1. Introduction
Interleukin 11 (IL-11) is a member of the IL-6 family with several, well-documented modes of biological action, namely and most notably, stimulatory and maturational actions on megakaryocytopoiesis within hematopoietic tissues and related thrombocytopoiesis, along with anti-inflammatory and cytoprotective effects on both gastrointestinal (GI) crypts and hematopoietic progenitors [Citation1]. IL-11 belongs to the gp130 family of cytokines and besides IL-6, IL-11 is the only member of this family which acts on a homodimer of the ubiquitously expressed gp130 co-receptor. Responsiveness of cells is, therefore, determined by the presence of the IL-11 receptor (the IL-6 receptor in the case of IL-6). It might be possible that certain cells are not responsive to IL-11 due to the lack of the IL-11 receptor expression. In such a case, the use of a ‘Hyper-IL-11’ (soluble IL-11 receptor-α fused with IL-11 without any artificial linker to avoid induction of antibody production) might be not only possible but perhaps appropriate [Citation2].
IL-11 is a drug currently in clinical use (Neumega/Oprelvekin, Wyeth Pharmaceuticals Inc. Philadelphia, PA, USA) for preventing thrombocytopenia induced by chemotherapy. Its biologics licensing application was approved by the United States Food and Drug Administration (US FDA) on 25 November 1997. Recombinant human IL-11ʹs (rhuIL-11) therapeutic efficacy for the above mentioned indications has been thoroughly investigated [Citation3,Citation4]. However, recombinant human IL-11 has been generally overlooked and underappreciated as a potential radiation medical countermeasure (MCM) for the acute radiation syndrome (ARS) and radiation injuries in general [Citation5,Citation6,Citation7].
The active ingredient in Neumega, Oprelvekin, is produced in Escherichia coli by recombinant technology. This is a non-glycosylated protein of 177 amino acids with approximately 19,000 Da molecular weight. This polypeptide differs from the 178 amino acid native IL-11 molecule only in lacking the proline residue at the amino-terminus. This structural change has not demonstrated any measurable differences in bioactivity [Citation8]. The major hematopoietic property of Neumega is stimulation of thrombopoiesis and megakaryocytopoiesis. This agent has demonstrated thrombopoietic efficacy in animal models of compromised hematopoiesis, including myelosuppressed mice and nonhuman primates (NHP). Neumega improved platelet recoveries and platelet nadirs compared to untreated controls. Preclinical studies have demonstrated that megakaryocytes developed with the treatment of Neumega are structurally normal. Furthermore, platelets produced in response to Neumega treatment were functionally and morphologically normal and had a normal life span [Citation8].
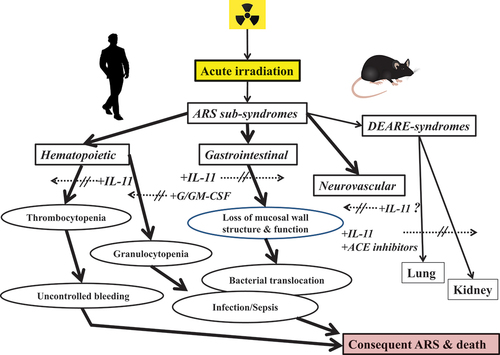
In preclinical studies of radiation injuries, it has been shown that the administration of IL-11 significantly improves both structure and function of GI and hematopoietic tissues in animals exposed to acute lethal doses of radiation. Specifically, IL-11 treatment fosters GI crypt survival and also reduces mucosal injury after total-body irradiation (TBI) [Citation9], while IL-11 in combination with granulocyte colony-stimulating factor (G-CSF) enhances recovery of blood granulocytes and select myeloid compartments of the bone marrow of acutely irradiated, pancytopenic mice [Citation10]. These early studies employed primarily a preclinical murine model of acute radiation injury as well as more chronic radiation injuries demonstrating that the combination treatment regimen of IL-11 and G-CSF was clearly superior to either recombinant treatment. This appeared to be the case not only in terms of hastening recovery of hematopoietic tissues but also in improving overall blood profiles. The prominent features of the recombinant IL-11, specifically in terms of radioprotective efficacy and related toxicology, are provided below as well as in .
Table 1. Major radioprotective attributes of recombinant IL-11 and related analogs.
2. Efficacy of IL-11 as a radiation MCM
The effects of rhuIL-11 on thrombocytopenia and neutropenia have been investigated preclinically using both small and large animal models of radiation injury [Citation6,Citation9–11]. In one study employing acutely, but sublethally irradiated rhesus NHPs, results suggested that rhuIL-11 (administered parenterally shortly before or after irradiation at doses ranging from 30 to 120 µg/kg) promotes megakaryocyte development and ameliorates radiation-induced myelosuppression [Citation11]. Specifically, this study demonstrated that early treatments but not late treatments (~2–4 weeks post-exposure) resulted in higher platelet nadirs than those noted within the placebo-treated animals.
