Abstract
A series of coumarin analogs, designed and synthesised as potential fluorescent zinc probes were evaluated for their biological activity as anti-inflammatory and antioxidant agents. The effect of the synthesised compounds on inflammation, using the carrageenin-induced rat paw oedema model, was studied. In general, the compounds were found to be potent anti-inflammatory agents (26.5–64%). Compound 5 was found to interact significantly with 1,1-diphenyl-2-picryl-hydrazyl stable free radical (DPPH) whereas the remainder were inactive in this assay. The compounds inhibit in general the soybean lipoxygenase and scavenge superoxide anion radicals. The anti-inflammatory activity seems to be connected with their reducing activity. Their RM values were determined as an expression of their lipophilicity. Theoretical calculations of their lipophilicity as clog P were performed indicating that only a poor relationship exists between their lipophilicity and anti-inflammatory activity.
Introduction
Coumarins form an elite class of compounds, which occupy a special place in nature. Pharmacologically, coumarins are flavonoids along with a range of other compounds. Coumarins and their derivatives have been found to exhibit a variety of biological and pharmacological activities and have roused considerable interest because of their potential beneficial effects on human health Citation1-3. As a result, coumarins and their derivatives have been the subject of extensive investigations.
Many coumarin derivatives have the special ability to scavenge reactive oxygen species (ROS) and to influence processes involving free radical-injury [Citation1,Citation2,Citation4]. They have also been found to inhibit lipid peroxidation [Citation5,Citation6] and to possess anti-inflammatory activity [Citation1,Citation5,Citation6]. Moreover, coumarin and related derivatives have been used as inhibitors of lipoxygenase (LOX) Citation5-8, and cyclooxygenase (COX) [Citation1] pathways of arachidonic acid metabolism.
It has been previously presented that 7-amino substituted coumarins play a significant role as biologically active compounds in various diseases [Citation9] and as substrates for P-450 isozymes [Citation10].
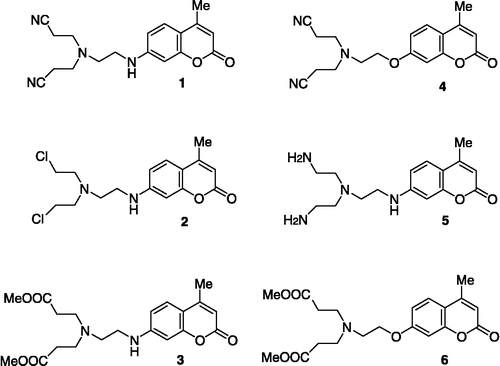
During the last two decades, substituted coumarins have been synthesized and used as fluorescent ion sensors [Citation11]. Substituents including polycarboxylate and polyamino chelators, cryptands, crown ethers and polyaza macrocyclics have been used as ion-selective binding sites. Recently, a series of 7-amino- and 7-hydroxy-substituted coumarins (), have been prepared and tested as potential zinc indicators [Citation12,Citation13]. Compound 5 exhibited the highest affinity for the zinc ion, responding to zinc ion concentrations as a ratiometric indicator. The term “fluorescent ratiometric dyes, sensors or indicators” refers to compounds able to detect concentrations of analytes (e.g. Ca2 + ) via fluorescent ratio measurements instead of absolute fluorescent values [Citation14].
Considering the variety of the substituents and the similarity of these structures (1–6) to known active compounds we were prompted to examine the above mentioned coumarin congeners: a) as possible anti-inflammatory agents, b) for their ability to inhibit soybean lipoxygenase involved in the arachidonic acid cascade and, c) for their antioxidant behaviour. The structural modifications involved within this series were, a) the 7-amino or 7-hydroxy substitution of the coumarin core structure, b) the disubstituted ethylenediamine moiety, c) the presence of a bis-carboxylate ester, bis-amino or bis-cyano group in combination to (a) and (b), that could constitute new templates for anti-inflammatory activity. The suggested structural variations could affect both their efficacy and tolerability partly due to differences in their physicochemical properties which determine their distribution in the body and their ability to pass through and to enter internal membranes [Citation15,Citation16]. An additional advantage of the compounds under study is the complete absence of acidic functions. There is currently an increased interest in the development of effective, non-acidic anti-inflammatory agents, since the used acidic NSAIDs cause the development untoward side effects in a significant fraction of the population [Citation17,Citation18].
