ABSTRACT
Background
Rasagiline is a monoamine oxidase B inhibitor for the treatment of Parkinson’s disease (PD). This study assessed the safety and effectiveness of rasagiline in patients with PD in routine clinical practice in Japan.
Research design and methods
This multicenter, prospective, observational study (148 sites) enrolled patients (1 November 2018−31 October 2020) with PD. Patients received rasagiline orally 1 mg once daily; maximum observation period was 24 months. The incidence of adverse drug reactions (ADRs) was evaluated; effectiveness was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III total score.
Results
The safety analysis set comprised 961 patients (mean age, 72.50 years; 53.80% female; mean duration of PD, 6.82 years). Mean treatment duration was 14.74 months, with 42.25% receiving rasagiline for ≥ 19 months; 189 (19.67%) had ≥ 1 ADR. Common ADRs were dyskinesia (4.06%), orthostatic hypotension (2.29%), hallucination (1.87%), visual hallucination, nausea, fall (1.56% each), dizziness (1.35%), and somnolence (1.25%). Mean (standard deviation) UPDRS Part III total score was 28.5 (14.35) at baseline and 25.5 (14.98) at the final assessment.
Conclusions
No new concerns in safety and effectiveness regarding rasagiline in Japanese patients with PD were raised.
Trial registration
ClinicalTrials.gov: NCT03727139; Japan Pharmaceutical Information Center Clinical Trials Information: JapicCTI-184181.
1. Introduction
Parkinson’s disease (PD) is a neurological disease characterized by motor symptoms (tremor, slow movement, rigidity, imbalance) and non-motor symptoms (fatigue, pain, depression, sleep disorders, autonomic nervous disorders, and cognitive dysfunction) [Citation1]. PD symptoms are the result of the loss of dopaminergic neurons and dopamine [Citation2]. The global prevalence of PD in 2019 was > 8 million individuals, with higher prevalence occurring in patients aged over 65 years [Citation3]; in 2016 the prevalence of PD in Japan was 256,455 patients [Citation4]. Current pharmacotherapies for PD only alleviate symptoms, and no cure is available; treatments include levodopa, dopamine agonists, and monoamine oxidase B (MAO-B) inhibitors [Citation5].
Rasagiline is a selective and irreversible MAO-B inhibitor that inhibits the breakdown of dopamine [Citation6] and is an approved treatment of PD. Other MAO-B inhibitors approved for the treatment of PD include selegiline [Citation7] and safinamide [Citation8]. The safety and efficacy of rasagiline have been evaluated in several randomized placebo-controlled clinical trials, either as monotherapy in patients with early-stage PD [Citation9,Citation10] or as an adjunct to levodopa in patients with PD and motor fluctuations (i.e. wearing-off phenomena) [Citation11,Citation12]. In addition, the safety and efficacy of rasagiline have also been evaluated in Japanese patients both as monotherapy in a phase 3 trial in patients with early PD [Citation13] and as adjunctive therapy to levodopa in a phase 2/3 trial in patients with PD and wearing-off phenomena [Citation14]. However, information about safety and effectiveness for long-term use of rasagiline is limited because most of the clinical trials conducted to date are relatively short term (up to 26 weeks). An open-label extension study of initial rasagiline monotherapy up to 6.5 years has provided implications on the long-term tolerability of rasagiline [Citation15]. Two studies have assessed the long-term use of rasagiline for up to 52 weeks in Japanese patients: an open-label phase 3 extension study in Japanese patients with early PD [Citation16] and an open-label phase 3 study of rasagiline in combination with levodopa in Japanese patients with PD and wearing-off phenomena [Citation17]. No major safety issues were identified with long-term use of rasagiline in these Japanese studies, consistent with previous observations based on clinical trials [Citation18]. However, the safety and efficacy of rasagiline in these studies do not fully reflect the real-world clinical settings, where anti-PD drugs are prescribed to patients with PD of various demographics, including elderly patients, whose numbers are increasing in aging societies.
This post-marketing surveillance study was conducted to investigate the safety and effectiveness of the long-term use of rasagiline in patients with PD in routine clinical practice in Japan. Consistency of the safety of rasagiline in this real-world study compared with that from clinical trials of shorter duration is discussed.
2. Patients and methods
2.1. Study design
This was a multicenter, prospective, observational study conducted between 1 November 2018 and 31 October 2021. Patients with PD treated with rasagiline as part of routine medical care, excluding those with any contraindication for rasagiline as described in the prescribing information [Citation19], were eligible for inclusion in the study. Patient enrollment occurred between 1 November 2018 and 31 October 2020. No patient was enrolled on or after 1 November 2020, even if rasagiline was prescribed to the patient by 31 October 2020. The observation period was primarily set to 12 months. For patients enrolled during the first 12 months of the study, the maximum duration of observation could be extended up to 24 months in accordance with the patients’ enrollment period. If administration of rasagiline was discontinued for any reason, participation in the study was terminated at that time.