Additional preclinical studies have shown that the recombinant IL-11 can provide therapeutic protection from acute irradiation not only to the blood-forming system but also to the GI and other vital organ systems (e.g. lung and kidney) (). For example, acutely irradiated (9 or 10 Gy) mice were administered recombinant IL-11 by oral gavage daily for 5 days beginning 24 h after TBI and were shown to have significantly improved intestinal mucosal surface area and crypt survival compared to the untreated control animals [Citation6]. Furthermore, coliform bacterial translocation was inhibited in IL-11 treated animals and serum citrulline levels also rebounded faster in the treated animals compared with untreated control. Systemic absorption of IL-11 was low, and hematopoietic recovery was not observed.
Figure 1. Recombinant IL-11 used either singly or in combination with other recombinants for preventive treatments of ARS and related radiation injuries.
2.1. New analogs and modes of delivery of the recombinant
PEGylated forms of the IL-11 recombinant have been developed and preclinically tested for their radioprotective efficacy [Citation12,Citation13,Citation15. Single subcutaneous (sc) doses (0.3 mg/kg) of one such analog, BBT-059 (a product of Bolder BioTechnology, Boulder, CO) delivered to CD2F1 mice either 24 h pre-, 4 or 24 h post-irradiation significantly increased the survival of mice following acute, potentially lethal gamma-irradiation []. The dose reduction factorCitation13 of this agent administered 0.3 mg/kg at 24 h pre-exposure was 1.28 []. Furthermore, recovery of radiation-Citation14induced neutropenia and thrombocytopenia corresponded to noted recoveries of multiple bone marrow cell lineages, including megakaryocytic elements.
Oral gavage, as well as direct, intra-luminal gastric infusions of both the parent recombinant as well a modified, PEGylated form of the recombinant have been tested preclinically using acutely irradiated rodents [Citation6,Citation16]. These alternative routes of drug delivery not only alleviate adverse systemic effects of the recombinant but also provide substantial radioprotection to the GI systems of acutely irradiated animals to supralethal doses of ionizing radiation [Citation6,Citation16].
2.2. Effects of combining recombinant IL-11 with other radioprotective recombinants, a polypharmaceutical approach
Combined regimens composed of three PEGylated recombinant growth factors (PEG-rhuG-CSF/BBT-015, PEG-rmGM-CSF/BBT-007, rhuPEG-IL-11/BBT-059) were tested for both survival benefit and for improvements of hematopoietic function in acutely and lethally irradiated rats in addition to studies with single agent in mice [Citation13,Citation17]. In brief, the combination of PEGylated recombinants proved to be more efficacious than any single agent. The study highlighted that a single dose of a combined, three agent regimen significantly improved the measured endpoints of the study (i.e. hematopoietic functions and survival) [Citation13,Citation17]. The latter study served to confirm and to extend earlier studies showing markedly enhanced radioprotective efficacy with use of similar combinations of non-PEGylated recombinant growth factors. [
These PEGylated recombinants (i.e. BBT-015, BBT-007 and BBT-059) in combination with yet another MCM, an angiotensin-converting-enzyme (ACE) inhibitor (Lisinopril), were shown to enhance radiomitigation of late-arising, life-threatening injuries within organ systems that are not generally associated with ARS (i.e. pulmonary and kidney/urinary systems) using a WAG/RijCmcr rat model of ‘delayed effects of acute radiation’ (DEARE) [Citation17]. This radiomitigation by the recombinants was correlated with the enhanced recovery in complete blood counts shortly following irradiation [Citation17].
In brief, recombinant IL-11 treatments delivered systemically by sc injections or non-systematically by either oral gavage or by direct gastric infusions have demonstrated significant radiomitigative efficacy for two major radiosensitive organ systems, the hematopoietic and GI systems, during the development of the ARS. This radiomitigative action of recombinant IL-11 can be significantly augmented by the addition of other hematopoietic growth factors or vascular-targeting agents for managing ARS and also delayed effects of acute radiation exposure (DEARE)-related sub-syndromes.
