Abstract
Novel 1,3,9-triazacyclopenta[b]fluorene-4,10-diones and 1,3,9-triazacyclopenta[b]fluorene, analogue of ellipticine, were synthesised, and evaluated in vitro for their antiproliferative activity on various breast cancer cell lines.
Introduction
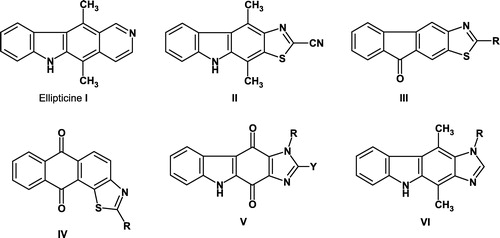
Ellipticine (I), 5,11-dimethyl-6H-pyrido[4,3-b]carbazole, is a naturally occurring alkaloid which is known for its promising antitumour properties and has been attracting considerable interest [Citation1,Citation2]. A number of structure-activity studies have already been made to determine the essential structural requirements associated with its biological activity Citation3-5. In order to overcome some limitations, such as low water solubility or cardiovascular side effects, in the therapeutic use of ellipticine and early pyridocarbazole congeners, a number of analogues have been synthesised and evaluated so far. In particular, bioisosteres were prepared consisting in replacement of the pyridine nucleus by other heterocycles like pyrroles, pyrimidines or thiophenes Citation6-8. On account of our interest in original heterocyclic systems with potential pharmacological value, we recently described the synthesis and the anticancer activity of novel tetracyclic thiazolo-analogues of ellipticine. Among all the compounds prepared, one of the most promising structures was the 4,10-dimethyl-9H-1-thia-3,9-diazacyclopenta[b]fluorene-2-carbonitrile (II) that showed a significant cytotoxic activity without a real effect on the cell cycle () [Citation9].
Similarly, we also described the synthesis of novel thiazolofluorenones (III) and anthraquinones (IV), which exhibit interesting in vitro cytotoxic activity [Citation10] against the murine L1210 leukaemia cell line.
All these results prompted us to re-investigate the incidence of pharmacomodulations at the level of the heterocyclic ring combined with the carbazole skeleton, and we decided to explore the synthesis of novel 1,3,9-triazacyclopenta[b]fluorene-4,10-diones (V), and 1,3,9-triazacyclopenta[b]fluorene (VI) to determine whether the imidazole ring was a suitable bioisostere (). The antiproliferative effects of these new substituted polyheterocyclic compounds are described and will be compared with some thiazolo-analogues previously described [Citation9].
Materials and methods
Chemistry
Melting points were obtained on a Büchi capillary instrument and are uncorrected. IR spectra were recorded on a Perkin-Elmer 681 infrared spectrophotometer. 1H and 13C NMR spectra were recorded with a Bruker Avance 300 spectrometer (Centre Commun de RMN, Université Claude Bernard—Lyon 1). Chemical shifts are expressed in parts per million (ppm) relative to residual solvent peak. Low resolution mass spectra were recorded on a Perkin-Elmer SCIEX API spectrometer (ion spray). High resolution mass spectra were recorded on a Thermofinnigan MAT 95 XL (chemical ionisation, gas: isobutane) Thin Layer Chromatography (TLC) was conducted on precoated silica gel plates (Merck 60F254) and the spots visualised using an ultraviolet light. Flash chromatography was carried out on a column using flash silica gel 60 Merck (40–63 μm) using the indicated solvents (light petroleum: boiling range 40–60°C). All reactions requiring anhydrous conditions were conducted in flame-dried apparatus.
General procedure for the preparation of 3-[(imidazol-4-yl)hydroxymethyl]indoles
Under an inert atmosphere at − 78°C, a solution of n-BuLi (2.35 M in hexanes, 562 μL, 1.3 mmol) was added dropwise to a solution of 4,5-diiodoimidazole 1 (1.3 mmol) in anhydrous tetrahydrofuran (30 mL). The medium was stirred at − 78°C for 5 min, and a solution of 2 (461 mg, 1.5 mmol) in anhydrous tetrahydrofuran (30 mL) was added dropwise. The final mixture was stirred at − 78°C for 45 min and then hydrolysed by addition of a saturated aqueous solution of ammonium chloride. Extraction was performed with dichloromethane (3 × 20 mL). The combined organic phases were dried over magnesium sulfate and evaporated in vacuo. The crude residue was purified by column chromatography (eluent: light petroleum/ethyl acetate: 1/1) to afford the desired compound 3.
