Abstract
The in vivo and in vitro antiandrogenic activity of four aromatic esters 10a–10d, one aliphatic ester 10e based on the pregna-4,16-diene-6, 20-dione structure and two aromatic 17c, 17d and two aliphatic valeroyloxy esters 17a, 17b based on the more saturated 4-pregnene-6,20-dione skeleton was examined. The biological activity of steroids 9, 10a–10e and 17a–17d, was determined using prostate glands from gonadectomized adult male golden hamsters.
In the in vitro studies, the relative binding affinity of these steroids to cytoplasmic androgen receptor (AR) of hamster prostate was determined from, the corresponding IC50 values obtained from the competitive binding plots. The standards dihydrotestosterone (DHT) and cyproterone (CA) acetate used have displaced [3H]DHT from the AR with an IC50 value of 3.2 and 4.4 nM respectively. All steroidal compounds synthesized in this study showed a binding affinity for the androgen receptor, present in the cytosol from prostate hamster; compounds 10a–10c showed the highest affinities for this receptor.
The in vivo experiments showed that all steroidal derivatives were subcutaneously active, since they decreased the weight of the prostate gland in gonadectomized hamsters treated with DHT, and are antagonists for the androgen receptor since they block the DHT-induced prostate weight gain. The derivatives having the more conjugated 4,16-pregnadiene-6, 20-dione system (10a–10c) exhibited a higher antiandrogenic activity than the corresponding steroids (17a–17d) based on the more saturated 4-pregnene-6,20-dione system.
1 Introduction
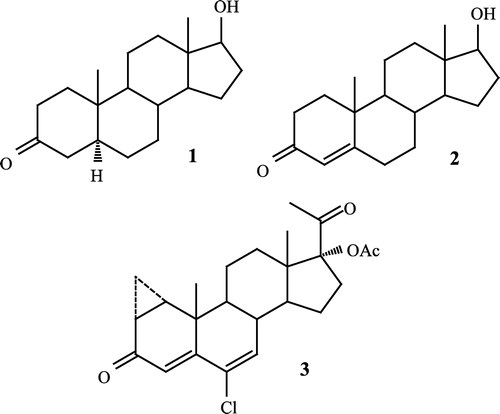
Androgen antagonists offer a potentially useful treatment for androgen mediated diseases such as prostate cancer, seborrhea, androgenic alopecia and benign prostatic hyperplasia [Citation1], the most important therapeutic application being in the treatment of prostate cancer and benign prostatic hyperplasia. Although surgery represents the most accepted treatment for prostate cancer (about 400,000 prostatectomies are performed each year in the USA) there are several other modalities available for the treatment of these diseases [Citation2]. Dihydrotestosterone 1 (DHT) (), the 5α-reductase metabolite of testosterone 2 (T) has been implicated as a causative factor in the progression of these diseases. Citation3-5 It has also been observed that this steroid interacts more efficiently with the androgen receptors than testosterone [Citation6]. Apparently DHT contains the optimal features for interaction with its protein receptor, notably a 17β-hydroxyl group, a 3-carbonyl group and the all trans 5α-reduced skeleton. At the present time there are several commercially available antiandrogens that can inhibit the action of dihydrotestosterone by competing for the high affinity binding site on the androgen receptor molecule. One of the most currently used antiandrogen is cyproterone acetate 3 [Citation7], which has successfully been used for the treatment of prostate cancer and other androgen-dependent afflictions. Although several steroidal and nonsteroidal compounds have been reported [Citation8] as antiandrogens during the last decade, the steroidal compounds have attracted more attention.
The structure activity relationships [Citation9] of a series of pregnane derivatives determined in our laboratory, indicated that an endocyclic double bond at C-4 or C-4, C-6 double bonds conjugated with the C-3 carbonyl group increases the ability of the steroid to form a complex with the androgen receptor. Although this concept is just an evolving hypothesis, it explains very well the high antiandrogenic activity of several dienediones and trienediones synthesized in our laboratory. Apparently the sp2 hybridization at C-3, C-4, C-5 and C-6 carbon atoms in the pregnane skeleton makes the steroidal molecule more coplanar and as a result of this, it enhances the steroid-receptor complex formation.
On the basis of these results obtained from similar compounds synthesized in our laboratory Citation8-17 during the last decade, these results prompted us to synthesize the new steroidal derivatives described in and . These compounds have at C-4, C-5, and C-6 a trigonal hybridization which should flatten the A–B rings and according to our hypothesis, should show an antiandrogenic activity.
2 Experimental
2.1 Chemical and radioactive material
Solvents were laboratory grade or better. (1, 2, 4, 5, 6, 7- 3H) dihydrotestosterone [3H] DHT specific activity 110–150 Ci/mmol, was provided by Perkin Elmer Life Sciences, Inc. (Boston, Ma). Radio inert cyproterone acetate (CA) and 5α-DHT were supplied by Steraloids (Wilton NH, U.S.A). dl-Dithiothreitol was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A).