Patients were enrolled using a centralized enrollment method via a web-based electronic data collection system. The study physician or designee entered data for patient enrollment into the system by 14 days after the date of prescription of rasagiline (with the day of prescription defined as ‘Day 0’ and the next day as ‘Day 1’). Administration and discontinuation of rasagiline and adverse events (AEs) were recorded in the system.
All patients (or their legally acceptable representatives) were required to provide verbal or written consent to permit data provision for the study; patients who provided consent were assigned an identification number. This study was conducted in compliance with the Japanese Ministerial Ordinance on Good Post-Marketing Study Practice (GPSP). Institutional Review Board and Ethics Committee approvals were not required according to the GPSP Ordinance. This study was registered at ClinicalTrials.gov (identifier: NCT03727139) and with the Japan Pharmaceutical Information Center (identifier: JapicCTI-184181).
2.2. Treatment
Rasagiline was administered orally 1 mg once daily as a tablet according to the dosage described in the prescribing information [Citation19]. A lower dose (i.e. 0.5 mg) could be considered for elderly and other patients under observation.
2.3. Collected information
Patient information was collected at the time of enrollment and included the date of prescription of rasagiline, patient identification number, patient initials, sex, and age (at the time of prescription of rasagiline). Patient background information at the time rasagiline was initiated included duration of PD, inpatient/outpatient status (at the start of treatment with rasagiline), concurrent diseases (presence or absence and details), height, body weight, modified Hoehn and Yahr (HY) severity scale stage, presence or absence of wearing-off phenomena (in patients given levodopa at the time of administration of rasagiline), and presence or absence of dyskinesia (in patients given levodopa at the time of administration of rasagiline).
Treatment information included detailed use of rasagiline (dosage at initiation), detailed use of levodopa (presence or absence, dosage at initiation of rasagiline administration, therapy dates), detailed use of drugs to treat PD other than rasagiline and levodopa (presence or absence, name of the drug, therapy dates), detailed use of concomitant drugs other than drugs to treat PD (presence or absence, name of the drug, reason for use), and detailed use of concomitant therapies other than pharmacotherapy for PD (presence or absence, name of concomitant therapy, therapy dates). Treatment details were collected from the initiation of treatment with rasagiline and up to 24 months (or discontinuation) of treatment with rasagiline. Treatment adherence with rasagiline was assessed at 6, 12, 18, and 24 months or at discontinuation of treatment with rasagiline.
For safety, AE data were collected from initiation of treatment with rasagiline until up to 24 months (or discontinuation) of treatment. Data collected were AE term, date of onset, seriousness and reason for the assessment as serious, action taken with rasagiline, outcome assessment date, outcome, and causal relationship to rasagiline. If the event outcome was ‘not resolved’ or ‘unknown,’ the event was to be followed for as long as possible. Abnormal worsening of target disease, e.g. worsening beyond the expected natural course of the disease, was to be handled as an AE.
Motor symptoms were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III (motor examination) total score at baseline, 6, 12, 18, and 24 months or at discontinuation of treatment with rasagiline.
2.4. Statistical analysis
The planned sample size for this study was 1000 patients and was based on AE data from the Japanese clinical studies of rasagiline for the important identified risks [Citation13,Citation14,Citation17]. Assuming that orthostatic hypotension, somnolence and sudden onset of sleep, and psychiatric symptoms such as hallucinations and dyskinesia would occur with an incidence of 1% or above, the planned sample size of 1000 would enable the evaluation of these events. With a sample size of 1000, at least five cases of an AE occurring with an incidence of 1% can be detected with a probability of at least 95%.
The safety analysis population was defined as patients who received rasagiline and were evaluable for safety, excluding those meeting the following conditions: administration of rasagiline before the contract period; enrollment outside the enrollment period; enrollment ≥15 days after the date of prescription of rasagiline; prescription of rasagiline outside the contract period; no information on presence or absence of AEs; and withdrawal of consent. The efficacy analysis population was defined as a subset of the patients in the safety analysis population who were evaluable for efficacy, excluding those meeting the following conditions: no target disease; and administration of contraindicated medication for rasagiline (PD medication). Categorical data are reported as n (%) of patients. Quantitative data are reported as the number of patients, mean, and standard deviation (SD).
Adverse drug reactions and infections (hereinafter referred to as ADRs), defined as AEs assessed as causally ‘related’ to rasagiline by the survey physician, were coded using the Medical Dictionary for Regulatory Activities/Japanese edition Version 24.1 and summarized by preferred term and system organ class. ADRs occurring during the observation period were summarized by frequency, with stratification of patients according to background and treatment factors (age [<65 years; ≥65 to <75 years; ≥75 years; <80 years; ≥80 years], HY severity scale [<3; ≥3], daily dose of rasagiline [1 mg; 0.5 mg; other], daily dose of levodopa [0 mg; >0 to <200 mg; 200 to <400 mg; ≥400 mg], body weight [<50 kg; 50 to <65 kg; ≥65 kg], treatment stage [rasagiline alone; rasagiline plus other PD medications; combined with only levodopa without wearing-off phenomena; combined with levodopa and other PD medications, without wearing-off phenomena; combined with levodopa, with wearing-off phenomena], presence or absence of dyskinesia in patients given levodopa at initiation of treatment with rasagiline, and presence or absence of dementia as judged by the survey physician at the initiation of the survey). Discontinuation of rasagiline due to ADRs was also analyzed and summarized by frequency.
