ABSTRACT
Background
Endothelin receptor antagonists (ERAs) are associated with liver injury. We used data from previous oncology clinical trials to determine the liver safety profile of zibotentan, which is currently in clinical development (in combination with dapagliflozin) for chronic kidney disease and cirrhosis.
Research design and methods
Six global, double-blinded, phase 2b and 3 clinical trials from the zibotentan oncology development program were pooled to analyze liver safety. Descriptive statistics, proportion of liver-related adverse events, liver biochemistry parameter elevation, and shifts from baseline were analyzed, with individual case assessment.
Results
A total of 1532 patients received zibotentan for 285 days (mean), and 1486 patients received placebo for 320 days (mean). The frequency of any hepatic disorder preferred term was similar across zibotentan monotherapy (22/947 patients, 2.3%) and placebo monotherapy arms (30/881 patients, 3.4%). A total of 4 (0.4%) patients receiving zibotentan monotherapy experienced ALT elevations >5× ULN versus 8 (0.9%) receiving placebo. Of the seven patients receiving zibotentan who met criteria for potential Hy’s Law, there were no cases of drug-induced liver injury.
Conclusions
We found no evidence of zibotentan-related liver biochemistry changes among cancer-treated patients, suggesting that hepatotoxicity of ERAs is molecule-dependent, and allowing exploration of zibotentan for new indications.
1. Introduction
Endothelin-1 is the most potent vasoconstrictor and a key driver of vasoconstriction in pulmonary artery hypertension. The first drug in the endothelin receptor antagonist (ERA) class, bosentan, a mixed endothelin A and B receptor antagonist, although effective in reducing pulmonary artery pressure, can cause drug-induced liver injury (DILI). Dose-dependent transaminase elevations are observed with bosentan use, which can progress to severe liver damage [Citation1–3]. Close monitoring of liver function is recommended during bosentan use by the US Food and Drug Administration (FDA) and the European Medicines Agency.
Ambrisentan, an endothelin antagonist with greater endothelin A receptor specificity, was subject to post-marketing liver monitoring initially, but subsequently relaxed as increased patient exposure did not reveal clinically relevant liver damage [Citation4–6]. For sitaxentan, a mixed antagonist, marketing authorization was withdrawn after case reports of fatal hepatotoxicity [Citation7]. It is unclear why bosentan and sitaxentan are associated with DILI. Bile salt export pump inhibition has been postulated as a possible mechanism; in addition, both agents have been used at relatively high doses and are more lipophilic compared to others in the class, factors thought to be associated with increased risk of liver injury [Citation8–11]. Sparsentan, a mixed endothelin and angiotensin receptor antagonist, has recently received accelerated approval from the FDA for immunoglobulin A (IgA) nephropathy based on reduction in proteinuria. It is, however, subject to a liver risk evaluation and mitigation strategy [Citation12]. Of note is the relatively high 400 mg daily dose of sparsentan and the high lipophilicity of the molecule, higher than bosentan and sitaxentan [Citation13–15].
Against this backdrop of potential hepatotoxicity, efforts to explore ERAs in new diseases have been limited despite preclinical, epidemiological, and experimental study evidence that increased levels of endothelin-1 have a role in several conditions associated with endothelial dysfunction, such as liver cirrhosis, proteinuric renal disease, microvascular angina, and microvascular stroke. Two ERAs are now in later-stage clinical trials in such indications (atrasentan and zibotentan); both agents have lower proposed daily doses and low levels of lipophilicity compared with older ERAs [Citation16,Citation17]. ERAs as a class are also known teratogens in pre-clinical models and therefore require additional risk minimization measures for safe use in women of child-bearing potential [Citation18,Citation19].
Zibotentan is currently in clinical development (in combination with dapagliflozin) for cirrhosis and chronic kidney disease indications. The results of the ZENITH CKD phase 2b study investigating the effects of different doses of zibotentan in combination with dapagliflozin, an SGLT2 (sodium-glucose linked transporter) inhibitor, showed a reduction of high proteinuria associated with chronic kidney disease [Citation20]. Furthermore, dose optimization of zibotentan in combination with dapagliflozin appeared to mitigate fluid retention associated with use of the ERA class, including zibotentan [Citation20,Citation21]. Of note in the ZENITH CKD phase 2b trial, no cases of suspected drug-induced liver injury were seen and there were small reductions in aminotransferases and alkaline phosphatase over the course of the study [Citation20].
