ABSTRACT
Introduction
Direct oral anticoagulants (DOACs) are considered high risk medicines and are frequently associated with medication errors. The nature of incidents and associated outcomes of such incidents are poorly understood.
Areas covered
Using a national patient safety reporting database, the National Reporting and Learning System (NRLS), this study aimed to report the contributory factors and outcomes including severe harm and deaths related to all safety incidents involving DOACs reported in England and Wales between 2017–2019. Reason’s accident causation model was used to classify the incidents.
Expert opinion
A total of 15,730 incident reports were analyzed. A total of 25 deaths were reported with a further 270 and 55 incidents leading to moderate and severe harm, respectively. A further 8.8% (n = 1381) of incidents were associated with low degree of harm. The majority of the incidents involved active failures (n = 13776; 87.58) including duplication of anticoagulant therapies, patients being discharged without DOACs, non-consideration of renal function, and lack of commencement of DOACs post-surgery suggesting preventability of such reported incidents. This study shows that medication incidents involving DOACs have the potential to cause severe harm and deaths, and there is a need to promote guideline adherence through education, training, and decision support technologies.
1. Introduction
Direct oral anticoagulants (DOACs) have been recognized as one of the most commonly concerned drug classes in relation to safety incidents [Citation1,Citation2]. A recent study showed approximately 12% of all severe and fatal medication errors in hospitals constituted the use of anticoagulants including DOACs [Citation3]. The lack of long-term clinical experiences and the need for careful consideration of risk and benefit profiles make DOACs, the likely candidates for medication errors, particularly prescribing errors [Citation4]. DOACs are now the most commonly prescribed oral anticoagulants in the UK accounting to over 60% of total quantity prescribed [Citation5]. A recently published systematic review of 32 studies concluded that one in five prescriptions of DOACs have errors in prescribing [pooled prescribing error rate of 20% (95% CI 15–25%; I2 = 96%; 95% PrI 4–43%)] [Citation6]. However, the review identified that data on circumstances, contributory factors, and likelihood of harm were scant.
Medication errors can be detrimental to patient safety, yet they are a common occurrence in clinical practice [Citation7]. A recent estimate suggested that 66 million out of 237 million medication errors which occurred in England in the year are potentially clinically significant [Citation8]. The WHO ‘Global Patient Safety Challenge: Medication Without Harm’ had set a global challenge which aimed to reduce severe avoidable medication-related harm by 50% by 2022 [Citation9]. Errors can occur throughout the medication use processes including storage, distribution, prescribing, compounding, preparing, dispensing and administration, and during monitoring [Citation10].
The National Health Service (NHS) England aims to promote safety culture and to monitor spontaneously reported safety incidents by healthcare professionals (HCPs) or patients through the National Reporting and Learning System (NRLS) [Citation11]. The NRLS allows safety incidents in relation to healthcare and healthcare interventions to be reported from all healthcare settings from England and Wales, collated and analyzed to prevent future incidents and promote patient safety. The NRLS database offers excellent opportunity to identify circumstances and contributory factors in relation to medication errors involving DOACs.
The NRLS defines a ‘patient safety incident’ as ‘any unintended or unexpected incident, which could have or did lead to harm for one or more patients receiving health care’ [Citation12]. The focus of this paper was on patient safety incidents that were specifically related to DOACs use. Incidents are likely to have resulted due to errors in the process of prescribing, preparing, dispensing, administering, monitoring, or through suboptimal advice. Additionally, safety incidents also include near misses and never events. Near misses are defined as prevented medicine-related patient safety incidents which could have led to patient harm, while ‘never events’ are serious, largely preventable patient safety incidents that should not occur if HCPs have implemented existing national guidance or safety recommendations as in case of administration of medication by the wrong route and overdose of high-risk medication despite the published policy [Citation12]. This study aimed to analyze and report the nature, severity of harm, and types of all medication incidents related to DOACs submitted through all clinical settings across England and Wales to the NRLS database. An in-depth evaluation of all incidents associated with severe harm and deaths were conducted.
2. Methods
2.1. Study design
This study constitutes a mixed-methods analysis of retrospectively collected NRLS data in relation to DOACs safety incidents, contributory factors, degree of harm, and prevention strategies as recommended by organizations responsible for reporting of errors.