Effectiveness was evaluated by mean (SD) change from baseline over time in UPDRS Part III total score; this is shown as a line graph for the total efficacy population and with stratification according to age (<65 years; ≥65 to <70 years; ≥70 to <75 years; ≥75 to <80 years; ≥80 years) and is tabulated by patient background and treatment factors (age among patients receiving rasagiline 1 mg; body weight; duration of PD; modified HY severity scale; wearing-off phenomena; dyskinesia; UPDRS Part III total score at initiation of treatment with rasagiline; daily rasagiline dose; daily levodopa dose).
Treatment duration was defined as therapy end date minus therapy start date plus 1. Disease duration (years) was calculated as the date of first administration of rasagiline minus the date of disease onset plus 1 divided by 365.25. If the month and day of disease onset were unknown, January 1 was used; if the month was known but the day was unknown, 1 was used as the day.
For observation items, data that were evaluable (i.e. not missing and determined to be included in the analysis) were handled in accordance with the following rules: data within the allowable time window were used; and if there were multiple evaluable data within the same allowable time window, the data closest to the standard implementation time point of the examination, observation, or assessment was used. The allowable time windows after administration of rasagiline were: baseline (at initiation with rasagiline), Day −1 (time window, Days −30 to 1); at 6 months of treatment, Day 182 (time window, Days 2–273); at 12 months of treatment, Day 365 (time window, Days 274–456); at 18 months of treatment, Day 547 (time window, Days 457–638); at 24 months of treatment or discontinuation, Day 730 (time window, Days 639–821); final assessment, time window Days 2–821.
3. Results
3.1. Patient disposition
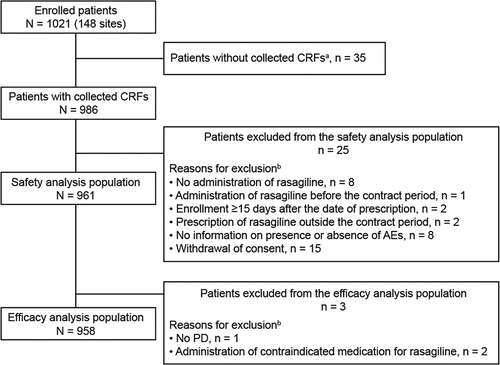
A total of 1021 patients were enrolled across 148 sites, case report forms were collected for 986 patients, and 961 patients were included in the safety analysis set; 25 patients were excluded for ≥ 1 reason (). The main reasons for exclusion were withdrawal of consent (15 patients), no administration of rasagiline (eight patients), and no information on the presence or absence of AEs (eight patients). A total of 958 patients were included in the efficacy analysis population; three patients were excluded due to no target disease (one patient) and administration of contraindicated medication for rasagiline (two patients).
3.2. Patient demographics and baseline characteristics
The demographics and characteristics of the 961 patients included in the safety analysis set are presented in . Overall, 517 (53.80%) patients were female, mean (SD) age was 72.50 (9.04) years, 793 (82.52%) patients were aged ≥65 years, and mean (SD) body weight was 54.84 (11.46) kg. The mean (SD) duration of PD was 6.82 (5.41) years, with 455 (47.35%) patients having a modified HY severity scale of stage 3. Overall, 759 patients (78.98%) had a concurrent disease. At the time rasagiline treatment was initiated, 865 (90.01%) patients were using levodopa, and the mean (SD) UPDRS Part III total score was 28.50 (14.36).
Table 1. Patient characteristics at baseline.
3.3. Treatment characteristics
3.3.1. Rasagiline
The mean (SD) daily dose of rasagiline was 0.86 (0.23) mg, and the specific daily dose was 1 mg for 679 (70.66%) patients and 0.5 mg for 280 (29.14%) patients (). Mean (SD) treatment duration was 14.74 (9.00) months; treatment duration was ≥19 months for 406 (42.25%) patients. The reason for discontinuation of treatment with rasagiline was most commonly AE onset (204 patients; 21.23%), followed by no return to study site (including referral to another hospital; 97 patients; 10.09%), inadequate effectiveness (62 patients; 6.45%), other (30 patients; 3.12%), and achievement of treatment goal (three patients; 0.31%). The percentage of patients who were at least 90% compliant was 89.13% (812/911) at 6 months, 83.01% (645/777) at 12 months, 96.53% (417/432) at 18 months, and 93.12% (379/407) at 24 months or discontinuation of treatment (Supplementary Table S1).