Since zibotentan exposure is dependent on renal and liver function, lower zibotentan doses were used for the CKD study as compared with past oncology clinical trials. In the ongoing ZENITH High Proteinuria phase 3 trial, 0.25–0.75 mg daily doses, adjusted by glomerular filtration rate, are being explored. These doses correspond to between 1/40th and approximately 1/13th of the 10 mg zibotentan oncology dose.
Another indication for the zibotentan/dapagliflozin combination currently in phase 2 clinical development is liver cirrhosis with portal hypertension, aiming to reduce portal pressure and its associated complications. Again, zibotentan is combined with dapagliflozin to balance body fluid volume (NCT05516498). Given the known serious liver signal with earlier ERA compounds, coupled with their potential for benefit in conditions with an unmet clinical need including an end-stage liver disease indication, the development of ERAs remains complex. This unique situation, where data from a previously terminated indication is available, allows exploration of available relevant safety data to inform the liver safety profile and risk mitigation in ongoing and future development programs in new indications.
Zibotentan is a potent and specific endothelin A receptor antagonist, with no endothelin B receptor activity [Citation22]. Zibotentan has a low daily dose (with most patients in oncology clinical trials receiving 10 mg daily), with low lipophilicity and no significant reactive metabolite formation; these factors are considered to be important in determining the risk of severe DILI, where compounds with higher lipophilicity (logP ≥ 3), daily dose ≥ 100 mg of oral medications, and with reactive metabolites are associated with increased risk [Citation10]. Furthermore, zibotentan does not appear to cause bile salt export pump (BSEP) inhibition as demonstrated by the absence of progressive or degenerative hepatocellular effects in chronic toxicology studies (data on file); BSEP inhibition has been proposed as an important mechanism for bosentan hepatotoxicity [Citation9]. Zibotentan was originally developed for the treatment of patients with castration-resistant prostate cancer (CRPC) and other cancers, used in phase 2b and 3 studies at doses up to 15 mg per day [Citation23–28]. The oncology program was discontinued in 2012 for insufficient efficacy and not due to safety reasons. In this integrated analysis of over 1500 patients from 6 phase 2b and phase 3 oncology studies, we review evidence to support the liver safety and tolerability profile of zibotentan. Using an integrated data set to create a larger sample size increases the detection of potential less frequent (or rare) liver safety issues. Understanding the liver safety profile of zibotentan is critical to exploring other potential therapeutic indications, including those where ERA use could be broad, such as in chronic kidney disease, and to inform potential development in patient populations with preexisting liver conditions, such as portal hypertension and underlying cirrhosis.
2. Patients and methods
Global, double-blinded, phase 2b and 3 clinical trial data from the zibotentan oncology clinical development program, which was active between 2004 and 2012 and performed/funded by AstraZeneca, were pooled into an integrated data set. All global Phase 2b/3 studies were conducted with the required ethics approval and followed the principles of the Declaration of Helsinki. All participants provided consent for the primary Phase 2b/3 global studies. Participant data were used in this review if participants consented to allow data reuse beyond the planned study endpoints and were from countries with supportive legislation to permit re-use. Therefore, results in this analysis may vary from the publication describing the full study population and primary endpoint [Citation23–28].
All phase 2b and phase 3 trials from the development program were included in this analysis; earlier phase clinical trials (designated as either phase 1 or 2a) were excluded due to significant differences in design, such as short duration studies, open-label design, lack of comparator arm, dose escalation studies, or healthy volunteer/clinical pharmacology such as drug–drug interaction studies; these factors limited the usefulness of such early phase studies in addressing the research question. Limiting the review to all global phase 2b and 3 studies, without further selection, resulted in largely consistent study design features such as dosing, duration, control arm and liver-related eligibility criteria for participants. The primary publications report that demographics and baseline characteristics were generally similar between treatment and control groups, though in two studies, some baseline disease characteristics showed minor imbalances, such as presence of more frequent bone metastases, lower rates of smoking, increased duration of advanced disease, and higher frequency of lymph node and bone metastases in zibotentan-treated patients compared with controls [Citation24,Citation28].