3. Safety issues and related toxicological assessments
Recombinant IL-11 (rhuIL-11/Oprelvekin/Neumega) and related analogs have been assessed both preclinically and clinically for drug tolerance and for signs and symptoms of drug toxicity (). Both small and large animal models have been used to assess toxicity, with reports indicating that the recombinant is well tolerated and not generally associated with significant toxicity in either irradiated or unirradiated animals. Nevertheless, there appears to be disconnect in the results reported from toxicological/safety assessments conducted preclinically versus those conducted clinically. In humans treated systemically with recombinant IL-11, substantial adverse side effects have been noted and reported [Citation4]. Under conventional treatment conditions (e.g. sc injections of 50 µg/kg/d for 10–21 d), greater than 10% of the subjects experienced one or more adverse side effects, a number of which are considered significant and need to be clinically managed (e.g. 77% exhibited nausea/vomiting, 59% edematous reactions, and 48% neutropenic fevers). Further, very rare, but serious, life-threatening clinical conditions have been associated with rhuIL-11 treatments, especially when those treatments were delivered systemically (e.g. the rare occurrence of a vascular leak syndrome and also multi-organ system failure) [Citation18,Citation19]. In attempting to mitigate these side-effects of systematically administered IL-11 recombinant, enteric-coated oral formations have been developed and tested clinically using an intraluminal route of administration. Despite the relatively high doses of the recombinant delivered, there were no clinical signs of toxicity and corresponding lack of detectable levels of drug systemically [Citation20].
Table 2. Safety and toxicological features of recombinant IL-11 and related analogs.
Besides the above-reported effort to reduce systemic toxicity and to improve the safety profile of the recombinant, several additional approaches have been pursued; e.g. most notably, the PEGylation of the IL-11 recombinant in order to extend the drug’s effective half-life, along with possible oral delivery. Initial reports of preclinical assessments of these rhuIL-11 analogs appear promising: single sc injections of a mono-PEGylated rhIL-11 to cynomolgus NHPs demonstrated a prolonged 67 h half-life of the recombinant compared to the much shorter half-life of ~3 h for the un-PEGylated recombinant [Citation12]. The latter extended half-life of the recombinant was complemented expectedly by an extended period of enhanced thrombopoiesis [Citation12]. Toxicokinetic evaluations of single and multiple sc doses demonstrated that systemic drug exposure was positively correlated with drug dosing, suggesting that efficacy and toxicity were mechanistically linked. However, major adverse events and immunogenicity were indeed observed with high sc doses of the recombinant (e.g. single bolus sc doses of 6.25 mg/kg) and were associated with significant but transient toxicities (i.e. of the heart, kidney, liver, lung and consequent to edematous responses), while multiple-lower doses (0.3 mg/kg weekly) developed only mild to moderate toxicities.
As indicated earlier, comparable preclinical safety evaluations have been conducted using small rodents treated with both modified (e.g. PEGylated) and un-modified forms of the recombinant IL-11 [Citation13–15]. Consistent with previous preclinical toxicological evaluations of the IL-11 (in small rodents), results of a comprehensive 14-d toxicity study failed to reveal any significant signs or symptoms of drug toxicity despite a fourfold escalation (from 0.3 to 1.2 mg/kg) of the effective drug dose delivered to test animals [Citation13].
4. Expert opinion
The current situation is less than optimal in terms of the availability of needed medicinals to prevent, mitigate, or treat life-threatening ionizing radiation injuries consequent to excessive and unwanted radiation exposures that arise from nuclear/radiological events, by accident or otherwise. At present, there are no US FDA-approved preventive agents for ARS and only a handful of agents available to clinically manage acute radiation exposures. The few agents available are mainly recombinant hematopoietic growth factors (e.g. Neupogen, Neulasta, Leukine, and Nplate) that selectively target the myeloid elements of the blood-forming system. Although these agents have received US FDA approval for use for this indication (to mitigate the ARS), they tend to be encumbered (in terms of mass casualty field-use) not only by additional clinical support requirements and by the limited scope of drug effectiveness for select types of organ system injuries but also by other factors, such as complex drug delivery schedules and uncertainties of the drug’s survival benefit. In this regard, there is a clear and unambiguous need to expand the medical resources for the effective prevention and to manage ARS among individuals that are either at risk to or are actually exposed to high doses of ionizing radiation. Accordingly, we suggest here that recombinant IL-11 be seriously reconsidered for additional work in its full development and, in turn, for its eventual regulatory approval. The latter would be based on the US FDA Animal Rule, a pathway that allows for the demonstration of efficacy of new MCMs using appropriate animal models of ARS. Although pivotal preclinical studies of the agent’s efficacy still remain to be conducted, the underlying radioprotective efficacy of the IL-11 recombinant is well founded and is not the primary problem associated with getting this agent indicated and approved for use in humans with ARS. The primary problem has been and continues to be one of ‘safety.’ As discussed earlier, delivering the drug systemically (via sc/intravenous infusions) will invariably produce an array of adverse side effects, ranging from mild to severe, within sizable fractions of the treated population. However, as we also pointed out, by delivering the selectively modified recombinant either orally or by direct intraluminal (gastric), these safety issues appear to become non-consequential. Furthermore, as suggested earlier, the therapeutic potential of recombinant IL-11 might be substantially enhanced by combining this recombinant with other efficacious recombinants bearing therapeutic potentials for different injury/organ systems. Nevertheless, additional research will be required in order to verify these initial findings concerning the safety of new delivery systems, especially in the context of the radioprotective efficacy of the agent itself.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
VK Singh and TM Seed performed literature searches, drafted the manuscript, revised, and finalized for publication.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Uniformed Services University of the Health Sciences, or the Department of Defense. The mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification. We apologize to those having contributed substantially to the topics discussed herein that we were unable to cite because of space constraints. We are thankful to Ms. Alana Carpenter for editing the manuscript.