2.2. Data Source and inclusion criteria
The NRLS is presently managed by NHS Improvement, which receives over three million patient safety incident reports each year [Citation13]. Incidents reported to NRLS between 1 January 2017 to 31 December 2019 were included. Five DOACs that are licensed and used in the NHS in England and Wales were considered for inclusion namely: dabigatran, rivaroxaban, edoxaban, apixaban, and betrixaban. Reports without drug name or not related to DOACs were excluded. A complete list of search terms is available in Supplementary material . The data of incident reports included patients age, drug name, indication, severity, type and sub-type of medication errors, and location, as well as free-text data usually describing ‘what happened,’ ‘apparent causes,’ and ‘actions preventing reoccurrence.’
Table 1. Medication incidents related to direct oral anticoagulants (DOACs) categorized as per the Reason’s accident causation model.
Data on severity resulting from harm as entered by the reporter in the database were classified as- ‘no harm occurred;’ to ‘low,’ ‘moderate,’ or ‘severe harm,’ which respectively caused minimal, temporal, or permanent harm to one or more persons or fatal incidents[Citation13]. The primary author (AA) checked all entries for completeness, accuracy of descriptions around severity of harm, and applying the inclusion/exclusion criteria. A sample of 10% of these data was validated by two other authors (VP and ZA).
2.3. Data Analysis
Frequency tables were generated to report incidents including drug name, age, care setting of occurrence, description of what happen, medication use process stage, medication error category, and degree of harm. The narrative descriptions of contributory factors and prevention strategies in relation to all incidents resulting in moderate harm, severe harm, or death of the patient were analyzed based on Reason’s model [Citation14]. Reason’s Accident Causation model is a theoretical framework and is most commonly used to inform investigation on the nature and causes of incidents. Incidents can be classified into active failures (such as slips or lapses), those caused by error-producing conditions and latent failures (or system related). Two members of the research team (AA and VP) familiarized themselves by reading the incident-free text description. Incidents were considered irrelevant if the error was not directly associated with DOACs. All free texts data related associated with medication safety incidents involving death and severe and moderate harm were thematically analyzed.
2.4. Ethical approval
The University of Birmingham’s Ethics Committee reviewed and approved this study (ERN_20–0551). NHS Improvement granted approval to share the NRLS data with University of Birmingham (Ref:5199/10 December 2020).
3. Results
A total of 24,322 incident reports were identified from the search. During the data cleaning phase, 8592 incidents were excluded as they were duplicate, irrelevant, or insufficient as shown in . A total of 15,730 (64.7%) anonymized DOACs medications incident reports were quantitatively analyzed and categorized according to Reason’s accident causation model into active failures (n = 13776; 87.58%), followed by error-provoking conditions (n = 1601; 10.18%) and latent failures (n = 353; 2.24%) as shown in .
Figure 1. National Reporting and Learning Dataset used for the evaluation of incidents related to directly-acting oral anticoagulants.
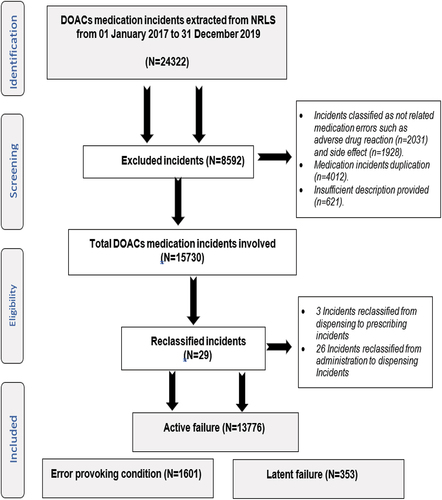
The majority of the active failures were related to mistakes (n = 6286; 45.63%). For instance, ‘Patient was prescribed Apixaban 25 mg BD. The dose was supposed to be 2.5 mg instead of 25 mg.’ Error-provoking conditions contributed to the over a tenth of incidents (n = 1601; 10.18%), and within this category, poor documentation constituted the highest reasons (n = 704; 43.97%). For instance, ‘nurse mentioned that 6 doses of dabigatran had not been signed in anticoagulant chart since admission … missed doses?’ Latent failure constituted 2.24% of incidents (n = 353) mainly owing to the lack of training (n = 203; 57.51%). For instance, ‘The patient was prescribed Apixaban, but dalteparin still continued because … trainings of anticoagulants medication a bit lacking.’