Assessment of liver safety included patient demographics and characteristics, adverse events (AEs), laboratory data, medical history, and concomitant medications; many different data variables were examined as there are a number of alternative medical diagnoses or concomitant medications that can be associated with aminotransferase elevations [Citation29]. For enhanced detection of potential liver injury, further analyses were performed in line with recent specialist recommendations, including liver biochemistry parameter risk difference, shifts from baseline, and mean change from baseline over time [Citation29–31]. Liver-related AEs were identified by using preferred terms (PT) from the ‘drug related hepatic disorders – comprehensive search’ Standardized MedDRA version 26.0 Query (SMQ). Cases meeting the definition for potential Hy’s Law (alanine aminotransferase/aspartate aminotransferase [ALT/AST] ≥3× upper limit of normal [ULN] with concurrent or subsequent total bilirubin ≥ 2× ULN) were identified and reviewed.
All analyses were performed using the safety analysis set consisting of all randomized patients who received at least one dose of double-blind study medication and who had consent for data reuse as described above. Patients were evaluated according to the actual treatment received. Analysis of adverse events and laboratory parameters were performed for an on-treatment period, defined as events that occurred on or after the first dose of study medication until 28 days after the last dose; 28 days post last dosing was considered sufficient to detect drug-related effects given the short elimination half-life of zibotentan of 7–10 hours.
Demographic and baseline liver biochemistry characteristics were summarized by treatment groups for each study and for the pooled studies using descriptive statistics. The extent of exposure to study medication was defined as the difference between the day of the last dose and the day of the first dose plus 1 day, regardless of treatment interruption, and analyzed descriptively. Liver-related AEs were presented by System Organ Class (SOC) and PT. Patients with multiple events in the same PT category are counted only once in that category. Patients with events in more than one PT category are counted once in each of those categories. Risk differences between treatment groups for liver laboratory parameters were calculated along with their confidence intervals. Shift tables from baseline to maximum value and mean change from baseline over time for liver laboratory parameters were also presented descriptively. Box plots were generated for maximum ALT relative to ULN or baseline ALT by treatment groups. Potential Hy’s Law cases were screened using scatter plots (i.e. Hepatocellular Drug-Induced Liver Injury Screening Plots [Citation30]). All analyses were exploratory in nature and no adjustments for multiplicity were applied. There was no imputation for missing data.
Patients receiving concomitant chemotherapy were also analyzed as a separate treatment subgroup, as many chemotherapy agents are associated with aminotransferase elevations. Zibotentan dosing was predominantly 10 mg daily, with a small number of patients in one study dosing at 15 mg (n = 88); these patients were pooled for analysis with the 10 mg zibotentan monotherapy group. Analyses were therefore grouped into the following pooled categories: zibotentan monotherapy (10 or 15 mg daily, compared with placebo monotherapy-treated patients), zibotentan 10 mg plus chemotherapy (compared with placebo plus chemotherapy), and all zibotentan-treated patients (compared with all placebo-treated patients [ footnote]).
Table 1. Study design, demographics, baseline characteristics, and exposure for the six oncology clinical trials included in the analysis.
3. Results
3.1. Study populations and baseline characteristics
The pooled analysis comprised eligible patients from three phase 2b and three phase 3 studies (). Four of the studies were in CRPC, one in non-small cell lung cancer (NSCLC), and one in ovarian cancer. Chemotherapeutic agents used in combination with zibotentan/placebo were docetaxel, pemetrexed, or carboplatin/paclitaxel combination. Liver biochemistry eligibility criteria were considered similar enough across studies to allow meaningful pooling across these different trial populations. In five of six studies, patients were excluded from these zibotentan trials with ALT/AST >2.5× ULN, (>5× ULN in the presence of hepatic metastases); one phase 2b trial excluded those with ALT/AST >1.5× ULN. Five studies excluded patients with total bilirubin (TBILI) >1.5× ULN; one study used a threshold of >2× ULN.
The total analysis population was 3018 patients, of which 1532 received zibotentan (with or without chemotherapy) for a mean of 285 days (standard deviation [SD] 224 days) and 1486 received placebo (with or without chemotherapy) for a mean of 320 days (SD 213 days) (Supplementary Table S1). Mean age was 69 years for zibotentan-treated patients and 70 years for patients receiving placebo, with 96% of participants on zibotentan and on placebo being male. Mean baseline liver chemistries for ALT, AST, and bilirubin were similar and in the normal range (Supplementary Table S1). Mean alkaline phosphatase (ALP) was similar across patients receiving zibotentan or placebo but above ULN, as expected due to the prevalence of bone metastases across the study population, predominantly in CRPC. As exposure time was similar between zibotentan and placebo-treated patients, there were no adjustments for exposure when analyzing frequency of events.