Table 2. Use and discontinuation of rasagiline.
3.3.2. Levodopa use
Levodopa use throughout the observation period was reported for almost all patients (908/961, 94.48%), with half receiving the drug at a daily dose of 200 to <400 mg (Supplementary Table S2). The mean (SD) duration of levodopa administration was 20.64 (20.03) months; treatment duration was ≥19 months for 430 (47.36%) patients.
3.3.3. Other drugs
Other drugs used to treat PD (other than rasagiline and levodopa) were reported by 661 (68.78%) patients (Supplementary Table S3). Of the patients who received other PD drugs, most were administered rotigotine (51.59%, 341/661 patients), zonisamide (44.63%, 295/661 patients), and istradefylline (32.98%, 218/661 patients).
3.4. Safety
3.4.1. ADRs
A total of 248 ADRs were reported in 189 (19.67%) patients (). ADRs with an incidence of ≥ 1% were dyskinesia (4.06%, 39/961 patients), orthostatic hypotension (2.29%, 22/961 patients), hallucination (1.87%, 18/961 patients), visual hallucination, nausea, fall (1.56% each, 15/961 patients each), dizziness (1.35%, 13/961 patients), and somnolence (1.25%, 12/961 patients). An overdose was associated with dizziness in one patient.
Table 3. Incidence of ADRs.
3.4.2. ADRs by age
The incidence of ADRs was numerically lower for patients aged <65 years compared with patients aged ≥65 years (). The incidence of ADRs was 16.07% (27/168 patients) for patients aged <65 years, 19.94% (71/356 patients) for patients aged ≥ 65 to <75 years, and 20.82% (91/437 patients) for patients aged ≥75 years. The incidence of ADRs was similar when comparing patients aged <80 years with those ≥80 years (19.41% [145/747 patients] vs 20.56% [44/214 patients], respectively; Supplementary Table S4). Overall, the types of common ADRs (occurring at an incidence of ≥ 1%) did not vary substantially across different age categories, although numerical differences in the incidence of some specific ADRs across the age groups should be noted (). Of note is the higher incidence of psychiatric disorders in patients ≥80 years compared with those <80 years (7.48% [16/214 patients] vs 4.42% [33/747 patients], respectively; Supplementary Table S4).
Table 4. Incidence of ADRs by age occurring at a frequency ≥ 1% (by preferred term) in either subgroup or in the total safety population.
3.4.3. ADRs by modified HY severity scale
The incidence of ADRs was 18.45% (62/336 patients) for patients with a modified HY severity scale < 3, and 20.32% (127/625 patients) for patients with a modified HY severity scale ≥ 3 (Supplementary Table 5). The types of common ADRs were similar across the two groups except for psychiatric disorders, which occurred at a numerically higher frequency in patients with a modified HY severity scale ≥ 3 compared with patients with a modified HY severity scale < 3 (6.08% vs 3.27%, respectively).
3.4.4. ADRs by daily dose of rasagiline and daily dose of levodopa
The incidence of ADRs was numerically higher with 0.5 mg compared with 1 mg of rasagiline (26.07% [73/280 patients] vs 16.79% [114/679 patients]), and the types of common ADRs did not differ largely between the doses (Supplementary Table S6).
The incidence of ADRs increased with higher daily doses of levodopa (Supplementary Table S7). The incidence of ADRs was 7.55% (4/53 patients), 11.11% (8/72 patients), 15.62% (72/461), and 28.00% (105/375 patients) for patients given a daily levodopa dose of 0 mg, >0 to <200 mg, 200 to <400 mg, and ≥400 mg, respectively. ADRs of psychiatric disorders and nervous system disorders occurred at numerically higher frequencies in patients receiving daily doses of levodopa ≥400 mg compared with those receiving daily doses of 200 to <400 mg (8.27% [31/375 patients] vs 3.69% [17/461 patients] for psychiatric disorders and 12.27% [46/375 patients] vs 4.56% [21/461 patients] for nervous system disorders).
3.4.5. ADRs by body weight
The incidence of ADRs decreased with increased body weight; the types of ADRs were more similar between the <50 kg and 50 to <65 kg subgroups compared with the ≥65 kg subgroup (Supplementary Table S8). The incidence of ADRs was 26.95% (69/256 patients) for patients <50 kg, 19.33% (63/326 patients) for patients 50 to <65 kg, and 15.00% (21/140 patients) for patients ≥65 kg.