3-[(3-Ethoxymethyl-5-Iodo-3H-Imidazol-4-yl)Hydroxymethyl]Indole-1,2-Dicarboxylic Acid 1-tert-butyl Ester and 2-ethyl Ester (3a)
This compound was prepared from 1a and 2. Yield: 49%; mp = 132–134°C (Et2O); νmax (KBr)/cm− 1 3500–2800, 1733; δH (300 MHz, CDCl3) 0.98 (t, 3H, J 7.0 Hz, CH3), 1.36 (t, 3H, J 7.0 Hz, CH3), 1.64 (s, 9H, 3 CH3), 3.21–3.32 (m, 2H, CH2), 4.26–4.37 (m, 2H, CH2), 4.62 (d, 1H, J 7.0 Hz, OH), 5.22 (AB system, 2H, J 10.7 Hz, CH2), 6.29 (d, 1H, J 7.0 Hz, CH), 7.09–7.19 (m, 2H, Har.), 7.32 (t, 1H, J 8.0 Hz, Har.), 7.55 (s, 1H, Har.), 8.06 (d, 1H, J 8.0 Hz, Har.); δC (75 MHz, CDCl3) 14.0 (CH3), 14.5 (CH3), 28.1 (3 CH3), 62.2 (CH2), 63.6 (CH), 64.6 (CH2), 75.6 (CH2), 85.3 (C), 87.3 (C), 115.4 (CH), 121.0 (CH), 121.5 (C), 123.6 (CH), 126.2 (CH), 126.7 (C), 127.7 (C), 133.1 (C), 135.5 (C), 140.8 (CH), 149.2 (C), 164.2 (C); m/z 570 (M+H)+; HRMS (CI) for C23H29IN3O6: calculated: 570.1101, found: 570.1100.
2.1.1.2 3-[(2-Chloro-3-Ethoxymethyl-5-Iodo-3H-Imidazol-4-yl)Hydroxymethyl]Indole-1,2-Dicarboxylic Acid 1-tert-butyl Ester and 2-ethyl Ester (3b)
This compound was prepared from 1b and 2. Yield: 50%; mp = 114–116°C (Et2O); νmax (KBr)/cm− 1 3700–3200, 1733 broad s; δH (300 MHz, CDCl3) 1.06 (t, 3H, J 7.0 Hz, CH3), 1.36 (t, 3H, J 7.2 Hz, CH3), 1.64 (s, 9H, 3 CH3), 3.43 (q, 2H, J 7.2 Hz, CH2), 4.15 (d, 1H, J 7.1 Hz, OH), 4.27–4.38 (m, 2H, CH2), 5.28 (AB system, 2H, J 11.1 Hz, CH2), 6.24 (d, 1H, J 7.1 Hz, CH), 7.18 (t, 1H, J 7.7 Hz, Har.), 7.30 (d, 1H, J 7.7 Hz, Har.), 7.36 (t, 1H, J 7.7 Hz, Har.), 8.08 (d, 1H, J 7.7 Hz, Har.); δC (75 MHz, CDCl3) 14.0 (CH3), 14.6 (CH3), 28.1 (3 CH3), 62.3 (CH2), 64.3 (CH), 64.8 (CH2), 73.9 (CH2), 84.7 (C), 85.4 (C), 115.4 (CH), 120.9 (C), 121.0 (CH), 123.7 (CH), 126.3 (CH), 126.7 (C), 127.8 (C), 135.4 (2 C), 135.5 (C), 149.2 (C), 164.1 (C); m/z 604 (M+H)+ for 35Cl, 606 (M+H)+ for 37Cl; HRMS (CI) for C23H28ClIN3O6: calculated: 604.0711, found: 604.0711.