2.2 Synthesis of steroidal compounds 5–10e
2.2.1 3β-Acetoxy-5α,6α-epoxypregn-16-ene-20-one 5
A solution of steroid 4 (1 g, 2.8 mmol) and m-chloroperbenzoic acid (1.4 g, 8.1 mmol) in chloroform (50 ml) was stirred for 30 min at room temperature. The reaction mixture was neutralized with an aqueous solution of sodium bicarbonate to pH 7. The organic phase was separated, dried over anhydrous sodium sulfate and the solvent was removed in vacuum. The crude product was recrystallized from ethyl acetate and hexane; yield 886 mg, 2.38 mmol (85%) of pure product 5, mp 170–172°C. UV (nm): 238 (ϵ = 10,240). IR (KBr) cm− 1: 1732, 1665,1590. 1H-NMR (CDCl3): 0.8 (3H, s), 1.1 (3H, s), 2.0 (3H, s), 2.2 (3H, s), 4.6 (1H,m), 5.3 (1H,d, J = 3H), 6.6 (1H, q, J = 2H). 13C-NMR (CDCl3)δ: 15.8 (C-18), 16.9 (C-19), 21.3 (C-21), 27.1 (CH3 acetoxy), 63.2 (C-6), 65.3 (C-5), 144.1 (C-16), 155.2 (C-17), 170.5 (ester carbonyl), 196.7 (C-20). MS (m/z): 372 (M+).
2.2.2 3β-Acetoxy-5α-hydroxypregn-16-ene-6, 20-dione 6
To a solution of steroid 5 (1 g, 2.68 mmol) in acetone (50 ml) was added dropwise a solution of chromium trioxide (1.05 g, 10.5 mmol) in water (5 ml) at 0°C during 10 min. The mixture was allowed to warm to room temperature and again the same amount of chromium trioxide was added during 20 min. Ice-water (150 ml) was added and the precipitated crystalline compound was filtered; it was dried at 70°C for 3 h. Yield 852 mg, 2.19 mmol (82%) of pure product 6, mp 244–245°C. UV(nm): 237 (ϵ = 10,100). IR (KBr) cm− 1: 3401, 1725, 1665, 1040. 1H-NMR (CDCl3)δ: 0.82 (3H, s), 1.11 (3H, s), 2.01 (3H, s), 2.26 (3H, s), 5.03 (1H, m), 6.68 (1H, q, J = 2 Hz). 13C-NMR (CDCl3)δ: 13.8 (C-18), 15.7 (C-19), 21.3 (C-21), 27.0 (CH3 acetoxy), 70.5 (C-3), 80.3 (C-5), 143.8 (C-16), 155.0 (C-17), 171.0 (ester carbonyl), 196.6 (C-20), 211.7 (C-6). MS (m/z) 388 (M+).
2.2.3 3β-Acetoxypregna-4,16-diene-6, 20-dione 7
To a solution of steroid 6 (1 g, 2.57 mmol) in pyridine (10 ml) was added dropwise thionyl chloride (1 ml, 13.79 mmol) and the clear solution was stirred for 45 min at room temperature. Ice-water (100 ml) was added and the precipitated crystalline compound was filtered and washed with water. It was recrystallized from ethyl acetate-hexane; yield 626 mg, 1.69 mmol (65%) of pure product 7, mp 193–195°C. UV (nm): 237 (e = 10,200). IR (KBr) cm− 1: 1730, 1691, 1042. 1H-NMR (CDCl3)δ: 0.93 (3H, s), 1.06 (3H, s), 2.07 (3H, s), 2.27 (3H, S), 5.31 (1H,m), 6.09 (1H, m), 6.70 (1H, q, J = 2 Hz). 13C-NMR (CDCl3)δ: 15.7 (C -18), 19.5 (C-19), 21.1 (C-21), 27.0 (CH3, acetoxy), 69.1 (C-3), 129.0 (C-4), 143.7 (C-16), 147.8 (C-5), 154.8 (C-17), 175.5 (ester carbonyl), 196.5 (C-20), 201.7 (C-6). MS (m/z) 370 (M+).
2.2.4 3β-Hydroxypregna-4,16-diene-6,20-dione 8
A solution of steroid 7 (1 g, 2.7 mmol) in methanol (150 ml) and sodium hydroxide (10 ml, 2%) was stirred for 30 min at room temperature. Ice-water (100 ml) was added and the precipitated crystalline compound was filtered and recrystallized from ethyl acetate-hexane.Yield 531 mg, 1.62 mmol (60%) of pure product 8, mp 168–1700C. UV (nm): 239 (ϵ = 10,300). IR (KBr) cm-1: 3430, 1688, 1665. 1H-NMR (CDCl3)δ: 0.92 (3H, s), 1.04 (3H, s), 2.27 (3H, s), 4.25 (1H, t, J = 2 Hz), 6.18 (1H, t, J = Hz), 6.71 (1H, t, J = 2 Hz). 13C-NMR (CDCl3)δ: 15.8 (C-18), 19.7 (C-19), 27.0 (C-21), 67.1 (C-3), 133.2 (C-4), 143.9 (C-16), 154.8 (C-17), 172.3 (ester carbonyl), 196.6 (C-20), 202.3 (C-6). MS (m/z):328 (M+).