3.2. Liver-related AE analysis
reports patients experiencing any liver-related AEs, as defined by the SMQ ‘Drug related hepatic disorders – comprehensive search’ and summarizes AEs occurring in more than one patient per pooled treatment group. Overall, the frequency of any drug-related hepatic disorder PT was similar for zibotentan monotherapy (22 patients, 2.3%) compared with placebo monotherapy (30 patients, 3.4%); the frequency of AEs was higher in the chemotherapy arms, where the placebo/chemotherapy group showed a higher number of AEs (53 patients, 8.8%) than the zibotentan/chemotherapy group (32 patients, 5.5%). Frequencies of specific PTs were similar across treatment groups. There were no reported AEs with the PT of DILI, and there was a single report of hepatotoxicity in the placebo/chemotherapy group. Liver-related AEs leading to treatment interruption or permanent discontinuation were low and comparable across treatment groups (two patients on zibotentan ≥10 mg monotherapy compared with three patients on placebo monotherapy, and four patients on zibotentan/chemotherapy compared with five patients on placebo/chemotherapy).
Table 2. Number of patients with any liver-related adverse event by preferred term, occurring in more than one patient per pooled treatment group.
3.3. Analysis of laboratory parameters
Analysis of maximum on-treatment ALT, AST, ALP, and bilirubin () showed that 0.4% patients treated with zibotentan monotherapy, compared with a total of 0.9% in the placebo monotherapy group, experienced ALT elevations greater than 5× ULN on study. ALT elevations > 5× ULN were more frequent in those treated with chemotherapy but matched between zibotentan and placebo-control arms (zibotentan 15 patients [2.6%] vs placebo 15 patients [2.5%]). Maximum on-treatment values for AST, ALP, and bilirubin elevations were similar between patients treated with zibotentan or placebo, with or without chemotherapy.
Table 3. Liver biochemistry parameters and risk difference on treatment.
Liver biochemistry parameter risk differences were calculated; these allow an exploratory comparison between the frequency of liver biochemical elevations in each treatment group. There were no notable or consistent changes in the risk difference between treatment groups for ALT, AST, ALP, or TBILI (); however, these risk difference safety analyses are exploratory in nature and confidence intervals for the risk differences are not adjusted for multiplicity.
Using absolute thresholds (e.g. ALT > 3× ULN) to assess liver safety can over-estimate the frequency of aminotransferase elevations in patients with abnormal baseline liver biochemistry. Therefore, categorical shifts in liver biochemistry from baseline value (using toxicity grades described in the Common Terminology Criteria for AEs version 5 [CTCAE]) were reviewed to focus on clinically relevant changes from baseline values (Supplementary Table S2). The numbers of patients experiencing at least a two-grade shift in ALT were similar between zibotentan and placebo monotherapy-treated patients (15 patients compared with 18 patients, respectively).
Analysis of maximum values and categorical shifts from baseline focuses more on those patients with outlier values for liver biochemistry. To equally address population-level liver biochemistry, mean change from baseline in liver biochemistry over time for ALT, AST, ALP, and bilirubin was examined; zibotentan treated patients showed generally similar or lower mean ALT, AST, ALP, and total bilirubin values over time compared with placebo-treated patients ().
Figure 1. Mean liver biochemistry data change from baseline in (a) Alanine aminotransferase, (b) Aspartate aminotransferase, (c) Alkaline phosphatase, and (d) total bilirubin over time.