3.4.6. ADRs by treatment stage
The incidence of ADRs was numerically higher for patients receiving rasagiline combined with levodopa compared with rasagiline monotherapy or in combination with other PD medications (Supplementary Table S9). The incidence of ADRs in patients receiving rasagiline alone was 5.63% (4/71 patients), in patients receiving rasagiline plus other PD medications was 4.00% (1/25 patients), and in those receiving rasagiline combined with levodopa only and without wearing-off phenomena was 18.92% (35/185 patients). The incidence of ADRs in patients receiving rasagiline combined with levodopa and other PD medications, without wearing-off phenomena, was 14.83% (31/209 patients), and in those receiving rasagiline combined with levodopa, with wearing-off phenomena, was 25.05% (118/471 patients). In general, the types of ADRs differed among patients stratified by treatment stage, but small sample sizes in some treatment stages should be noted.
3.4.7. ADRs by the presence or absence of dyskinesia
When stratified by the presence or absence of dyskinesia among patients who were given levodopa at initiation of treatment with rasagiline, the incidence of ADRs was numerically higher in patients with concurrent dyskinesia compared with those with no dyskinesia, and the types of common ADRs did not largely differ between the subgroups (Supplementary Table S10). The incidence of ADRs was 19.31% (135/699 patients) for patients with no dyskinesia and 29.52% (49/166) for patients with dyskinesia.
3.4.8. ADRs by the presence or absence of dementia
The incidence of ADRs was numerically higher in patients with dementia (25.71% [18/70 patients]) compared with those without dementia (19.19% [171/891] patients). The types of common ADRs did not largely differ between the subgroups; of note, the incidence of psychiatric disorders was numerically higher for patients with dementia (12.86% [9/70 patients]) compared with patients without dementia (4.49% [40/891 patients]) (Supplementary Table S11).
3.5. Effectiveness
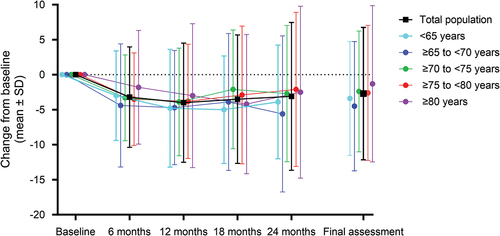
In the efficacy analysis population, the mean (SD) UPDRS Part III total score was 28.50 (14.35) at baseline, which decreased to 25.50 (14.98) at the final assessment (). The change from baseline in the UPDRS Part III total score showed greatest decrease at 12 months of treatment, and then moderately increased, but remained decreased from baseline at all time points of assessment. Age was evaluated as a potential factor likely to affect the effectiveness of rasagiline; however, analysis with stratification by age showed no consistent trend (). When analyzed by patient background and treatment factors, a decrease in the mean UPDRS Part III total score from baseline at the final assessment was observed for all subgroups (Supplementary Table S12). Among those patients receiving rasagiline at a daily dose of 1 mg, mean (SD) change in UPDRS Part III total score was greatest for patients aged ≥ 65 to < 70 (−5.1 [9.92]) and smallest for those aged ≥ 80 (−1.0 [12.49]) (Supplementary Table S13).
4. Discussion
This post-marketing surveillance study of more than 950 Japanese patients with PD confirms that the safety of rasagiline for up to 24 months in real-world clinical practice is similar to that established in clinical trials. This is the first report of the long-term safety and effectiveness of rasagiline in a large number of Japanese patients with PD treated with rasagiline in routine clinical practice; furthermore, patients aged ≥80 years and those with dementia were included. Novel severe ADRs occurring at a high incidence were not observed, indicating that no new safety concerns were identified despite the inclusion of patients of ≥80 years age, with comorbidities, using rasagiline in combination with other drugs to treat PD, and using concomitant therapies. Decreases in mean UPDRS Part III total score, indicating improvements in motor symptoms, were observed at all time points after initiation of treatment with rasagiline and at the final assessment time point. Overall, these results provide practical information on the use of rasagiline for the treatment of Japanese patients with PD.
Orthostatic hypotension, somnolence, sudden onset of sleep, psychiatric symptoms such as hallucination, and dyskinesia are listed as precautions for use of rasagiline [Citation19,Citation20]. Approximately one-fifth of the patient cohort in this study had at least one ADR, consisting mainly of nervous system disorders, psychiatric disorders, and vascular disorders, consistent with the known risks of rasagiline. Based on the information obtained in this real-world study of Japanese patients with PD, the addition of a new precautionary statement for rasagiline is not considered necessary.
In the phase 2/3 clinical study of rasagiline in Japanese patients with PD with wearing-off phenomena, the incidence of treatment-emergent AEs judged to be related to rasagiline was 44.4% for 0.5 mg and 51.2% for 1 mg; the most common treatment-emergent AEs were nasopharyngitis, dyskinesia, fall, and contusion [Citation14]. In this study of Japanese patients with PD (with and without wearing-off phenomena), the incidence of ADRs (AEs considered related to rasagiline) in clinical practice was 19.67%; nasopharyngitis and contusion were not identified as common ADRs. In two post-marketing observational studies of rasagiline treatment (as either monotherapy or combination therapy) for patients with PD in Germany, the frequency of AEs/ADRs was low (≤8.0%); the most common ADRs/AEs were nausea, dizziness, and headache [Citation21,Citation22]. ADRs that were common in this Japanese real-world study, such as dyskinesia, orthostatic hypotension, hallucination, visual hallucination, and somnolence, were not observed or occurred in < 0.3% of patients in the German studies [Citation21,Citation22]. Although different ethnic backgrounds could explain these differences in safety results, this is unlikely because similar AEs were observed in clinical studies of rasagiline conducted in Japanese and Western patient populations [Citation10,Citation16]. More likely, the large number of patients with advanced stage of disease treated with combination therapy may have contributed to the different safety profile observed. The low ratio of rasagiline monotherapy in current clinical practice in Japan may also account for the difference observed.