3.1. Degree of harm
The NRLS definition of harm is provided in Supplementary material , and the results of the quantitative analysis for the degree of harm are demonstrated in Supplementary material . While the majority of the incidents (n = 13999; 89%) were associated with no harm and low degree of harm (n = 1381; 8.8%), a total of 325 incidents were related to moderate or severe harm [moderate: 270 incidents (1.70%) and 55 (0.30%) severe harm]. A further 25 (0.20%) incidents led to deaths.
Table 2. Detailed descriptions of incidents leading to deaths with the assigned medication process stage, error type and nature of contributing factor.
Table 3. Examples of incidents associated with severe harm.
The majority of the incidents known to have caused either moderate harm, severe harm, or deaths occurred in age category 76 to 85 years (104; 29.7%). In total, 44.6% (n = 156) were reported to relate to prescribing, followed by administration (80; 22.8%) and dispensing of medication (60; 17.1%). Wrongful omission of DOACs (70; 20%), followed by contra-indication to the use of the medicine in relation to concomitant drugs prescribed or patients’ clinical conditions (52; 15.4%) and wrong/unclear dose or strength of DOACs (43; 12.5%) were the three most common types of medication error reported among these incidents.
3.2. Incidents leading to deaths
Majority of the 25 incidents leading to deaths were reported to have been contributed by active failures caused by mistakes (n = 13; 52%). Free text data in relation to every incident leading to death were analyzed and presented in . For example, a physician prescribed apixaban to a patient (who was 56–65 years old) only on the drug chart and wrote ‘see anticoagulation chart’ without writing the same order on the anticoagulation chart. This mistake in prescribing was assumed to have led to death because of the omitted medicine used by the patient before admission.
3.3. Incidents leading to severe to moderate harm
Qualitative analysis of the examples of severe errors are depicted in and represent slips, mistakes, and latent failures. One of the examples documented in the database is related to the lack of experience and training of cardiologist staff in regard DOACs causing latent failure because of contraindication to drugs or condition. In this case, the patient (who was 66–75 years old) was admitted with chest pain and was on apixaban for managing previous stroke. The patient received fondaparinux to treat ACS while already on apixaban. Afterward, the patient was readmitted with hemorrhagic stroke because of the errors in prescriptions due to the lack of experience from the cardiologists. Further examples are presented in .
Most of the incidents leading to moderate harm (84.8%, 229) were caused by active failure of which the majority were caused by mistakes (49.3%; n = 113). Poor documentation was the most prevalent reason in relation to error provoking conditions (35.1%; n = 35). Heavy workload and lack of training contributed equally (n = 2) and were linked to the occurrence of the latent failures. For example, patient received coadministration of dalteparin with apixaban for evening dose, and this was reported to have happened because of the heavy workload of the nurses that led to losing concentration in managing the patient’s medications and lack of consulting the physician after prescribing dalteparin while the patient was already on apixaban. Further examples are demonstrated in .
Table 4. Examples of incidents associated with moderate harm.
3.4. Medications involved and indication
Apixaban represented the highest number of reported incidents accounting for the majority of the incidents (n = 8127; 52.0%) followed by rivaroxaban (n = 5658; 36.0%), edoxaban (n = 937; 6.0%), dabigatran (n = 787;5.0%), and unspecified of DOACs (n = 221; 1.0%) as shown in Supplementary material .
3.5. Indication for medication use
Atrial fibrillation showed highest number related to the medication incidents (59.50%; n = 9340) followed by deep vein thrombosis (DVT) (n = 4625; 29.40%), pulmonary embolism (PE) (n = 1510; 9.60%), and other conditions (n = 255; 2.0%) as shown in Supplementary material figure S2.
3.6. Incidents per stage of medication use process
The highest number of medication incidents were reported to occur during the prescribing stage (n = 6614; 42.0%). The second highest category of reported medication incidents was reported to involve administration stage (n = 4581; 29.10%), followed by medication dispensing (n = 2557; 16.30%), monitoring/follow-up (n = 571; 3.60%), and advice (n = 357; 2.30%), and the least number was reported in the supply stage or OTC drugs use stage (n = 48; 0.30%). A total of 6.4% (n = 1002) incidents were categorized as ‘other’ which included storage, response to treatment, order communication, and product labeling and packaging. Detailed data and examples of incidents per stage of medication use process are provided in Supplementary material .
Table 5. Proportion and examples of safety incidents per stage of medication error category.