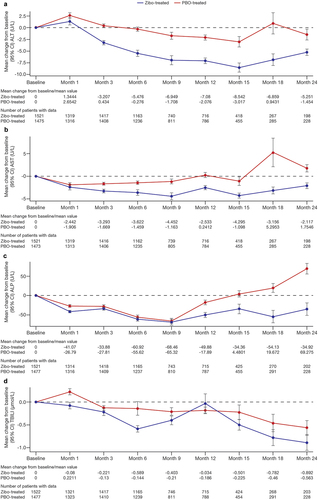
3.4. Detection of DILI cases
Assessment of DILI often focuses on potential Hy’s Law cases, the most concerning DILI cases, as jaundice as a result of hepatocellular dysfunction from DILI carries an increased risk of DILI-related fatality. Potential Hy’s Law cases are defined as any post-baseline TBILI elevation to ≥2× ULN occurring on or within 30 days after a post-baseline ALT or AST elevation to ≥3× ULN, where concurrent ALP is <2× ULN. This aims to identify patients with hepatocellular DILI significant enough to disrupt bilirubin metabolism and excretion, associated with a 10% risk of death from liver failure or requirement for transplantation [Citation32]. No cases in either treatment arm met this definition for potential Hy’s Law cases. There were a small and similar number of cases in both zibotentan- and placebo-treated groups that met Hy’s law criteria for ALT/AST and TBILI, but all had elevated ALP > 2× ULN; seven cases (0.5%) in zibotentan-treated patients and four (0.3%) in placebo-treated patients (Supplementary Fig. S1, Supplementary Table S3). However, given that baseline ALP values can be elevated in this patient population with a high proportion of bone metastases, and therefore potentially mask hepatocellular DILI through non-liver related ALP secretion, the seven cases on zibotentan treatment were reviewed (, Supplementary Table S4) for medical history, AEs, concomitant medications, and liver biochemistry tests, to identify any cases suggestive of hepatocellular DILI.
Table 4. Narratives of the seven zibotentan cases with alanine aminotransferase or aspartate aminotransferase >3× upper limit of normal and total bilirubin ≥2× upper limit of normal.
Of the seven patients, four (patients 1, 3, 6, and 7) had a cholestatic pattern of liver injury, (with at least a 2-fold increase in ALP at the time of the liver biochemistry event, compared with baseline value), and peak ALT and bilirubin occurring at the time, or within 21 days, of subsequent death or confirmed disease progression. Although there was no information available on the presence or absence of bone metastases in these patients, and no gamma-glutamyl transferase values to help interpret ALP elevation, these cases were considered most likely to be disease-related. Patient 2 had a cholestatic pattern of liver injury with >2-fold change in ALP from baseline on day 27, with no liver-related AEs reported, and was lost to follow-up. Patient 4 had a clear alternative cause of bile duct stone, and liver biochemistry normalized on continued study drug treatment. The final of seven cases (patient 5) had an abnormal ALT at baseline, with ongoing fluctuation throughout the study, and an AE of ALT and AST elevation reported as unrelated to study drug. Peak ALT was reported at study day 29, and the first bilirubin elevation ≥ 2× ULN occurred at study day 148, peaking at day 169, at which time ALT was 4.4× ULN. The patient continued treatment until death on study day 195. Although there is limited information, the presence of baseline abnormalities, and continuation of study treatment at the time of bilirubin elevation, without liver-related AEs reported, suggests an underlying alternative cause. In summary, six of the seven cases had sufficient information for assessment, and cases appeared related to underlying disease or to preexisting liver pathology; there are no key cases suggestive of hepatocellular DILI cases for zibotentan.
Cases meeting the definition for ‘Temple’s Corollary’ were also specifically examined for the detection of hepatocellular DILI; ALT/AST elevations >3× ULN but without total bilirubin elevations above 2× ULN (Supplementary Table S3), where an imbalance in frequency between treatment groups can indicate potential for hepatocellular DILI. This aims to detect cases of DILI with mild/moderate ALT/AST elevations where stopping the drug due to the liver test abnormalities prevents severe DILI with bilirubin elevation, and the appearance of these cases in the Hy’s Law quadrant. A similar number of cases of ALT > 3× ULN (without bilirubin >2× ULN) were observed for zibotentan and placebo monotherapy groups (21 vs 24 cases, respectively) and fewer cases in the zibotentan/chemotherapy group compared with the placebo/chemotherapy group (36 vs 57 cases). An absence of an imbalance further adds to the weight of evidence that there appears to be no increased liver safety liability with zibotentan compared with placebo.
To provide an additional assessment of dosing effect, box plots were used to illustrate absolute or relative changes from baseline compared across treatment groups (Supplementary Fig. 2). Consistent with the findings above, these plots do not suggest any notable difference in the maximum ALT on-treatment for zibotentan-treated patients compared with those receiving placebo.