2.2.5 Pregna-4,16-diene-3, 6, 20-trione 9
To a solution of steroid 8 (1 g, 3.04 mmol) in acetone (50 ml) was added dropwise a solution of chromium trioxide (1.05 g, 10.5 mmol) in water (5 ml) at 0° during 10 min. The mixture was allowed to warm up to room temperature and again the same amount of chromium trioxide was added during 20 min. Ice-water (150 ml) was added and the precipitated crystalline compound was filtered and dried at 70°C for 3 h. Yield 800 mg, 2.45 mmol (80.6%) of pure product 9, mp 207–2090C. UV (nm): 250 (e = 11,200). IR (KBr) cm− 1: 1687, 1680, 1600. 1H-NMR (CDCl3)δ: 0.95 (3H,s), 1.10 (3H,s), 2.10 (3H, s), 6.2 (1H, d, J = 2 Hz). 13C-NMR(CDCl3)δ: 15.1 (C-18), 17.5 (C-19), 27.1 (C-21), 129.0 (C-4), 148.2 (C-5), 197.0 (C-20), 202.3 (C-6), 205.8 (C-3). MS (m/z) 326 (M+).
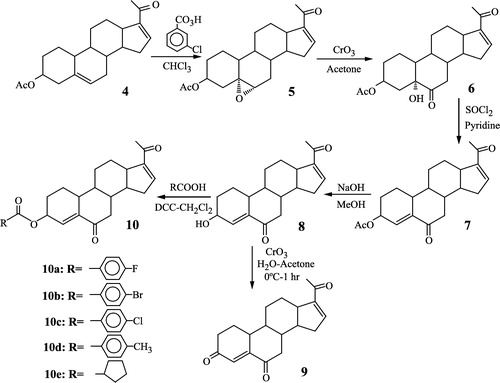
2.2.6 Preparation of compounds 10a–10e
These esters were prepared according to the following procedure:
A solution containing steroid 8 (1 g, 3.0 mmol), the corresponding acid (7 mmol), dicyclohexylcarbodiimide (1 g, 5 mmol) and 4-dimethylaminopyridine (0.6 g, 4.91 mmol) in methylene chloride (100 ml) was stirred for 1.5 h at room temperature. Water (100 ml) was added and the reaction mixture was thrice extracted with chloroform. The organic phase was washed with water, dried over anhydrous sodium sulfate and the solvent was removed in vacuum. The crude product was dissolved in ethyl acetate and filtered through a column containing silica gel to remove the dicyclohexyl urea. The organic solvent was removed in vacuum; a white crystalline ester was obtained.
2.2.6.1 3β-p-Fluorobenzoyloxypregna-4,16-diene-6,20-dione 10a
Yield 65%, mp 244–246°C. UV(nm): 236 (ϵ = 10,100). IR (KBr) cm− 1: 1723, 1690, 1665, 1300). 1H-NMR (CDCl3)δ: 0.92 (3H,s), 1.10 (3H,s), 2.28 (3H,s), 5.57 (1H,m), 6.21 (1H, t, J = 2 Hz), 6.71 (1H,t,J = 2 Hz), 7.11 (2H,m), 8.07 (2H,m). 13C-NMR (CDCl3)δ: 15.7 (C-18), 19.6 (C-19), 27.1 (C-21), 70.0 (C-3), 132.3 (C-4), 143.7 (C-16), 148.1 (C-5), 154.8 (C-17), 175.0 (ester carbonyl), 196.5 (C-20), 201.8 (C-6). MS (m/z): 450 (M+).
2.2.6.2 3β-p-Bromobenzoyloxypregna-4,16-diene-6,20-dione 10b
Yield 54.9%, mp 241–2420C. UV(nm) 244 (ϵ = 10,300). IR (KBr): 1721, 1692, 1688, 756. IR (KBr)cm− 1: 1721, 1692, 1668, 756. 1H-NMR (CDCl3)δ: 0.94 (3H, s,), 1.15 (3H,s), 2.26 (3H, s), 5.60 (1H, m), 6.25 (1H, t, J = 2 Hz), 6.73 (1H, t, J = 2 Hz), 7.13 (2H, m), 7.89 (2H, m). 13C-NMR (CDCl3)δ: 15.8 (C-18), 19.7 (C-19), 27.2 (C-21), 70.3 (C-3), 131.1 (C-4), 143.8 (C-16), 148.5 (C-5), 154.9 (C-17), 177.0 (ester carbonyl), 196.6 (C-20), 201.8 (C-6). MS (m/z): 510 (M+).