3.7. Safety incidents per medication error category
The most common errors resulting in an incident were omission (n = 3437; 21.90%) followed by contraindication to the use of the medicine in relation to drugs or conditions (n = 2223; 14.1%), followed by wrong/unclear dose or strength (n = 2188; 13.90%) and wrong drug/medicine (n = 1714; 10.9%). presents data on proportion of safety incidents per stage of medication error category with some examples.
3.8. Patient age
Patients aged 76 to 85 years were most commonly involved covering over a quarter of all incidents (28.53%; n = 4487), followed by patients above 85 years old (22.38%; n = 3520), as shown in Supplementary material figure S3.
3.9. Safety incidents per care setting
Cardiology wards in the hospitals had the highest proportion of medication reports for DOACs incidents (n = 7346; 46.70%), followed by acute medical ward (n = 3311; 21.10%). Community pharmacies had also a relatively high rate of error report (n = 1696; 10.80%). Results are provided in Supplementary material table S6.
4. Discussion
4.1. Statement of key findings
To our knowledge, this is the first study evaluating the medication incidents relating to DOACs using a large national reporting dataset. Of the 15,730 incidents analyzed, a total of 11% incidents were associated with some degree of harm, including severe and moderate harm, ontributing to deaths. The present study showed that apixaban and rivaroxaban were amongst the medications most associated with safety incidents. The majority of all incidents (68%) involved patients aged 66 years and above and occurred in cardiology and acute medical wards in hospitals.
4.2. Interpretation of findings
A recent study showed that apixaban was the most commonly prescribed oral anticoagulants in the UK in 2019 accounting for 38% of all prescribed items [Citation5]. This study by Afzal et al [Citation5] showed that while the highest number of adverse drug reactions reported to the Medicines and Healthcare Regulatory Agency (MHRA) in the UK amongst all anticoagulants was apixaban (643 events reported in 2019), the number of ADRs per 100,000 items were similar to other DOACs such as rivaroxaban. This suggests that the higher number of incidents related to apixaban in our study was linked with higher prescribing volume rather than its adverse event profile.
The findings of our study corroborate well with the results of other studies around DOAC errors. A study conducted in a large University hospital in the West Midlands region of England reported prescribers’ active failure contributed to the majority of DOAC-related incidents [Citation15]. Another retrospective study investigated severe medication errors (MEs) reported to the National Supervisory Authority for Welfare and Health (Valvira) in Finland and conveyed similar results as most of the errors occurred in prescribing, administration, and monitoring phases of the medication process due to mainly active failure causes [Citation16]. Omission error had the highest prevalence among medication errors category in the present study, similar to other studies conducted in tertiary care hospitals in England, Wales, and India [Citation17,Citation18].
4.3. Strengths and limitations
This study has the advantages of using a big national database with extensive information on safety incidents. Availability of free-text description of the safety incidents added value to our study through qualitative analysis. However, researchers noted a lack of a systematized way of narrative reporting. Due to the voluntary nature of the error reporting system, errors are likely to have been under-reported, leading to selection or reporting bias. Previous studies describe barriers such as lack of time, fear of blame, lack of knowledge, and forgetfulness amongst key barriers to incident reporting [Citation19,Citation20]. Furthermore, the researchers had limited information about patient’s clinical conditions, risk factors, and comorbidities. Therefore, independent evaluation of the causality of the harm with the DOACs could not be undertaken. Error-provoking conditions relating to charting mistakes might include other medications that could have also contributed to the adverse outcomes. Finally, we were unable to identify and classify the incidents linked to the actions of different HCPs and their hierarchy, including senior or junior doctors, nurses, and pharmacists, due to the narratives being often incomplete. Such evaluation will be useful in identifying target populations in future interventions.
5. Conclusion
The findings from this large-scale study involving a national incident reporting dataset suggests that medication incidents involving DOACs are a common occurrence and have led to severe harm and deaths. Most of the DOACs incidents were related to active failures, such as slips and mistakes, as well as through poor documentation and communication and lack of training. Given their proven effectiveness and ease of use, it is imperative to promote safer prescribing and use of DOACs. Health organizations should invest in the continuous professional development, promoting guideline adherence and interventions that have been shown effective in minimizing medication and specifically DOAC related errors.
6. Expert opinion
DOACs related incidents are common in healthcare settings, and this study shows that the incidents can lead to fatal outcomes as well as various degrees of harm with many of these being preventable. Based on Reason’s accident causation model, the present study also showed that the majority of DOACs-pertaining safety incidents were due to active failures of slips and mistakes followed by poor documentation and communication and lack of training. Examples include missed doses, duplication of anticoagulant therapies, patient being discharged without DOACs, and lack of commencement or adjustment of DOACs post-surgery. There is a need to promote guideline adherence through education, training, and decision support technologies, given the majority of DOAC errors related to active failures.