Materials and methods
UV-Vis spectra were obtained on a Perkin–Elmer Lambda 20 double beam spectrophotometer and on a Hitachi U-2001 spectrophotometer.
All the chemicals used were of analytical grade and commercially available 1,1-diphenyl-2-picrylhydrazyl (DPPH), nordihydroguairetic acid (NDGA) are purchased from the Aldrich Chemical Co. Milwaukee, WI, (USA). Arachidonic Acid (AA), nicotinamido-adenine-dinucleotide (NADH), nitrotetrazolium Blue (NBT), porcine heme, butylated hydroxytoluene (BHT), soybean lipoxygenase, linoleic acid sodium salt and indomethacin were obtained from Sigma Chemical, Co. (St. Louis, MO, USA). N-Methylphenazonium-methyl sulfate (PMS) was purchased from Fluka. Carrageenin, type K, was offered by MEBGAL
Physicochemical studies
Reversed phase TLC (RPTLC) was performed on silica gel plates impregnated with 55% (v/v) liquid paraffin in light petroleum ether [Citation6]. Mobile phase was methanol/water mixture (70/30, v/v). The plates were developed in closed chromatography tanks saturated with the mobile phase at 24°C. Spots were detected under UV light or by iodine vapours. RM values were determined from the corresponding Rf values (from ten individual measurements) using the equation RM = log [(1/Rf) − 1] ().
Table I. Lipophilicity values: experimentally determined RM and theoretically calculated Clog P values; inhibition % of induced carrageenin rat paw edema (CPE %); In vitro % inhibition of soybean lipoxygenase (LOX).
Biological assay
In vivo
Inhibition of carrageenin-induced oedema (CPE) [Citation6]
Oedema was induced in the right hind paw of Fisher 344 rats (150–200 g) by the intradermal injection of 0.1 mL 2% carrageenin in water. Both sexes were used but pregnant females were excluded. The animals, which had been bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during their maintenance but were entirely fasted during the experiment period. Our studies were in accordance with recognised guidelines on animal experimentation (Guidelines for the care and use of laboratory animals published by the Greek Goverment 160/1991, based on EU regulations 86/609).
The tested compounds, 0.01 mmol/Kg body weight, were suspended in water with a few drops of Tween 80 and ground in a mortar before use and were given intraperitoneally simultaneously. The rats were euthanized 3.5 h after carrageenin injection. The difference between the weight of the injected and uninjected paws was calculated for each animal. The change in paw weight was compared with that in control animals (treated with water) and expressed as a percent inhibition of the oedema CPE % values (). Indomethacin in 0.01 mmol/Kg (47%) was administered as a standard drug for comparative reasons. Values CPE % are the mean from two different experiments with a standard error of the mean less than 10% (p < 0.01 compared with control values).
In vitro assays
Interaction of the tested compounds with 1,1-diphenyl-2-picryl-hydrazyl (DPPH) stable free radical [Citation6]
To a solution of DPPH (0.1 mM) in absolute ethanol an equal volume of the compounds (0.1 and 0.2 mM) dissolved in ethanol was added. Ethanol was used as control solution. After 20 min at room temperature the absorbance was recorded at 517 nm. NDGA and BHT were used as appropriate standards ().
Table II. Interaction % with DPPH (RA %); % superoxide radical scavenging activity (PMS %).
Soybean lipoxygenase activity [Citation6]
The tested compounds dissolved in DMSO (final concentration 0.1 mM) were incubated at room temperature with sodium linoleate (0.1 ml, 0.00454 g/5 ml diluted in Tris) and 0.2 ml of enzyme solution (1000 U/ml 0.9% NaCl-saline). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard (nordihydroguaretic acid 0.1 mM, 83.7%) (see ).
Non enzymatic assay of superoxide radicals- measurement of superoxide radical scavenging activity [Citation6,Citation19]
The superoxide producing system was set up by mixing phenazine methosulfate (PMS), NADH and air–oxygen. The production of superoxide was estimated by the nitroblue tetrazolium method. The reaction mixture containing 3 μM PMS, 25 μM NBT in 19 μM phosphate buffer pH 7.4 and the tested compounds, was preincubated for 2 min at room temperature before adding 78 μM NADH and the absorption measured at 560 nm against a blank containing PMS ().