2.2.6.3 3β-p-Chlorobenzoyloxypregna-4,16-diene-6,20-dione 10c
Yield 44.8, mp 205–2070C. UV(nm): 240 (e = 10,100). IR (KBr)cm− 1: 1756, 1691, 1664, 745. 1H-NMR (CDCl3)δ: 0.93 (3H, s), 1.06 (3H, s), 2.27 (3H, s), 5.48 (1H, m), 6.08 (1H, t, J = 2 Hz), 6.86 (1H, m), 7.15 (2H,m), 7.90 (2H, m). 13C-NMR(CDCl3)δ: 15.7 (C-18), 17.6 (C-19), 27.0 (C-21), 68.3 (C-3), 132.4 (C-49), 143.6 (C-16), 149.0 (C-5), 154.9 (C-17), 174.3 (ester carbonyl), 196.8 (C-20), 201.2 (C-6). MS (m/z): 424 (M+).
2.2.6.4 3β-p-Toluoyloxypregna-4,16-diene-6,20-dione 10d
Yield 73%, mp 223–2350C. UV(nm), 241 (ϵ = 10,400). IR (KBr)cm− 1: 1724, 1693, 1685, 748. 1H-NMR (CDCl3)δ: 0.94 (3H, s), 1.11 (3H, s), 2.1 (3H, s), 2.28 (3H, s), 5.56 (1H, m), 6.2 (1H, t, J = 2 Hz), 6.7 (1H, t, J = 2 Hz), 7.12 (2H, m), 7.8 (2H, m). 13C-NMR (CDCl3)δ: 15.7 (C-18), 19.6 (C-19), 21.6 (CH3 on phenyl ring), 27.1 (C-29), 69.6 (C-3), 134.6 (C-4), 145.6 (C-16), 150.0 (C-5), 155.1 (C-17), 174.3 (ester carbonyl), 194.8 (C-20), 203.1 (C-6). MS (m/z): 446.
2.2.6.5 3β-Cyclopentylcarbonyloxypregna-4,6-diene-6,20-dione 10e
Yield 46%, mp 170–1720C. UV(nm) 238 (e = 10,200). IR (KBr) cm− 1: 1720, 1685, 1665, 755. 1H-NMR(CDCl3)δ: 0.93 (3H, s), 1.06 (3H, s), 2.27 (3H, s), 5.32 (1H, m), 6.09 (1H, m), 6.71 (1H, m). 13C-NMR (CDCl3)δ: 15.7 (C-18), 17.6 (C-19), 27.0 (C-21), 70.1 (C-3), 129.4 (C-16), 143.7 (C-4), 147.6 (C-5), 154.8 (C-17), 176.4 (ester carbonyl), 196.5 (C-6), 201.7 (C-20). MS (m/z) 424 (M+).
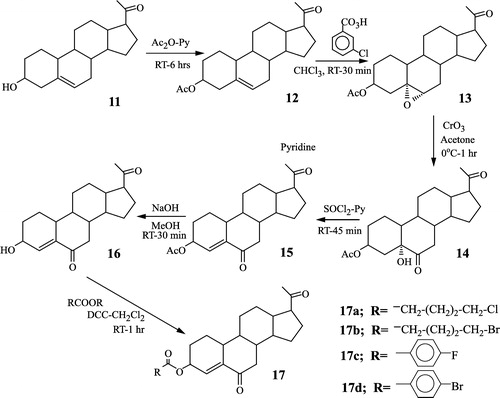
2.3 Synthesis of steroidal compounds 12–17d
The intermediates 12–17d () were synthesized using the procedure for the preparation of the C-16 unsaturated series (compounds 5–10e, ).
2.3.1 3β-Acetoxypregn-5-ene-20-one 12
Yield 50%, mp 147-149. IR (KBr) cm− 1: 1726, 1704. 1H-NMR (CDCl3)δ: 0.63 (3H, s), 1.02 (3H, s), 2.06 (3H, s), 2.2 (3H, s), 5.38 (1H, d, J = 2 Hz). 13C-NMR(CDCl3)δ: 13.2 (C-18), 19.3 (C-19), 31.8 (C-21), 73.8 (C-3), 122.3 (C-6), 139.7 (C-5), 170.4 (acetoxy carbonyl), 209.6 (C-20). MS (m/z) 358 (M+).
2.3.2 3β-Acetoxy-5α,6α-epoxypregnan-20-one 13
Yield 56%, mp 125–127°C- IR(KBr)cm− 1: 1726, 1702, 1034. 1H-NMR (CDCl3)δ: 0.56 (3H, s), 1.02 (3H, s), 2.03 (3H, s), 2.10 (3H, s), 3.09 (1H, d, J = 2 Hz), 4.77 (1H, m). 13C-NMR (CDCl3)δ: 13.2 (C-18), 15.8 (C-19), 31.5 (C-21), 63.3 (C-6), 71.2 (C-3), 170.2 (ester carbonyl), 209.4 (C-20). MS (m/z) 374 (M+).