Many of the reported incidents leading to harm were related to the lack of dose adjustments as per renal function. Dose adjustments is key to optimizing treatment and reducing the risk of bleeding. Creatinine clearance (CrCl) instead of eGFR has been advised to determine dosage adjustments for DOACs [Citation21]. Mandating renal functions in prescriptions is likely to minimize errors. Multifaceted interventions involving the use of clinical pharmacists in ward rounds and technological and educational support have been shown to be effective in reducing errors [Citation22,Citation23].
This study could be used as guidance for policy makers, safety professionals, as well as the clinical practitioners to pay attention for omission errors and contraindications while prescribing, dispensing, and administering DOACs. Given the significant impact that such preventable incidents can have on patient-related outcomes, some of the omissions or delays of DOACs could provoke significant patient harm that necessitate further and expensive health care. Health organizations should focus on training their clinicians on DOACs pharmacotherapy and on human factors associated with medication errors such as mistakes in prescribing and encouraging clinician to follow evidence-based clinical guidelines or pathways to improve prescribing practice of such medications. It is also important to perform clinical audits of reported safety incidents and disseminate recommendations for clinical practice improvements. Pharmacists can play an important role in ensuring safety practices through participation and leadership in anticoagulant stewardship programs, which emphasize the use of evidence base in decision making as well as appropriate prescribing, dispensing, and follow-up practices [Citation24]. In addition, pharmacists could play important role in prevention action through medication reviews and medication reconciliation upon admission, care transfer and on discharge, and through their wider roles in prescription and non-prescription medicines counseling and advice in the community [Citation25,Citation26] to help minimizing administration errors. In addition to implementing anticoagulation stewardship and providing anticoagulation management, pharmacist-based ambulatory care anticoagulation clinics can also promote patient safety [Citation27].
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions Statement
The authors confirm contribution to the paper as follows: study conception and design: A Alrowily, Z Jalal and V Paudyal; data collection: A Alrowily and V Paudyal; The primary author (A Alrowily) was responsible for cleaning up of data and applying the inclusion/exclusion criteria. A sample of 10% of these data was validated by two other authors V Paudyal and Z Jalal. Analysis and interpretation of results: A Alrowily, Z Jalal; Draft manuscript preparation: A Alrowily. All authors reviewed the results and approved the final version of the manuscript prior to submission.References
References
NRLS_Supplementary_EODS.docx
Download MS Word (361.1 KB)Data availability statement
All data corresponding to this work are provided with the manuscript.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2023.2223947
Additional information
Funding
- Piazza G, Nguyen TN, Cios D, et al. Anticoagulation-associated adverse drug events. Am j med. 2011;124(12):1136–1142. doi: 10.1016/j.amjmed.2011.06.009
- Institute for Safe Medication Practices. High-alert medications in acute care settings. 2014. cited 20Mar 2023. https://www.ismp.org/recommendations/high-alert-medications-acute-list.
- Mulac A, Taxis K, Hagesaether E, et al. Severe and fatal medication errors in hospitals: findings from the norwegian incident reporting system. Eur J Hosp Pharm. 2021;28(Suppl 2):e56–e61. doi: 10.1136/ejhpharm-2020-002298
- Barr D, Epps QJ. Direct oral anticoagulants: a review of common medication errors. J Thromb Thrombolysis. 2019;47(1):146–154. doi: 10.1007/s11239-018-1752-9
- Afzal S, Zaidi STR, Merchant HA, et al. Prescribing trends of oral anticoagulants in England over the last decade: a focus on new and old drugs and adverse events reporting. J Thromb Thrombolysis. 2021;52(2):646–653. doi: 10.1007/s11239-021-02416-4
- Al Rowily A, Jalal Z, Price MJ, et al. Prevalence, contributory factors and severity of medication errors associated with direct-acting oral anticoagulants in adult patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78(4):623–645. doi: 10.1007/s00228-021-03212-y
- Institute of Medicine (US). Committee on Quality of Health Care in America. To Err is Human: building a Safer Health System. Kohn LT, Corrigan JM, Donaldson MS, eds. (WA) (DC): National Academies Press (US); 2000.