General procedure for the preparation of 3-(imidazol-4-carbonyl)indoles
A solution of compound 3 (0.9 mmol) and excess manganese dioxide (2.12 g) in anhydrous dichloromethane (30 mL) was stirred for 15 h at room temperature. The solution was filtered over Celite® and the filtrate obtained was evaporated in vacuo. The crude residue was purified by column chromatography (eluent: light petroleum/ethyl acetate: 3/2) to afford ketone 4.
2.1.2.1 3-(3-Ethoxymethyl-5-Iodo-3H-Imidazol-4-Carbonyl)Indole-1,2-Dicarboxylic Acid 1-tert-butyl Ester and 2-ethyl Ester (4a)
This compound was prepared from 3a. Yield: 66%; mp = 110–112°C (Et2O); νmax (KBr)/cm− 1 1747, 1635; δH (300 MHz, CDCl3) 1.18 (t, 3H, J 7.0 Hz, CH3), 1.26 (t, 3H, J 7.0 Hz, CH3), 1.64 (s, 9H, 3 CH3), 3.59 (q, 2H, J 7.0 Hz, CH2), 4.15–4.20 (m, 2H, CH2), 5.53–5.59 (m, 2H, CH2), 7.32 (t, 1H, J 7.9 Hz, Har.), 7.45 (t, 1H, J 7.9 Hz, Har.), 7.61 (d, 1H, J 7.9 Hz, Har.), 7.76 (s, 1H, Har.), 8.13 (d, 1H, J 7.9 Hz, Har.); δC (75 MHz, CDCl3) 13.8 (CH3), 14.9 (CH3), 27.9 (3 CH3), 62.4 (CH2), 65.5 (CH2), 76.6 (CH2), 86.3 (C), 93.4 (C), 115.2 (CH), 121.2 (CH), 122.6 (C), 124.6 (CH), 126.3 (C), 127.1 (CH), 133.3 (C), 133.4 (C), 135.7 (C), 142.4 (CH), 148.6 (CO), 161.3 (CO), 181.5 (CO); m/z 568 (M+H)+; HRMS (CI) for C23H27IN3O6: calculated: 568.0945, found: 568.0946.
2.1.2.2 3-(2-Chloro-3-Ethoxymethyl-5-Iodo-3H-Imidazol-4-Carbonyl)Indole-1,2-Dicarboxylic Acid 1-tert-butyl Ester and 2-ethyl Ester (4b)
This compound was prepared from 3b. Yield: 84%; oil; νmax (neat)/cm− 1 1750, 1640; δH (300 MHz, CDCl3) 1.17 (t, 3H, J 7.0 Hz, CH3), 1.30 (t, 3H, J 7.1 Hz, CH3), 1.65 (s, 9H, 3 CH3), 3.61 (q, 2H, J 7.0 Hz, CH2), 4.24 (broad q, 2H, J 7.1 Hz, CH2), 5.61 (s, 2H, CH2), 7.33 (t, 1H, J 7.9 Hz, Har.), 7.46 (t, 1H, J 7.9 Hz, Har.), 7.63 (d, 1H, J 7.9 Hz, Har.), 8.13 (d, 1H, J 7.9 Hz, Har.); δC (75 MHz, CDCl3) 13.9 (CH3), 14.9 (CH3), 27.9 (3 CH3), 62.5 (CH2), 65.4 (CH2), 74.8 (CH2), 86.5 (C), 91.2 (C), 115.3 (CH), 121.2 (CH), 121.9 (CH), 124.7 (CH), 126.3 (C), 127.1 (CH), 133.7 (C), 135.5 (C), 135.6 (C), 139.0 (C), 148.6 (CO), 161.3 (CO), 180.6 (CO); m/z 602 (M+H)+ for 35Cl, 604 (M+H)+ for 37Cl; HRMS (CI) for C23H26ClI N3O6: calculated: 602.0555, found: 602.0554.