4. Discussion
This comprehensive analysis of 1532 zibotentan-treated oncology patients from 6 clinical trials did not show evidence of drug-related liver biochemistry changes. The overall frequencies of drug-related hepatic disorder AEs were higher in placebo than zibotentan groups, with similar duration of exposure. There was no imbalance in the number of patients with ALT > 3× ULN for zibotentan monotherapy compared with placebo monotherapy, and the frequency of patients with ALT > 5× ULN was lower for zibotentan compared with placebo monotherapy and similar for the zibotentan/chemotherapy group and placebo/chemotherapy group. There were no imbalances in the frequency of categorical ALT elevations between groups, further supported by calculating risk differences, and a similar proportion of shifts in liver biochemistry from baseline was observed between zibotentan and placebo-treated groups. A review of each zibotentan case with ALT and bilirubin elevations meeting potential Hy’s law criteria did not identify any cases suggestive of drug-induced injury. Therefore, combining multiple analysis methods across AE and liver biochemistry data increases confidence that the risk of aminotransferase elevations and DILI may relate to properties of the specific ERA molecule, rather than being a class effect across all ERAs. These findings complement previously published data on ambrisentan and a recent post-hoc analysis of the SONAR study investigating atrasentan, which showed reduced aminotransferases in patients given active treatment. Mechanistic evidence suggests this may be mediated through positive effects on hepatic insulin sensitivity [Citation33–35]. In a meta-analysis of abnormal liver function in 4894 patients, bosentan, but not macitentan, significantly increased the risk of abnormal liver function [Citation33]. Taken together, these data suggest that the liver safety profile of ERAs is molecule-specific.
A key strength of this analysis is sample size with adequate duration of exposure; the mean exposure to study drug was 285 days, which covers the first few months of drug exposure where DILI is most likely to occur [Citation36]. Participants in these studies were exposed to higher doses of zibotentan (mostly 10 mg) than currently being studied in chronic kidney disease and liver cirrhosis, though these populations require lower doses due to increased drug exposure in these conditions [Citation37]. A further strength was having a significant proportion of the total patients being treated with either zibotentan monotherapy or placebo monotherapy; this allows more accurate incidence rates to directly compare frequency of liver biochemistry abnormalities without confounding effects from other investigational study drugs or standard of care medications. The use of all available phase 2b and 3 studies from the development program, without further selection, is also a strong point. This analysis used both AE and laboratory data analysis, including population-level analysis, outlier analysis, and individual case reviews. The use of multiple different analysis methods, all with similar conclusions, further supports that this comprehensive review of liver safety did not suggest evidence of DILI with zibotentan in this data set.
Limitations include the historical nature of the data sets, which may not align with current data collection standards, and changes in MedDRA coding which could impact the AE analysis (MedDRA versions 11.1–14.0 were used across the six studies). Retrospectively applying the current ‘Drug related hepatic disorders’ SMQ to previous MedDRA versions could lead to failing to capture past AE terms that have significantly changed over time with MedDRA updates. However, examining liver biochemistry data adds confidence that any serious liver pathology coded with an obsolete MedDRA term would have been detected through the liver biochemistry review. Though this is a large data set, it does not categorically rule out liver toxicity for zibotentan, as sample size is still insufficient to exclude rare, drug-related hepatic events. A further limitation was that analysis could only include patients consenting to data reuse at the time of informed consent, or whose countries did not exclude data reuse; liver safety findings in excluded patients cannot be eliminated, though primary analysis and publication of the full study data sets do not report liver injury [Citation23–28]. Notably, data sets lacked information on the presence of liver metastases at baseline, or development of new liver metastases on study, an important parameter in understanding the liver safety profile of a drug. The pooled data set consisted of predominantly male patients, as most studies were in CRPC. For non-acetaminophen DILI, there is some suggestion that females may be more susceptible than males to hepatotoxic drug reactions, but other data suggest that females could be more predisposed to developing more severe liver injury/acute liver failure as a consequence of DILI, rather than being at an increased risk [Citation38,Citation39]. Furthermore, the exclusion of earlier phase studies, including healthy-volunteer clinical pharmacology studies and dose escalation studies in patients, may have reduced the ability to detect liver-related changes; however, many of these studies were of short duration on small numbers of participants under more exploratory conditions, and it would not be expected that the addition of these early phase study results would have improved the overall research conclusions.
The historical assumption that idiosyncratic hepatotoxicity is intrinsic to the mechanism of action of ERAs is challenged by these data and the recently published liver safety analyses for atrasentan [Citation34]. A hepatic safety signal has not been reported for atrasentan or zibotentan based on published data so far. Hepatoxicity within the ERA class may be molecule-specific, potentially relating to BSEP inhibition, lipophilicity, ability to form reactive metabolites, and dose. Elevated endothelin-1 activity is thought to play a key role in the pathogenesis of chronic kidney disease, portal hypertension and liver cirrhosis, and microvascular angina. These findings present further evidence that ERA hepatotoxicity is molecule-specific, not class-specific, which for the first time brings treatment of these diseases with an agent such as zibotentan into relevance.