- Elliott RA, Camacho E, Jankovic D, et al. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual Saf. 2021;30(2):96–105. DOI: 10.1136/bmjqs-2019-010206
- World Health Organisation. Global Patient safety challenge: medication without harm. 2017. Accessed 20 Mar 2023. http://www.who.int/patientsafety/medication-safety/medication-without-harm-brochure/en/.
- McDowell SE, Ferner HS, Ferner RE. The pathophysiology of medication errors: how and where they arise. Br J Clin Pharmacol. 2009;67(6):605–613. doi: 10.1111/j.1365-2125.2009.03416.x
- Department of Health. Building a safer NHS for patients. London: Department of Health, 2001. Accessed 20 Mar 2023. Available from: http://www.dh.gov.uk/assetRoot/04/05/80/94/04058094.pdf
- National Reporting and Learning System. Organisation patient safety incident reports. 2019. Accessed 20 Mar 2023. Available from: http://www.nrls.npsa.nhs.uk/patient-safetydata/organisation-patient-safety-incident-reports/
- NHS Improvement. Guidance notes on national reporting and learning system official statistics publications. 2017. Accessed 20 Mar 2023. Available from: https://improvement.nhs.uk/documents/1728/Guidance_notes_on_NRLS_officia_statistics_Sept_2017.pdf
- Reason J. A systems approach to organizational error. Ergonomics. 1995;38(8):1708–1721. doi: 10.1080/00140139508925221
- Haque H, Alrowily A, Jalal Z, et al. Direct oral anticoagulant-related medication incidents and pharmacists’ interventions in hospital in-patients: evaluation using reason’s accident causation theory. Int J Clin Pharm. 2021;43(6):1693–1704. doi: 10.1007/s11096-021-01302-6
- Linden-Lahti C, Takala A, Holmström AR, et al. What severe medication errors reported to health care supervisory authority tell about medication safety? J Patient Saf. 2021 1;17(8):e1179–e1185. doi: 10.1097/PTS.0000000000000914
- Härkänen M, Vehviläinen-Julkunen K, Murrells T, et al. Medication administration errors and mortality: incidents reported in England and Wales between 2007_2016. Res Social Adm Pharm. 2019;15(7):858–863. doi: 10.1016/j.sapharm.2018.11.010
- Sheikh D, Mateti UV, Kabekkodu S, et al. Assessment of medication errors and adherence to WHO prescription writing guidelines in a tertiary care hospital. Future J Pharm Sci. 2017;3(1):60–64. doi: 10.1016/j.fjps.2017.03.001
- Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19–31. doi: 10.2165/00002018-200932010-00002
- Paudyal V, Al-Hamid A, Bowen M, et al. Interventions to improve spontaneous adverse drug reaction reporting by healthcare professionals and patients: systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(9):1173–1191. doi: 10.1080/14740338.2020.1807003
- GOV.UK. Prescribing medicines in renal impairment: using the appropriate estimate of renal function to avoid the risk of adverse drug reactions. 2019. Accessed 20 Mar 2023. https://www.gov.uk/drug-safety-update/prescribing-medicines-in-renal-impairment-using-the-appropriate-estimate-of-renal-function-to-avoid-the-risk-of-adverse-drug-reactions
- Naseralallah LM, Hussain TA, Jaam M, et al. Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: a systematic review and meta-analysis. Int J Clin Pharm. 2020;42(4):979–994. doi: 10.1007/s11096-020-01034-z
- Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet. 2012;379(9823):1310–1319. 7. doi: 10.1016/S0140-6736(11)61817-5
- Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022 17;6(5):e12757. doi: 10.1002/rth2.12757
- Paudyal V, Hansford D, Cunningham S, et al. Over-the-counter prescribing and pharmacists’ adoption of new medicines: diffusion of innovations. Res Soc Admin Pharm. 2013 1;9(3):251–262. doi: 10.1016/j.sapharm.2012.05.001
- Paudyal V, Hansford D, Cunningham S, et al. Pharmacists’ perceived integration into practice of over-the-counter simvastatin five years post reclassification. Int J Clin Pharm. 2012;34(5):733–738. doi: 10.1007/s11096-012-9668-5
- The Joint Commission. Sentinel event alert: managing the risks of direct oral anticoagulants. 2019. cited 20 Mar 2023. https://www.jointcommission.org/resources/sentinel-event/sentinel-event-alert-newsletters/sentinel-event-alert-61-managing-the-risks-of-direct-oral-anticoagulants