General procedure for the preparation of triazacyclopenta[b]fluorene-4,10-diones
Under an inert atmosphere at − 78°C, a solution of n-BuLi (2.24 M in hexanes, 103 μL, 0.2 mmol) was added dropwise to a solution of 4 (0.2 mmol) in anhydrous tetrahydrofuran (5 mL). After 1 h of stirring at − 78°C, the reaction mixture was allowed to warm to room temperature (1 h) and was then hydrolysed by addition of a saturated aqueous solution of ammonium chloride. The solution was extracted with dichloromethane (3 × 10 mL), the combined organic layers were collected, dried over magnesium sulfate and concentrated in vacuo. The crude residue was purified by flash chromatography (eluent: dichloromethane/methanol: 98/2) to afford quinone 5 as a red solid.
2.1.2.4 3-Ethoxymethyl-3h,9h-1,3,9-triazacyclopenta[b]fluorene-4,10-dione (5A)
This compound was prepared from 4a. Yield: 48%; mp>210°C (MeOH); νmax (KBr)/cm− 1 1676, 1649; δH (300 MHz, DMSO-d6) 1.12 (t, 3H, J 7.0 Hz, CH3), 3.58 (q, 2H, J 7.0 Hz, CH2), 5.75 (s, 2H, CH2), 7.28–7.38 (m, 2H, Har.), 7.52 (d, 1H, J 8.0 Hz, Har.), 8.06 (d, 1H, J 8.0 Hz, Har.), 8.28 (s, 1H, Har.), 12.99 (s, 1H, NH); δC (75 MHz, DMSO-d6) 14.7 (CH3), 64.2 (CH2), 74.8 (CH2), 113.9 (CH), 115.3 (C), 121.4 (CH), 123.8 (C), 124.0 (CH), 125.9 (CH), 132.6 (C), 137.1 (C), 137.6 (C), 141.9 (C), 143.8 (CH), 173.6 (CO), 174.6 (CO); m/z 296 (M+H)+; HRMS (CI) for C16H14N3O3: calculated: 296.1035, found: 296.1035.
2.1.2.5 2-Chloro-3-ethoxymethyl-3h,9h-1,3,9-triazacyclopenta[b]fluorene-4,10-dione (5B)
This compound was prepared from 4b. Yield: 52%; mp>210°C (MeOH); νmax (KBr)/cm− 1 1670, 1650; δH (300 MHz, DMSO-d6) 1.12 (t, 3H, J 7.0 Hz, CH3), 3.62 (q, 2H, J 7.0 Hz, CH2), 5.78 (s, 2H, CH2), 7.29–7.39 (m, 2H, Har.), 7.52 (d, 1H, J 7.9 Hz, Har.), 8.05 (d, 1H, J 7.9 Hz, Har.), 13.07 (s, 1H, NH); δC (75 MHz, DMSO-d6) 14.8 (CH3), 64.6 (CH2), 74.0 (CH2), 114.1 (CH), 115.2 (C), 121.5 (CH), 123.9 (C), 124.4 (CH), 126.2 (CH), 133.7 (C), 136.5 (C), 137.7 (C), 139.2 (C), 139.8 (C), 172.6 (CO), 173.9 (CO); m/z 330 (M+H)+ for 35Cl and 332 (M+H)+ for 37Cl; HRMS (CI) for C16H13ClN3O3: calculated: 330.0645, found: 330.0645.
General procedure for the preparation of 3H,9H,1,3, 9-triazacyclopenta[b]fluorene-4,10-diones
A solution of 5 (0.17 mmol) and 1 M HCl (1 mL) in 1,4-dioxane (3 mL) was stirred at 80°C for 2 h. After cooling, the solution was neutralised by 1 M NaOH and extracted with dichloromethane (2 × 5 mL). The combined organic phases were dried over magnesium sulfate and evaporated in vacuo to afford quinone 6 as a red solid.
2.1.2.7 3H,9H-1,3,9-Triazacyclopenta[b]Fluorene-4,10-Dione (6a)
This compound was prepared from 5a. Yield: 80%; mp>210°C (washing with CH2Cl2); δH (300 MHz, DMSO-d6) 7.25–7.36 (m, 2H, Har.), 7.50 (d, 1H, J 7.7 Hz, Har.), 7.93 (s, 1H, Har.), 8.15 (d, 1H, J 7.7 Hz, Har.); m/z 238 (M+H)+; HRMS (CI) for C13H8N3O2: calculated: 238.0617, found: 238.0617.