Age effects on the safety of rasagiline have been previously examined by post hoc subgroup analyses of pivotal clinical trials [Citation23,Citation24]. Although some AEs occurred more frequently in older (≥70 years) patients receiving rasagiline compared with younger patients (<70 years), this effect was considered not influenced by rasagiline treatment because this was true for the placebo-treated population as well [Citation23]. A pooled analysis of clinical studies to demonstrate the efficacy and tolerability of rasagiline as an adjunct to levodopa revealed that the incidence of most AEs in elderly patients (≥70 years) was generally similar to that in younger patients treated with rasagiline [Citation24]. Consistent with these clinical studies, in this current post-marketing observational study of Japanese patients with PD, the incidence of ADRs was numerically higher in older patients (≥65 years) compared with younger patients (<65 years), but the incidence of ADRs in patients ≥75 years was similar to those aged ≥ 65 to <75 years, and similar between those aged ≥80 years and those aged <80 years, suggesting that the incidence of ADRs does not always increase with age. This is also consistent with the post-marketing observational study conducted in Germany, which reported that the tolerability of rasagiline (as either monotherapy or combination therapy) in patients with PD did not appear to be affected by age [Citation21]. The common ADRs reported in this current Japanese study were similar across the age groups except for visual hallucinations, which were more frequent in patients aged ≥75 years, and dyskinesia, which tended to be more frequent in patients aged <75 years. This same trend was observed when ADRs were analyzed for patients aged ≥80 years and those aged <80 years; hallucinations were more frequent in older patients (≥80 years), and dyskinesia was more frequent in those aged <80 years. These findings in the safety profile of rasagiline in elderly patients are important because patients aged ≥80 years were not included in the rasagiline clinical trials in Japan [Citation13,Citation14,Citation16].
Factors other than age that are likely to affect the safety of rasagiline in Japanese patients with PD were also investigated in this study. Although drug metabolism was not examined in this observational study, high exposure due to low body weight, compromised drug metabolism capacity, and other reasons may increase the risk of ADRs. In this study, the incidence of ADRs was also numerically higher in patients who received rasagiline in combination with levodopa compared with rasagiline monotherapy, in patients with concurrent dyskinesia compared with those without, in those with a modified HY severity scale ≥ 3 compared with patients with less severe disease, and in patients with dementia compared with those without dementia. These results suggest that patients with advanced disease tend to have a relatively higher risk of ADRs than those with early PD. This difference in ADRs by disease stage is consistent with a previous analysis showing different AEs in clinical trials of rasagiline as monotherapy and as an adjunct to levodopa [Citation25]. Furthermore, some ADRs such as dyskinesia and hallucination observed in subgroups in advanced stage of disease could also be observed with disease progression as complications [Citation26]. In this context, patients with PD of advanced severity should be monitored carefully for ADRs caused by rasagiline. Nevertheless, it should be noted that in this study, the ADRs frequently observed in these high-risk patients were predominantly the same as those with less severe forms of PD, namely, dyskinesia, hallucination, orthostatic hypotension, somnolence, fall, and nausea.
The efficacy of rasagiline for the treatment of PD has been assessed in a number of clinical studies by evaluating changes in the UPDRS and the Movement Disorder Society-UPDRS (MDS-UPDRS) score [Citation27,Citation28]; the UPDRS and MDS-UPDRS are instruments used to rate the severity and progression of PD [Citation29–31]. Significant improvement in the change in UPDRS total score with rasagiline compared with placebo has been shown [Citation27]. Furthermore, significant improvement in UPDRS/MDS-UPDRS Part III (motor examination) scores with rasagiline, and rasagiline in combination with levodopa, has been shown [Citation27,Citation28]. In this study of Japanese patients with PD treated with rasagiline (as monotherapy, adjunct to levodopa, or in combination with other PD drugs), the mean UPDRS Part III total scores indicated an improvement in motor symptoms at all time points. Moreover, age did not appear to alter the trend for improved motor symptoms over time with rasagiline treatment, supporting the previous findings of clinical trials that showed that efficacy was unaffected by age [Citation24]. Overall, the effectiveness of rasagiline assessed by changes in UPDRS Part III total score was supported by this real-world study of Japanese patients with PD.