5. Conclusions
In conclusion, this pooled analysis across six oncology studies did not identify notable or consistent differences in liver safety parameters between zibotentan-treated and placebo-treated patients. As anticipated with an oncology population, patients with significant changes in liver biochemistry often had coexisting disease progression. Though this is a large data set, it does not categorically rule out liver toxicity for zibotentan, as sample size is still insufficient to exclude rare, drug-related hepatic events; further data are required from ongoing clinical trials to continue to characterize liver safety. This work adds to the weight of evidence that liver biochemistry changes, and liver safety profile, with ERAs may be molecule-specific rather than a class effect, and therefore certain ERAs may be better suited to expanding into broader alternative indications such as chronic kidney disease, portal hypertension, and microvascular angina.
Declaration of interests
All authors are employees and shareholders of AstraZeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution statement
AF contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, visualization, writing the original draft, and review/editing. SM-M contributed to data curation, formal analysis, methodology, visualization, and review/editing. OM contributed to conceptualization, data curation, writing the original draft, and review/editing. MS contributed to conceptualization, data curation, writing the original draft, and review/editing. JO contributed to review/editing. ML contributed to formal analysis and review/editing. SR contributed to data curation, formal analysis, and review/editing. MB contributed to review/editing. PA contributed to conceptualization, project administration, writing the original draft, and review/editing. All authors had access to the data and AF and MS verified the data in the article. All authors reviewed and approved the final version and were responsible for the decision to submit the publication.
Data sharing statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supplementary Information.docx
Download MS Word (474.4 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2024.2328816.
Additional information
Funding
References
- Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001 Oct 6;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X
- Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002 Mar 21;346(12):896–903. doi: 10.1056/NEJMoa012212
- Eriksson C, Gustavsson A, Kronvall T, et al. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension: a case-report and review of the literature. J Gastrointestin Liver Dis. 2011 Mar;20(1):77–80.
- Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008 Jun 10;117(23):3010–9. doi: 10.1161/CIRCULATIONAHA.107.742510
- Patel KR, Blair CJ, Tislow JD. Hepatic safety of ambrisentan alone and in combination with tadalafil: a post-hoc analysis of the AMBITION trial. Pulm Circ. 2018 Oct;8(4):2045894018797273. doi: 10.1177/2045894018797273
- Ben-Yehuda O, Pizzuti D, Brown A, et al. Long-term hepatic safety of ambrisentan in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2012 Jul 3;60(1):80–1. doi: 10.1016/j.jacc.2012.03.025
- Galiè N, Hoeper MM, Gibbs JS, et al. Liver toxicity of sitaxentan in pulmonary arterial hypertension. Eur Respir J. 2011 Feb;37(2):475–6.
- Kenna JG, Stahl SH, Eakins JA, et al. Multiple compound-related adverse properties contribute to liver injury caused by endothelin receptor antagonists. J Pharmacol Exp Ther. 2015 Feb;352(2):281–90.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001 Apr;69(4):223–231.
- Chen M, Borlak J, Tong W. A model to predict severity of drug-induced liver injury in humans. Hepatology. 2016 Sep;64(3):931–40. doi: 10.1002/hep.28678
- Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug‐induced liver injury. Hepatology. 2013;58(1):388–396. doi: 10.1002/hep.26208
- Travere Therapeutics. Filspari highlights of prescribing information 2023 [cited 2023 Aug 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216403s000lbl.pdf
- Drugbank Online. Sparsentan. 2023 [cited 2023 Aug 10]. Available from: https://go.drugbank.com/drugs/DB12548
- Drugbank Online. Bosentan. 2023 [cited 2023 Aug 10]. Available from: https://go.drugbank.com/drugs/DB00559
- Drugbank Online. Sitaxentan. 2023 [cited 2023 Aug 10]. Available from: https://go.drugbank.com/drugs/DB06268
- Drugbank Online. Zibotentan. 2021 [cited 2023 Aug 10]. Available from: https://go.drugbank.com/drugs/DB06629
- Drugbank Online. Atrasentan. 2021 [cited 2023 Aug 10]. Available from: https://go.drugbank.com/drugs/DB06199
- Clouthier DE, Hosoda K, Richardson JA, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125(5):813–824. doi: 10.1242/dev.125.5.813
- Spence S, Anderson C, Cukierski M, et al. Teratogenic effects of the endothelin receptor antagonist L-753,037 in the rat. Reprod Toxicol. 1999 Jan;13(1):15–29.