2.1.2.8 2-Chloro-3h,9h-1,3,9-triazacyclopenta[b]fluorene-4,10-dione (6B)
This compound was prepared from 5b. Yield: 83%; mp>210°C (washing with CH2Cl2); δH (300 MHz, DMSO-d6) 7.27–7.38 (m, 2H, Har.), 7.51 (d, 1H, J 7.7 Hz, Har.), 8.15 (d, 1H, J 7.7 Hz, Har.); m/z 272 (M+H)+ for 35Cl, 274 (M+H)+ for 37Cl; HRMS (CI) for C13H7ClN3O2: calculated: 272.0227, found: 272.0225.
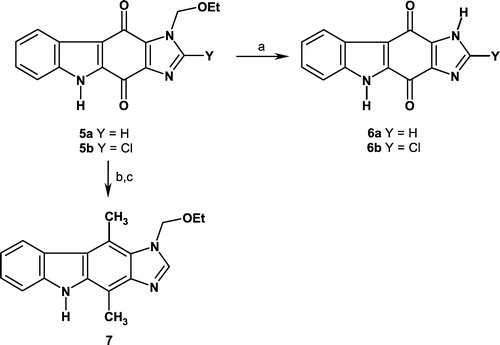
3-Ethoxymethyl-4,10-Dimethyl-3,9-Dihydro-1,3,9-Triazacyclopenta[b]Fluorene (7)
A solution of 5a (100 mg, 0.34 mmol) and MeLi (1.6 M in diethyl ether, 2.1 mL, 3.4 mmol) in anhydrous tetrahydrofuran (40 mL) was stirred at reflux for 3 h. After cooling, the solvent was evaporated under reduced pressure. The crude residue was dissolved in ethanol (20 mL) and NaBH4 (1.29 g, 34 mmol) was added portionwise under stirring to the reaction mixture. The final solution was then refluxed for 18 h. After cooling, the solvent was evaporated and acetone (30 mL) was added to the residue. The solution was stirred at room temperature for 20 min and evaporated in vacuo. The residue was taken up in water (40 mL) and ethyl acetate (15 mL), and the phases were separated. The aqueous phase was extracted twice with ethyl acetate (2 × 10 mL). The combined organic layers were dried over magnesium sulfate and concentrated in vacuo. The crude residue was purified by flash chromatography (eluent: dichloromethane/methanol: 98.5/1.5) to afford final compound 7 in 35% yield (35 mg). Mp >210°C (MeOH); δH (300 MHz, acetone-d6) 1.12 (t, 3H, J 7.0 Hz, CH3), 2.81 (s, 3H, CH3), 3.21 (s, 3H, CH3), 3.56 (q, 2H, J 7.0 Hz, CH2), 5.84 (s, 2H, CH2), 7.15 (t, 1H, J 7.9 Hz, Har.), 7.35 (t, 1H, J 7.9 Hz, Har.), 7.49 (d, 1H, J 7.9 Hz, Har.), 8.15 (s, 1H, Har.), 8.29 (d, 1H, J 7.9 Hz, Har.), 10.05 (s, 1H, NH); δC (75 MHz, acetone-d6) 11.5 (CH3), 15.0 (CH3), 15.2 (CH3), 63.6 (CH2), 76.4 (CH2), 107.0 (C), 111.2 (CH), 113.8 (C), 118.9 (CH), 120.6 (C), 123.3 (CH), 125.4 (C), 125.5 (CH), 128.3 (C), 137.5 (C), 142.6 (C), 144.7 (C), 146.5 (CH); m/z = 294 (M+H)+; HRMS (CI) for C18H20N3O: calculated: 294.1606, found: 294.1606.