2.3.3 3β-Acetoxy-5α-hydroxypregnan-6,20-dione 14
Yield 65.5%, mp 224–226°C. IR (KBr) cm− 1: 3455, 1737, 1715, 1690, 1323. 1H-NMR (CDCl3)δ: 0.60 (3H, s), 0.81 (3H, s), 2.01 (3H, s), 2.17 (3H, s), 2.79 (2H, d, J = 2 Hz), 5.04 (1H, m). 13C-NMR (CDCl3)δ: 13.4 (C-18), 13.9 (C-19), 31.4 (C-21), 70.5 (C-3), 17.2 (acetoxy carbonyl), 209.2 (C-20), 211.9 (C-6). MS (m/z) 390 (M+).
2.3.4 3β-Acetoxypregn-4-ene-6,20-dione 15
Yield 50.5%, mp 139–142°C. UV (nm) 237 (ϵ = 10,238). IR (KBr)cm − 1: 1737, 1698, 1243. 1H-NMR (CDCl3)δ: 0.60 (3H,s), 1.09 (3H, s), 2.07 (3H, s), 2.03 (3H, s), 2.60 (2H, d, J = 2 Hz). 13C-NMR(CDCl3)δ: 13.3 (C-18), 19.6 (C-19), 31.4 (C-21), 69.2 (C-3), 129 (C-4), 147.6 (C-5), 170.7 (ester carbonyl), 201.8 (C-6), 208.9 (C-20). MS (m/z) 372 (M+).
2.3.5 3β-Hydroxypregn-4-ene-6,20-dione 16
Yield 53.3%, mp 173–175°C. UV(nm) 240 (e = 10,450). IR(KBr) cm− 1: 3456, 1690, 1248. 1H-NMR (CDCl3)δ: 0.66 (3H, s), 1.01 (3H,s), 2.13 (3H, s), 4.25 (1H, m), 6.18 (1H, d, J = 2 Hz). 13C-NMR (CDCl3)δ: 13.3 (C-18), 19.8 (C-19), 31.4 (C-21), 67.2 (C-3), 133.1 (C-4), 146.3 (C-5), 202.4 (C-6), 208.0 (C-20). MS (m/z) 330 (M+).
2.3.6 3β-(5-Chlorovaleroyloxy)pregn-4-ene-6,20-dione 17a
Yield 59%, mp 96–97°C. UV (nm) 240 (e = 10,500). IR (KBr)cm− 1: 1728, 1690, 1640. 1H-NMR (CDCl3)δ: 0.68 (3H, s), 1.06 (3H, s), 2.24 (3H, s), 3.35 (2H, t, J = 2 Hz), 5.40 (1H, m), 6.12 (1H, d, J = 2 Hz). 13C-NMR(CDCl3)δ: 13.3 (C-18), 19.8 (C-199), 31.7 (C-21), 69.0 (C-3), 127.5 (C-4), 146.8 (C-5), 171.5 (ester carbonyl), 200.2 (C-6), 206.5 (C-20). MS (m/z) 492 (M+).
2.3.7 3β-(5-Bromovaleroyloxy)pregn-4-ene-6,20-dione 17b
Yield 58.6%, mp 94–95°C. UV (nm) 240 (e = 10,500). IR (KBr) cm− 1: 1728, 1690, 1640. 1H-NMR (CDCl3)δ: 0.68 (3H, s), 1.06 (3H, s), 2.24 (3H, s), 3.35 (2H,t, J = 2 Hz), 5.4 (1H, m), 6.12 (1H, d, J = 2 Hz). 13C-NMR (CDCl3)δ: 13.3 (C-18), 19.8 (C-19), 31.7 (C-21), 69.0 (C-3), 127.5 (C-4), 146.8 (C-5), 171.5 (ester carbonyl), 200.2 (C-6), 206.5 (C-20). MS (m/z) 492 (M+).
2.3.8 3β-(5-Fluorobenzoyloxy)pregn-4-ene-6,20-dione 17c
Yield 56.7%, mp 172–173oC. UV(nm) 236 (10,050). IR (KBr)cm− 1: 1716, 1702, 1630, 1270. 1H-NMR (CDCl3)δ: 0.67 (3H, s), 1.08 (3H, s), 2.15 (3H, s), 5.58 (1H, m), 6.21 (1H, m), 7.11 (2H, m), 8.07 (2H, m). 13C-NMR(CDCl3)d: 13.3 (C-18), 19.6 (C-19), 31.4 (C-21), 70.0 (C-3), 128.3 (C-4), 148.2 (C-5), 174.4 (ester carbonyl), 201.3 (C-6), 208.4 (C-20). MS (m/z) 458 (M+).