- Heerspink HJL, Kiyosue A, Wheeler DC, et al. Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet. 2023 Nov 2;402(10416):2004–2017. doi: 10.1016/S0140-6736(23)02230-4
- Heerspink HJL, Kohan DE, de Zeeuw D. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int. 2021 Feb;99(2):346–349. doi: 10.1016/j.kint.2020.09.026
- Morris CD, Rose A, Curwen J, et al. Specific inhibition of the endothelin a receptor with ZD4054: clinical and pre-clinical evidence. Br J Cancer. 2005 Jun 20;92(12):2148–52. doi: 10.1038/sj.bjc.6602676
- Nelson JB, Fizazi K, Miller K, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012 Nov 15;118(22):5709–18. doi: 10.1002/cncr.27674
- James ND, Caty A, Payne H, et al. Final safety and efficacy analysis of the specific endothelin a receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized phase II trial. BJU Int. 2010 Oct;106(7):966–73.
- Fizazi K, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013 May 10;31(14):1740–7. doi: 10.1200/JCO.2012.46.4149
- Miller K, Moul JW, Gleave M, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013 Jun;16(2):187–92.
- Cognetti F, Bagnato A, Colombo N, et al. A phase II, randomized, double-blind study of zibotentan (ZD4054) in combination with carboplatin/paclitaxel versus placebo in combination with carboplatin/paclitaxel in patients with advanced ovarian cancer sensitive to platinum-based chemotherapy (AGO-OVAR 2.14). Gynecol Oncol. 2013 Jul;130(1):31–7.
- Chouaid C, Nathan F, Pemberton K, et al. A phase II, randomized, multicenter study to assess the efficacy, safety, and tolerability of zibotentan (ZD4054) in combination with pemetrexed in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2011 May;67(5):1203–8.
- Avigan MI, Bjornsson ES, Pasanen M, et al. Liver safety assessment: required data elements and best practices for data collection and standardization in clinical trials. Drug Saf. 2014 Nov;37(Suppl 1):S19–31.
- Center for Drug Evaluation and Research (CDER) and Biomedical Informatics and Regulatory Review Science (BIRRS) Team. Standard safety tables and figures: integrated guide. 2022 [cited 2023 Aug]. Available from: https://www.regulations.gov/document/FDA-2022-N-1961-0046
- Merz M, Lee KR, Kullak-Ublick GA, et al. Methodology to assess clinical liver safety data. Drug Saf. 2014 Nov;37(Suppl 1):S33–45.
- U.S Department of Health and Human Services. Guidance for industry, drug-induced liver injury: premarketing clinical evaluation. 2009 [cited 2023 Sep 12]. Available from: https://www.fda.gov/media/116737/download
- Wei A, Gu Z, Li J, et al. Clinical adverse effects of endothelin receptor antagonists: insights from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J Am Heart Assoc. 2016 Oct 26;5(11). doi: 10.1161/JAHA.116.003896
- Kohan DE, Liew A, Tang SCW, et al. Effects of atrasentan on markers of liver function in patients with type 2 diabetes and chronic kidney disease. Diab Obes Metab. 2023 Aug;25(8):2410–2412.
- Berthiaume N, Carlson CJ, Rondinone CM, et al. Endothelin antagonism improves hepatic insulin sensitivity associated with insulin signaling in Zucker fatty rats. Metabolism. 2005 Nov;54(11):1515–23.
- Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005 Aug;129(2):512–21.
- Tomkinson H, Kemp J, Oliver S, et al. Pharmacokinetics and tolerability of zibotentan (ZD4054) in subjects with hepatic or renal impairment: two open-label comparative studies. BMC Clin Pharmacol. 2011 Mar 17;11(1):3. doi: 10.1186/1472-6904-11-3
- Francis P, Navarro VJ. Drug-induced hepatotoxicity. Treasure island (FL). eng. (StatPearls) StatPearls Publishing; 2022.
- Andrade RJ, Chalasani N, Björnsson ES, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019 Aug 22;5(1):58. doi: 10.1038/s41572-019-0105-0