Pharmacology
Cell culture
Three human breast carcinoma cell lines, MCF-7/6, MCF-7/AZ and MDA-MB-231 kindly provided by Dr. M. Mareel (Laboratoire de cancérologie expérimentale, Hôpital Universitaire, Ghent, Belgique), were used in the present study. MCF-7/AZ and MCF-7/6 cells are variants of the human mammary carcinoma cell family MCF-7. MCF-7/6 and MDA-MB-231 are invasive breast cancer cell lines, while MCF-7/AZ proliferation is slower. All cell lines were cultured at 37°C in a 5% CO2/95% air humidified atmosphere, in DMEM-HAM's F12 medium (1:1, v/v, Dutscher), supplemented with 10% heat inactivated fetal calf serum (v/v, Dutscher) to which was added penicillin 100 U mL− 1 and streptomycin 100 μg mL− 1.
Antiproliferative activity of 1,3,9-triazacyclopenta[b]fluorene derivatives on breast cancer cell lines
1,3,9-Triazacyclopenta[b]fluorene derivatives were dissolved in DMSO (Sigma-Aldrich) to give 10− 3 M stock solutions from which further dilutions were made in cell culture medium. In vitro drug sensitivity was performed by the CellTiter 96® non-radioactive cell proliferation assay (Promega, France) which allows determination of the fraction of viable cells remaining after drug treatment. On day 0, a 50 μL aliquot of medium containing 2.10− 9 or 2.10− 6 M of 1,3,9-triazacyclopenta[b]fluorene derivative was added to each well of 96-well plates. After equilibration at 37°C in a humidified 5% CO2 atmosphere, 50 μL of a 105 cell. mL− 1 suspension (5000 cells) was dispensed into all wells of the pre-equilibrated 96-well plate. After incubation at 37°C for 72 h in a humidified 5% CO2 atmosphere, 15 μL of MTT tetrazolium salt solution were added to each well. The plates were incubated for a further 4 h to allow for MTT metabolism to formazan by the succinate-tetrazolium reductase system active only in viable cells. A solubilisation/stop solution (100 μL) was added to stop the MTT assay and the optical densities were read on a plate reader (VERSAmax, Molecular Devices) at 570 nm. Data were then analysed to discern the % of growth inhibition through a comparison of samples with untreated cells (control, 0% growth inhibition). Data are presented as the mean percentage of growth inhibition ± S.E.M calculated from 24 measures from 3 independent experiments.
Results and discussion
Chemistry
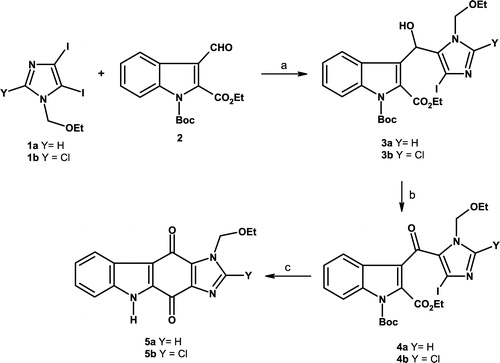
The starting materials 1, with the N-1 position of the imidazole ring protected by an ethoxymethyl (EOM) group, were prepared in good yields, according to our previously described paper [Citation11]. Selective halogen-metal exchange at position-5 of 1a–b was carried out with n-BuLi (1 equivalent) at − 78°C, then indole 2 was added to the solution to afford alcohols 3a–b in 49% and 50% yields respectively. The alcohols 3a–b were oxidised using MnO2 to give ketones 4a–b in 66–84% yield. The last halogen-metal exchange on 4a–b was performed in the presence of n-BuLi in THF at − 78°C to give quinones 5a–b in 48–52% yield through an intramolecular cyclisation. The ethoxymethyl (EOM) group deprotection in acidic medium afforded the final tetracyclic derivatives 6a–b in 80–83% yield (Scheme ).
Scheme 1 Synthesis of compounds 3–5. Reagents: (a) n-BuLi (1 eq), THF, − 78°C, 5 min, then 2 (1.2 eq), THF, − 78°C, 45 min, 3a = 49%, 3b = 50%; (b) MnO2 excess, CH2Cl2, r.t., 15 h, 4a = 66%, 4b = 84%; (c) n-BuLi (1 eq), THF, − 78°C, 1 h then r.t., 1 h, 5a = 48%, 5b = 52%.