Additional information
Funding
References
- Metcalfe RD, Putoczki TL, Griffin MDW. Structural understanding of interleukin 6 family cytokine signaling and targeted therapies: focus on interleukin 11. Front Immunol. 2020;11:1424.
- Dams-Kozlowska H, Gryska K, Kwiatkowska-Borowczyk E, et al. A designer hyper interleukin 11 (H11) is a biologically active cytokine. BMC Biotechnol. 2012;12(1):8.
- Kaye JA. The clinical development of recombinant human interleukin 11 (NEUMEGA rhIL-11 growth factor). Stem Cells. 1996;14(Suppl 1):256–260.
- Reynolds CH. Clinical efficacy of rhIL-11. Oncology. 2000;14:32–40.
- Hauer-Jensen M. Toward development of interleukin-11 as a medical countermeasure for use in radiological/nuclear emergencies. Dig Dis Sci. 2014;59(7):1349–1351.
- Burnett AF, Biju PG, Lui H, et al. Oral interleukin 11 as a countermeasure to lethal total-body irradiation in a murine model. Radiat Res. 2013;180(6): 595–602.
- Singh VK, Seed TM. Radiation countermeasures for hematopoietic acute radiation syndrome: growth factors, cytokines and beyond. Int J Radiat Biol. 2021;97(11):1526–1547.
- U.S. Food and Drug Administration. Neumega. 2006. cited 2022 Aug 29. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/103694_5065lbl.pdf.
- Potten CS. Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells. 1996;14(4):452–459.
- Seed TM, Inal CE, Deen JE. Assessment of a combined G-CSF plus IL-11 cytokine treatment for radiation-induced hematopoietic injury. 48th Annual Meeting of the Radiation Research Society; San Juan, Puerto Rico; 2001. p. 161.
- Hao J, Sun L, Huang H, et al. Effects of recombinant human interleukin 11 on thrombocytopenia and neutropenia in irradiated rhesus monkeys. Radiat Res. 2004;162:157–163.
- Yu KM, Lau JY, Fok M, et al. Preclinical evaluation of the mono-PEGylated recombinant human interleukin-11 in cynomolgus monkeys. Toxicol Appl Pharmacol. 2018;342:39–49.
- Plett PA, Chua HL, Sampson CH, et al. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2014;106:7–20.
- Kumar VP, Biswas S, Sharma NK, et al. PEGylated IL-11 (BBT-059): a novel radiation countermeasure for hematopoietic acute radiation syndrome. Health Phys. 2018;115:65–76.
- Sharma NK, Holmes-Hampton GP, Kumar VP, et al. Delayed effects of acute whole body lethal radiation exposure in mice pre-treated with BBT-059. Sci Rep. 2020;10(1):6825.
- Boerma M, Wang J, Burnett AF, et al. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res. 2007;67(19):9501–9506.
- Gasperetti T, Miller T, Gao F, et al. Polypharmacy to mitigate acute and delayed radiation syndromes. Front Pharmacol. 2021;12:634477.
- Antin JH, Lee SJ, Neuberg D, et al. A phase I/II double-blind, placebo-controlled study of recombinant human interleukin-11 for mucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29:373–377.
- Medscape. Oprelvekin (Rx). 2022. cited 2022 Oct 10. Available from: https://reference.medscape.com/drug/neumega-interleukin-11-oprelvekin-342165
- Cotreau MM, Stonis L, Strahs A, et al. A multiple-dose, safety, tolerability, pharmacokinetics and pharmacodynamic study of oral recombinant human interleukin-11(oprelvekin). Biopharm Drug Dispos. 2004;25(7):291–296.