2.3.9 3β-(p-Bromobenzoyloxy)pregn-4-ene-6,20-dione 17d
Yield 60%, mp 201–203°C. UV(nm) 245 (e = 10,240). IR (KBr) cm-1: 1717, 1704, 1635, 1275. 1H-NMR (CDCl3)d: 0.68 (3H, s), 1.09 (3H, s), 2.20 (3H, s), 5.63 (1H, m), 6.29 (1H, m), 7.68 (1H, m), 7.92 (2H, m). 13C-NMR (CDCl3), 13.25 (C-18), 18.8 (C-19), 32.2 (C-21), 69.8 (C-3), 131.6 (C-4), 146.2 (C-5), 165.8 (ester carbonyl), 201.8 (C-6), 210.2 (C-20). MS (m/z) 514 (M+).
2.4 Biological activity of the synthesized compounds
The biological activity of steroids 9, 10a–10e and 17a–17d, was determined in vivo and in vitro experiments using the prostate glands from gonadectomized adult male golden hamsters. The animals (150–200 g) were obtained from the Metropolitan University-Xochimilco of Mexico. Gonadectomies were performed under pentobarbital anesthesia 24 h before the experiments and the animals were sacrificed with CO2.
2.4.1 In vitro experiments
The prostate glands were immediately removed, blotted, weighed and soaked in cold TEMD (40 mM tris–HCl, 3 mM EDTA and 20 mM sodium molybdate, dithiotreitol 0.5 mM, 20% glycerol at pH 7.5) prior to their use. Unless specified, all procedures were carried out in an ice bath. Tissues used were homogenized with a tissue homogenizer (model 985-370; variable speed 5000–30,000 rpm, Biospec Products, Inc.)
Tissues were homogenized in 3 volumes of buffer TEMD and at 4°C with a tissue homogenizer. Homogenates were centrifuged at 140, 000 × g for 60 min [Citation12] in a SW 60 Ti rotor (Beckman Instruments, Palo Alto, CA).
The cytosolic fraction obtained from the supernatant liquid of the prostate homogenate centrifuged at 140,000 × g as described above, was stored at − 70°C. Prostatic cytosol proteins (5.4 mg of protein in 200 μl) were determined by the Bradford method [Citation18].
2.4.2 Competitive studies
For competitive studies, tubes containing 3.15 nM of [3H]DHT plus a range of increasing concentrations (10-9–10-3 M) of cold DHT and compounds 9, 10a–10e and 17a–17d in ethanol were prepared [Citation12,Citation14,Citation16]. The solvent was evaporated to dryness.
Aliquots of 200 μl of prostate cytosol (5.4 mg protein, determined by the Bradford method [Citation18]) were added and incubated (duplicate) for 24 h at 4°C in the tubes as previously described. Eight hundred μl of 0.1% dextran-coated 1% charcoal in TEDAM buffer (containing dithiotreitol) was then added and the mixture was incubated for 10 min at 4°C. To prepare the dextran-coated charcoal mixture, the dextran was agitated for 30 min before adding the charcoal to the mixture. The tubes were vortexed and immediately centrifuged at 800 × g for 10 min; aliquots (200 μl) were taken and submitted for radioactive counting. The IC50 of each compound was calculated according to plots of concentration versus percentage binding.
2.4.3 In vivo experiments
The effect of the new steroids 9, 10a–10e and 17a–17d ( and ) on the prostate of male hamsters, which had been gonadectomized 30 days prior to the experiment, was determined on 12 groups of 4 animals/experiment, which were selected at random. The animals were kept in a room with controlled temperature (22°C) and light–dark periods of 12 h. Food and water were provided ad libitum.
Daily subcutaneous injections of 400 μg of the steroids 9, 10a–10e and 17a–17d dissolved in 200 μl of sesame oil were administered for 6 days together with 200 μg of DHT. Two groups of animals were kept as control; one was injected with 200 μl of sesame oil and the other with 200 μg of DHT for 6 days. After the treatment, the animals were sacrificed by CO2 and the prostate gland was dissected and weighed. Four separate experiments were performed for each group of steroid-treated animals. The results () were analyzed using one-way analysis of variance with EPISTAT software
Table I. Relative binding affinity of the novel compounds to the hamster androgen receptor. RBA: Relative binding affinity. CA: Cyproterone acetate. DHT: Dihydrotestosterone.
3 Results
3.1 Synthesis of the steroidal derivatives 9 and 10a–10e ()
These compounds were prepared from the commercially available 16-dehydropregnenolone acetate 4. The reaction of 4 with m-chloroperbenzoic acid in chloroform at room temperature afforded the epoxy derivative 5. The opening of the oxirane ring in 5 with chromium trioxide in acetone-water yielded the hydroxyketone 6. Treatment of 6 with thionyl chloride in pyridine afforded the α, β-unsaturated ketone 7. The acetoxy group in 7 was hydrolyzed with methanol and sodium hydroxide to give the free alcohol 8 which on oxidation with chromium anhydride in acetone-water afforded the dienetrione 9. On the other hand, when the alcohol 8 was esterified with p-substituted benzoic acids and also with cyclopentanecarboxylic acid in the presence of trifluoroacetic anhydride and p-toluenesulfonic acid, it gave the desired esters 10a–10e.