Treatment of 5a with MeLi, and then NaBH4 afforded the ellipticine analogue 7 in 35% yield. Surprisingly, we were not able to remove the EOM group on compound 7 using classical acidic conditions, such as HCl aq. in 1,4-dioxane, HBr aq. or HBr in acetic acid (Scheme ).
Pharmacology
Growth inhibition of breast cancer cell lines by 1,3,9-triazacyclopenta[b]fluorene derivatives
1,3,9-Triazacyclopenta[b]fluorene antiproliferative activity is summarised in . Among the four molecules tested (5a–b, 6b and 7), 5a and 5b exhibited the best antiproliferative activity on the three cancer cell lines. This activity was good on MCF-7/6, with 22.7 and 25.6% of growth inhibition at 1 μM respectively, and moderate on MDA-MB-231 and MCF-7/AZ. Antiproliferative activity of 5a and 5b at 1 nM was very low. The presence of a chlorine atom in position 2 weakly enhanced antiproliferative activity on the three cancer cell lines as demonstrated by 5b. Replacement of the EOM group (5b) by hydrogen (6b) abolished activity, indicating that this substituent was determinant for interaction with a pharmacological target. In a same way, transformation of the carbazole-1,4-dione (5a) into its ellipticine-like analogue (7) dramatically decreased antiproliferative activity. These results can be compared with data previously published on thiazolocarbazoles [Citation9], fluorenones and anthraquinones [Citation10]. Among all the compounds studied, 3-ethoxymethyl-1,3,9-triazacyclopenta[b]fluorene-4,10-diones (5) appear as the best candidates screened.
Figure 2 Antiproliferative activity of 1,3,9-triazacyclopenta[b]fluorene derivatives 5a–b, 6 and 7 against MCF-7/6, MCF-7/AZ and MDA-MB-231 breast cancer cell lines.
![Figure 2 Antiproliferative activity of 1,3,9-triazacyclopenta[b]fluorene derivatives 5a–b, 6 and 7 against MCF-7/6, MCF-7/AZ and MDA-MB-231 breast cancer cell lines.](/cms/asset/ff4ccffa-896b-4c92-a0b7-f4c45af4e69e/ienz_a_121245_f0004_b.jpg)
In conclusion, we described in this paper a preliminary antitumoral evaluation of novel 1,3,9-triazacyclopenta[b]fluorene-4,10-diones which provides a promising basis for the development of anticancer agents. As for the thiazole counterparts previously described, design of new derivatives will be performed in our laboratory.
Acknowledgements
This research work was supported by a grant from the Ministère de l'Enseignement Supérieur et de la Recherche to C.M. T.B. thanks the “Comité de Charente et de Charente-Maritime de la Ligue Nationale Contre le Cancer” and the “Conseil Général de Charente Maritime” for financial support (L.P. fellowship).
References
- Sengupta SK, Cheng XCC. Cancer Chemotherapeutic Agents, WO Foye. ACS Professional Reference Books, Washington DC 1995; 246–251
- Garbett N, Graves DE. Curr Med Chem Anti-Cancer Agents 2004; 4: 149–172
- Moody CJ. Synlett 1994; 681–688
- Kansal VK, Potier P. Tetrahedron 1986; 42: 2389–2408
- Sainsbury M. Synthesis 1977; 437–448
- Martarello L, Joseph D, Kirsch G. Heterocycles 1996; 43: 367–379
- Peixoto FMC, Queiroz M-JRP, Kirsch G. J Chem Res (S) 1998; 172–173, J Chem Res (M), 801–812
- Joseph D, Martarello L, Kirsch G. J Chem Res (S) 1995; 448–449, J Chem Res (M), 2557–2568
- Testard A, Picot L, Fruitier-Arnaudin I, Piot JM, Chabane H, Domon L, Thiéry V, Besson T. J Enz Inhib Med Chem 2004; 19: 467–473
- Chabane H, Pfeiffer B, Renard P, Thiéry V, Guillaumet G, Besson T. J Enz Inhib Med Chem 2004; 19: 567–575
- Desforges G, Bossert C, Montagne C, Joseph B. Synlett 2004; 1306–1308