The strengths of this post-marketing surveillance study are that the results are real-world data that include a large number (>950) of Japanese patients with PD, the study population included patients with and without wearing-off phenomena, and the study design enabled the inclusion of patients with background characteristics who are rarely included in clinical trials, such as elderly patients and those with dementia. Furthermore, the long observation period (up to 24 months), which was longer than that of previous post-marketing studies of rasagiline [Citation21,Citation22], was also a key strength to confirm the safety profile of rasagiline in a real-world clinical setting.
The limitations of this study were the open-label design without a control or comparator group and the variable data capture outside the controlled environment of a clinical trial. When stratified by subgroups, patient background characteristics across the subgroups were not controlled for, and because of the small cohort sizes with unbalanced numbers of patients, statistical comparison between the subgroups could not be performed.
5. Conclusions
The results of this post-marketing surveillance study raised no new concerns regarding the long-term safety or effectiveness of rasagiline in Japanese patients with PD.
Declaration of interests
N. Hattori declares consulting fees from GlaxoSmithKline K.K., AbbVie Inc., Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., Kyowa Kirin Co., Ltd., Hisamitsu Pharmaceutical Co., Inc., Meiji Seika Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd, and FP Pharmaceutical Corporation; lecture fees from MSD K.K., Eli Lilly Japan K.K., Eisai Co., Ltd., FP Pharmaceutical Corporation, Otsuka Pharmaceutical Co., Ltd., Tsumura & Co., Kyowa Kirin Co., Ltd., GlaxoSmithKline K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Nihon Medi-Physics Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., and Daiichi Sankyo Company, Limited; honoraria from FP Pharmaceutical Corporation, Novartis Pharma K.K., Kyowa Kirin Co., Ltd., and AbbVie Inc.; research support from Otsuka Pharmaceutical Co., Ltd.; and grants from Astellas Pharma Inc., Eisai Co., Ltd., GlaxoSmithKline K.K., Sumitomo Dainippon Pharma Co. Ltd., Takeda Pharmaceutical Company Limited, Novartis Pharma K.K., Pfizer Japan Inc., Kyowa Kirin Co., Ltd., Medtronic Japan Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Boston Scientific Corporation, Kissei Pharmaceutical Co., Ltd, and Otsuka Pharmaceutical Co., Ltd. M. Kajita, S. Fujimoto, and M. Izutsu are employees of Takeda Pharmaceutical Company Limited. J. Fernandez declares stock options with Takeda Pharmaceutical Company Limited and GlaxoSmithKline, is a majority shareholder of Immunorock, Co., Ltd., and was an employee of Takeda Pharmaceutical Company Limited at the time of this study.
Reviewer disclosure
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors made a significant contribution to the work reported, and they participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. M. Kajita was involved in the data collection. All authors agree to be accountable for all aspects of the work.
Supplementary_Materials_REVISED.docx
Download MS Word (95.1 KB)Acknowledgments
The authors would like to thank all study participants. The authors would also like to thank Kohei Shimizu and Tadayuki Kitagawa (Biostatistics Takeda Pharmaceutical Company Limited) for their role in drafting the research, extracting and interpreting the results, creating an analysis plan, performing exploratory analyses for effectiveness, and for their review to ensure the accuracy of the interpretation of the analysis. A courtesy medical review of the manuscript was provided by Teva Pharmaceuticals.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization. Data requests should follow the process described in the Data Sharing section on https://clinicaltrials.takeda.com/and https://vivli.org/ourmember/takeda/.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2023.2293207.
Additional information
Funding
References
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3
- Triarhou LC. Dopamine and Parkinson’s disease. In: Madame Curie Bioscience Database Internet. Austin (TX): Landes Bioscience; 2000–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6271/
- Ou Z, Pan J, Tang S, et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health. 2021;9:776847. doi: 10.3389/fpubh.2021.776847
- GBD. Parkinson’s disease collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global burden of disease study 2016. Lancet Neurol. 2016;17(11):939–953.
- Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–2303. doi: 10.1016/S0140-6736(21)00218-X
- Lecht S, Haroutiunian S, Hoffman A, et al. Rasagiline - a novel MAO B inhibitor in Parkinson’s disease therapy. Ther Clin Risk Manag. 2007;3(3):467–474.
