable cost/benefit ratio. Hypothetically, personalized treatment with ICI, by selecting patients who likely will benefit from ICI, will increase the response rate of ICI and could save costs of treatment without clinical benefit. Furthermore, the exact pathophysiology of vitiligo in melanoma patients treated with ICI is not known. A better understanding of the type of immune reaction (e.g. innate immune system, antibody or T-cell mediated responses, or auto-inflammatory) against melanocytes in melanoma-associated vitiligo should be the focus of new studies. A better understanding of the immune responses against healthy and malignant melanocytes in these patients could potentially lead to different treatment regimens (e.g. combination therapy, dose-finding, etc.) and improved selection of patients who have increased chances for better response and survival rates upon ICI treatment.
Treatment with ICI leads to prolonged T-cell activation against the melanoma cells by elimination of negative feedback loops. However, initial anti-melanoma T-cell responses, albeit low or below detection, are needed to be enhanced by ICI. Research in immunotherapy in melanoma patients focuses on the combined treatment of ICI with immunization approaches, such as vaccinations, to initiate these anti-melanoma immune responses. Current research is mainly focused on neoantigens formed by mutations in melanoma cells, which serve as a target for these vaccinations. Vaccination with these neoantigens-based melanoma vaccines stimulates the cytotoxic T-lymphocyte responses and these responses could be further induced by combined treatment with ICI. However, every melanoma patient expresses a different subset of neoantigens that needs to be characterized to produce a personalized vaccine, which is a potentially time consuming and a costly treatment. Melanoma-associated vitiligo caused by ICI is initially induced by targeting the self-antigens in melanocytes. The development of melanoma-associated vitiligo due to ICI treatment shows that, in patients susceptible to developing vitiligo, the tolerance to self-antigens can be broken. The response and survival benefit of patients with melanoma-associated vitiligo may imply that these self-antigens could be very relevant for the clinical benefit of ICI treatment. Active induction of immunity against these self-antigens could also be a potential target for future therapy. For example, in previous studies, we have shown that combined treatment with immune-modifying agents targeting self-antigens (e.g. monobenzone and imiquimod) results in growth inhibition of melanoma in mice and partial regression of cutaneous metastasis in melanoma patients. Monobenzone is a skin depigmenting agent that causes systemic immune reactions against melanocytes, and this reaction is enhanced by immune response modifier imiquimod. Further research will reveal to what extent agents that stimulate immune responses against melanocyte self-antigens can confer durable clinical benefits to melanoma patients.
Article highlights
• Vitiligo induced by ICI is a good prognostic sign in melanoma.
• Treatment of vitiligo lesions in melanoma patients is not recommended.
This box summarizes key points contained in the article.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Ramondetta A, Ribero S, Conti L, et al. Clinical and pathological relevance of drug-induced vitiligo in patients treated for metastatic melanoma with anti-PD1 or BRAF/MEK inhibitors. Acta Derm Venereol. 2020;100(1):adv00001.
- Franklin C, Livingstone E, Roesch A, et al. Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol. 2017;43(3):604–611.
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
- Guan X, Wang H, Ma F, et al. The efficacy and safety of programmed cell death 1 and programmed cell death 1 ligand inhibitors for advanced melanoma: a meta-analysis of clinical trials following the PRISMA guidelines. Medicine (Baltimore). 2016;95(11):e3134.
- Li J, Gu J. Efficacy and safety of ipilimumab for treating advanced melanoma: a systematic review and meta-analysis. J Clin Pharm Ther. 2019;44(3):420–429.
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361.
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560–575.
- Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19(S1):31–39.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386(9988):74–84.
- Gawkrodger DJ, Ormerod AD, Shaw L, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159(5):1051–1076.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77(1): 1–13.
- Okamoto T, Irie RF, Fujii S, et al. Anti-tyrosinase-related protein-2 immune response in vitiligo patients and melanoma patients receiving active-specific immunotherapy. J Invest Dermatol. 1998;111(6):1034–1039.
- Palermo B, Campanelli R, Garbelli S, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117(2):326–332.
- Van Den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129(9):2220–2232.
- Rashighi M, Harris JE. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med. 2015;3(21):343.
- Wang XX, Wang QQ, Wu JQ, et al. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016;174(6):1318–1326.
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6(223):223ra23.
- Spritz RA, Andersen GH. Genetics of vitiligo. Dermatol Clin. 2017;35(2):245–255.
- Kroon MW, Vrijman C, Chandeck C, et al. High prevalence of autoimmune thyroiditis in children and adolescents with vitiligo. Horm Res Paediatr. 2013;79(3):137–144.
- Vrijman C, Kroon MW, Limpens J, et al. The prevalence of thyroid disease in patients with vitiligo: a systematic review. Br J Dermatol. 2012;167(6):1224–1235.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781.
- Lommerts JE, Teulings HE, Ezzedine K, et al. Melanoma-associated leukoderma and vitiligo cannot be differentiated based on blinded assessment by experts in the field. J Am Acad Dermatol. 2016;75(6):1198–1204.
- Teulings HE, Lommerts JE, Wolkerstorfer A, et al. Vitiligo-like depigmentations as first sign of melanoma; a retrospective case series from a tertiary vitiligo centre. Br J Dermatol. 2017;176(2):503-506.
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25.
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21(2):409–414.
- Teulings HE, Willemsen KJ, Glykofridis I, et al. The antibody response against MART-1 differs in patients with melanoma-associated leucoderma and vitiligo. Pigment Cell Melanoma Res. 2014;27(6):1086–1096.
- Le Gal FA, Avril MF, Bosq J, et al. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117(6):1464–1470.
- Fukuda K, Harris JE. Vitiligo-like depigmentation in patients receiving programmed cell death-1 inhibitor reflects active vitiligo. J Am Acad Dermatol. 2018;78(1):e15–e6.
- Larsabal M, Marti A, Jacquemin C, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017;76(5):863–870.
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74.
- Lo JA, Kawakubo M, Juneja VR, et al. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci Transl Med. 2021;13(581): eabd8636.
- Hartmann A, Bedenk C, Keikavoussi P, et al. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6(12):1053–1059.
- Fujimura T, Kambayashi Y, Tanita K, et al. HLA-DRB1*04:05 in two cases of Vogt-Koyanagi-Harada disease-like uveitis developing from an advanced melanoma patient treated by sequential administration of nivolumab and dabrafenib/trametinib therapy. J Dermatol. 2018;45(6):735–737.
- Chan L, Hwang SJE, Byth K, et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol. 2020;82(2):311–316.
- Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with Pembrolizumab. JAMA Dermatol. 2016;152(1):45–51.
- Nardin C, Jeand’heur A, Bouiller K, et al. Vitiligo under anti-programmed cell death-1 therapy is associated with increased survival in melanoma patients. J Am Acad Dermatol. 2020;82(3):770–772.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–1212.
- Muntyanu A, Netchiporouk E, Gerstein W, et al. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management. J Cutan Med Surg. 2021;25(1):59–76.