3.2 Synthesis of the steroidal derivatives 17a–17d
These compounds were prepared from the commercially available 3-hydroxy-5-pregnen-20-one 11 (). This sequence of reactions is very similar to that described above (). The reaction of 11 with acetic anhydride-pyridine afforded the corresponding acetoxy derivative 12 which with m-chloroperbenzoic acid in chloroform at room temperature gave the epoxy derivative 13. The opening of the oxirane ring in 13 with chromium trioxide in acetone-water yielded the hydroxyketone 14 which upon treatment with thionyl chloride in pyridine afforded the α,β-unsaturated ketone 15. The acetoxy group at C-3 in 15 was hydrolyzed with methanol and sodium hydroxide to give the free alcohol 16. Esterification with different aromatic and aliphatic acids in the presence of dicyclohexylcarbodiimide in chloroform at room temperature afforded the corresponding esters 17a–17d.
3.3 Biological activity
3.3.1 In vitro experiments
3.3.1.1 Relative binding affinity for the androgen receptor
The relative binding affinities of the steroids to cytoplasmic androgen receptor (AR) of hamster prostate were determined by standard dextran-coated charcoal adsorption techniques described above, and the results are shown in .
The relative binding affinity was calculated according to the following formula:
IC50 is the amount of the steroid required to inhibit the binding of [3H] DHT to the androgen receptor by 50% and was determined from the competitive binding plots. The reference standards DHT and cyproterone acetate (CA) displaced [3H] DHT from AR with an IC50 of 3.2 and 4.4 nM respectively. All steroids evaluated in this study showed affinity for the androgen receptor ().
3.3.2 In vivo experiments
After castration, the weight of the male hamster prostates significantly decreased (p < 0.005) compared to that of the normal glands. Treatment with vehicle alone did not change this condition, whereas s.c. injections of 200 μg of DHT for 6 days significantly increased (p < 0.005) the weight of the prostates in castrated male hamsters ().
Table II. Weight of prostate glands ± standard deviations from animals receiving for 6 days different s.c. treatments.Two groups of animals were kept as control, one was injected with 200 μl of sesame oil (Vehicle) and the second with 200 μg of DHT for 6 days (see Experimental Section).
When dihydrotestosterone (DHT) was injected together with compounds 9, 10a–10e and 17a–17d, the weight of the prostate decreased significantly (P < 0.05) in all cases ().
4 Discussion
Here, we assessed the antiandrogenic activity of four aromatic esters 10a–10d, one aliphatic ester 10e based on the pregna-4,16-diene-6, 20-dione structure () two aromatic 17c, 17d and two valeroyloxy esters 17a, 17b based on the more saturated 4-pregnene-6,20-dione skeleton (). The IC50 values of these compounds () increased progressively as the substituent on the phenyl group of the ester side chain at C-17 became more electropositive (compound 10a with a fluorine substituent has an IC50 value of 3.0 as compared to steroid 10d having a methyl substituent with an IC50 value of 4.0). On the other hand steroids 17a–17d having the more saturated 4-pregnene-6, 20-dione skeleton exhibited higher IC50 values as compared to the more unsaturated compounds 10a–10d. Steroid 17c with a fluorine substituent showed the lowest IC50 value in this series (3.7 nm) which is considerably higher than the corresponding fluoro steroidal derivative 10a (IC50 = 3.0 nm) in the more unsaturated system ().
also exhibited the relative binding affinity of these esters; compound 10a and 10c having a fluorine or chlorine substituent respectively, showed the highest binding affinity (100%) which was comparable to that of 1 (DHT). On the other hand 10b with a bromine substituent showed a relative binding affinity of 91.4%. These data indicated that the presence of halogens substituents in the ester moiety at C-3 as well as the double bond at C- 16, increased the binding affinity for the androgen receptor.
All steroidal derivatives were subcutaneously active since they decreased the weight of the prostate gland in gonadectomized hamsters (). Compounds 10a–10c having an electronegative substituent on the aromatic ring showed a higher antiandrogenic activity (lower weight of the prostate gland) as compared to 10d and 10e which have an electropositive methyl and cyclopentyl group in the ester moiety respectively. Apparently the increased electronegativity of the ester function polarizes the steroidal moiety and these more polar compounds form a stronger steroid-receptor complex probably by a dipole-dipole interaction. When the polarity of the molecule decreases (4-pregnene series, compounds 17a–17d) the antiandrogenic activity is reduced. In this case the fluorine and chlorine derivatives 17c and 17d exhibited a slightly higher activity than the corresponding aliphatic esters 17a and 17b which was, however, lower than the corresponding 10a and 10c of the 4, 16-pregnadiene series.