- Wang K, Liu ZH, Li XY, et al. Efficacy and safety of selegiline for the treatment of Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2023;15:1134472. doi: 10.3389/fnagi.2023.1134472
- Bette S, Shpiner DS, Singer C, et al. Safinamide in the management of patients with Parkinson’s disease not stabilized on levodopa: a review of the current clinical evidence. Ther Clin Risk Manag. 2018;14:1737–1745. doi: 10.2147/TCRM.S139545
- Parkinson Study Group. A controlled trial of rasagiline in early Parkinson disease: the TEMPO study. Arch Neurol. 2002;59(12):1937–1943. doi: 10.1001/archneur.59.12.1937
- Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361(13):1268–1278. doi: 10.1056/NEJMoa0809335
- Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol. 2005;62(2):241–248. doi: 10.1001/archneur.62.2.241
- Rascol O, Brooks DJ, Melamed E, et al. Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, lasting effect in adjunct therapy with Rasagiline given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet. 2005;365(9463):947–954. doi: 10.1016/S0140-6736(05)71083-7
- Hattori N, Takeda A, Takeda S, et al. Rasagiline monotherapy in early Parkinson’s disease: a phase 3, randomized study in Japan. Parkinsonism Relat Disord. 2019;60:146–152. doi: 10.1016/j.parkreldis.2018.08.024
- Hattori N, Takeda A, Takeda S, et al. Efficacy and safety of adjunctive rasagiline in Japanese Parkinson’s disease patients with wearing-off phenomena: a phase 2/3, randomized, double-blind, placebo-controlled, multicenter study. Parkinsonism Relat Disord. 2018;53:21–27. doi: 10.1016/j.parkreldis.2018.04.025
- Lew MF, Hauser RA, Hurtig HI, et al. Long-term efficacy of rasagiline in early Parkinson’s disease. Int J Neurosci. 2010;120(6):404–408. doi: 10.3109/00207451003778744
- Hattori N, Takeda A, Takeda S, et al. Long-term, open-label, phase 3 study of rasagiline in Japanese patients with early Parkinson’s disease. J Neural Transm (Vienna). 2019;126(3):299–308. doi: 10.1007/s00702-018-1964-3
- Hattori N, Takeda A, Takeda S, et al. Long-term safety and efficacy of adjunctive rasagiline in levodopa-treated Japanese patients with Parkinson’s disease. J Neural Transm (Vienna). 2019;126(3):289–297. doi: 10.1007/s00702-018-1962-5
- Perez-Lloret S, Rascol O. Safety of rasagiline for the treatment of Parkinson’s disease. Expert Opin Drug Saf. 2011;10(4):633–643. doi: 10.1517/14740338.2011.573784
- AZILECT® (rasagiline mesylate). Tablets for oral use [package insert]. Tokyo. Japan: Takeda Pharmaceutical Company Limited; 2022. Japanese.
- AZILECT® (rasagiline tablets). [Package insert]. North (UK PA): TEVA Pharmaceuticals USA, Inc.; 2014.
- Reichmann H, Jost WH. Efficacy and tolerability of rasagiline in daily clinical use–a post-marketing observational study in patients with Parkinson’s disease. Eur J Neurol. 2010;17(9):1164–1171. doi: 10.1111/j.1468-1331.2010.02986.x
- Reichmann H, Klasser M, Apfel R, et al. Efficacy and tolerability of rasagiline in daily clinical use—a post marketing observational study in patients with Parkinson’s disease focusing on non-motor symptoms and QoL. Basal Ganglia. 2015;5(4):101–106. doi: 10.1016/j.baga.2015.09.003
- Goetz CG, Schwid SR, Eberly SW, et al. Safety of rasagiline in elderly patients with Parkinson disease. Neurology. 2006;66(9):1427–1429. doi: 10.1212/01.wnl.0000210692.95595.1c
- Tolosa E, Stern MB. Efficacy, safety and tolerability of rasagiline as adjunctive therapy in elderly patients with Parkinson’s disease. Eur J Neurol. 2012;19(2):258–264. doi: 10.1111/j.1468-1331.2011.03484.x
- Solís-García Del Pozo J, Mínguez-Mínguez S, de Groot PWJ, et al. Rasagiline meta-analysis: a spotlight on clinical safety and adverse events when treating Parkinson’s disease. Expert Opin Drug Saf. 2013;12(4):479–486. doi: 10.1517/14740338.2013.790956
- Prange S, Danaila T, Laurencin C, et al. Age and time course of long-term motor and nonmotor complications in Parkinson disease. Neurology. 2019;92(2):e148–e160. doi: 10.1212/WNL.0000000000006737
- Chang Y, Wang L-B, Li D, et al. Efficacy of rasagiline for the treatment of Parkinson’s disease: an updated meta-analysis. Ann Med. 2017;49(5):421–434. doi: 10.1080/07853890.2017.1293285
- Kano O, Tsuda H, Hayashi A, et al. Rasagiline as adjunct to levodopa for treatment of Parkinson’s disease: a systematic review and meta-analysis. Parkinsons Dis. 2022;2022:4216452. doi: 10.1155/2022/4216452
- Fahn S, Elton R. Members of the UPDRS development committee. The Unified Parkinson’s disease Rating scale. In: Fahn S, Marsden C Calne D, et al. Eds. Recent developments in Parkinson’s disease. Vol. 2. Florham Park (NJ): Macmillan Health Care Information; 1987. p. 153–163, 293–304.
- Goetz CG, Fahn S, Martinez-Martin P, et al. Movement disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41–47. doi: 10.1002/mds.21198
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340