These data show very clearly that the novel compounds are antagonists of the androgen receptor, since these steroids block the DHT-induced prostate weight gain.
Structure-activity relationships [Citation9] determined in our laboratory with a variety of pregnane derivatives indicated that an increase of the conjugation in the steroidal molecule increases the antiandrogenic activity. This hypothesis is in complete agreement with the results obtained from this study which showed very clearly that the derivatives having the more conjugated double bonds, the 4,16-pregnadiene-6, 20-dione system (compounds 10a–10e), exhibited a higher antiandrogenic activity than the corresponding steroids (17a–17d) of the more saturated 4-pregnene-6, 20-dione system.
Acknowledgements
We gratefully acknowledge the financial support of Conacyt for the project 33450-M and DGAPA for project 200301
References
- Mallamo J, Pilling G, Wetzel J, Kowalczyc PJ, Bell MR, Kulling RK, Batzold FH, Juniewicz PE, Winneker RC, Luss HR. Antiendrogenic steroids sulphonyl heterocycles. Utility of electrostatic complementary in defining biosostenic sulphonyl heterocycles. J Med Chem 1991; 35: 1663–1670
- Labrie C, Trudel C, Lee S, Martel C, Cout J, Labrie F. Combination of antiandrogen and 5α-reductase inhibitor: A further step toward total androgen blockage. Endocrinology 1991; 128: 1163–1167
- Santen R. Hormonal therapy of prostate cancer: Choosing among several available options. Int J Andrology 1989; 12: 165–168
- Brooks R, Berman C, Nguyen C, Prahalada S, Primka RL, Rasmusson GH, Slater EE. Effect of castration, desflutamide, and 5α-reductase inhibitor, MK-906, on the growth of the dunning rat prostatic carcinoma, R-3327. Prostate 1991; 18: 215–219
- Bruchowsky N, Rennie PS, Bratzold FH, Goldenberg SL, Fletcher T, Mc Loughlin M. Kinetic parameters of 5α-reductase activity in stroma and epithelium of normal, hyperplastic and carcinomatous human prostates. J Clin Endocr Metab 1988; 67: 806–816
- Rasmusson G, Reynolds G, Steinberg N. Azasteroids: Structure-activity relationships for inhibition of 5α-reductase and for androgen receptor binding. J Med Chem 1986; 29: 2298–2315
- Horton R, Kato M, Sherino R. A rapid method for the estimation of testosterone in male plasma. Steroids 1967; 10: 245–249
- Bratoeff E, Ramírez E, Murillo E, Flores G, Cabeza M. Steroidal antiandrogens and 5α-reductase inhibitors. Curr Med Chem 1999; 6: 1107–1123
- Bratoeff E, Rubio M. Pharmacological and theoretical evaluation of new antiandrogens. Chem Today 1998; 16: 33–35
- Flores E, Bratoeff E, Cabeza M, Ramírez E, Quiroz A, Heuze I. Steroid 5α-reductase inhibitors. Mini Rev Med Chem 2003; 3(3)225–237
- Bratoeff E, Herrera H, Ramirez E, Solórzano K, Murillo E, Cabeza M. Antiandrogenic effect of 16-substituted, non-substituted and D-homopregnane derivatives. Chem Pharm Bull (Japan) 2000; 48(9)1249–1255
- Bratoeff E, Ramírez E, Flores E, Sánchez M, Heuze I, Cabeza M. New aromatic esters of progesterone as antiandrogens. J Enz Inhib Med Chem 2004; 19(2)99–105
- Cabeza M, Flores E, Heuze I, Sánchez M, Bratoeff E, Ramírez E, Francolugo V. Novel 17 substituted pregnandiene derivatives as 5α-reductase inhibitors and their binding affinity for androgen receptors. Chem Pharm Bull (Japan) 2004; 52(5)535–539
- Bratoeff E, Ramirez E, Valencia M, Sanchez M, Heuze I, Cabeza M. Molecular interactions of new pregnandione derivatives. Chem Pharm Bull (Japan) 2003; 51(10)1132–1136
- Cabeza M, Gutiérez E, Miranda R, Heuze I, Bratoeff E, Flores G, Ramírez E. Androgenic and anti-androgenic effects of progesterona derivatives with different halogens effects as substituents at C-6 position. Steroids 1998; 64: 413–421
- Cabeza M, Quiroz A, Bratoeff E, Murillo E, Ramírez E, Flores G. Synthesis and pharmacological evaluation of 4-halo progesterone derivatives as antiandrogens. Chem Pharm Bull (Japan) 1999; 47: 1232–1236
- Cabeza M, Heuze I, Bratoeff E, Ramírez E, Martinez R. Evaluation of new pregnane derivatives as 5α-reductase inhibitor. Chem Pharm Bull (Japan) 2001; 49(5)525–530
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1986; 72: 248–254