In vitro assays
Each experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10%.
Results and discussion
Biological studies
The in vivo anti-inflammatory effects of the tested coumarins were assessed by using the functional model of carrageenin-induced rat paw oedema and are given in , as percentage of weight increase at the right hind paw in comparison to the uninjected left hind paw. Carrageenin- induced oedema is a non-specific inflammation resulting from a complex of diverse mediators [Citation20]. Since oedema of this type are highly sensitive to NSAIDs, carrageenin has been accepted as a useful agent for studying new anti-inflammatory drugs [Citation21]. This model reliably predicts the anti-inflammatory efficacy of the NSAIDs and during the second phase it detects compounds that are anti-inflammatory agents as a result of inhibition of prostaglandin amplification [Citation22]. As shown in , all the investigated compounds induced protection against carrageenin—induced paw oedema. The protection ranged from 26.5–64.0% while the reference drug, indomethacin, induced 47% protection at an equivalent concentration. Compound 3 was the most potent (64.0%) whereas compound 1 had the lowest effect (26.5). The in vivo results might be affected by metabolism (compounds 1, 3, 4 and 6).
Compounds 1–6 were further evaluated for inhibition of soybean lipoxygenase (LOX) by the UV absorbance based enzyme assay [Citation6]. While one may not extrapolate the quantitative results of this assay to the inhibition of mammalian 5-LOX, it has been shown that inhibition of plant LOX activity by NSAIDs is qualitatively similar to their inhibition of the rat mast cell LOX and may be used as a simple qualitative screen for such activity [Citation23] (). Perusal of % inhibition values shows that compound 5 is the most active, within the set, followed by compounds 3 and 1.
Most of the LOX inhibitors are antioxidants or free radical scavengers, since lipoxygenation occurs via a carbon-centered radical. Some studies suggest a relationship between LOX inhibition and the ability of the inhibitors to reduce Fe+3 at the active site to the catalytically inactive Fe+2. LOXs contain a “non-heme” iron per molecule in the enzyme active site, as high-spin Fe+2 in the native state and as high spin Fe+3 in the activated state. Several LOX inhibitors are excellent ligands for Fe+3. Many flavonoids and coumarin derivatives inhibit soybean lipoxygenase [Citation1,Citation24]. This inhibition is related to their ability to reduce the iron species in the active site to the catalytically inactive ferrous form. Although lipophilicity is referred to as an important physicochemical property for LOX inhibitors [Citation25,Citation26], all the above tested derivatives do not follow this concept. It is possible for compound 5 to follow a different mechanism of inhibition by acting as a chelator for iron at the active site of LOX.
It seems that the molecular volume MgVol of the tested compounds (theoretically calculated values of the molecules using the C-QSAR program of Biobyte [Citation27]), a parameter which expresses steric effects, influences qualitatively the LOX % inhibition in vitro.
Coumarin is also included in the above biological study, indicating the importance of the presence of this ring on LOX inhibition.
In the second set of assays the compounds were studied in order to gain an insight into the mechanism of their anti-inflammatory activity. It is well known that free radicals play an important role in inflammatory processes. Consequently, compounds with antioxidant properties could be expected to offer protection in rheumatoid arthritis and inflammation and to lead to potentially effective drugs. In fact, many non-steroidal anti-inflammatory drugs have been reported to act either as inhibitors of free radical production or as radical scavengers. Thus, these derivatives were tested with regard to their antioxidant ability and in comparison with well known antioxidant agents e.g. nordihydriguaiaretic acid (NDGA), BHT, and caffeic acid (Tables and ).
The time course of the interaction of the compounds with the stable free radical DPPH, as affected by two concentrations, is given in . This interaction is indicative of their radical scavenging ability in an iron-free system. Compounds 1, 2, 3, 4 and 6 exhibited very low activity. Compound 5 showed the highest interaction (82.5%) at 0.2 mM, followed by a moderate response by compound 2 (20.8%) at 0.2 mM. It is not clear if the interaction was time- and concentration-dependent. In general, this interaction expresses the reducing activity of the tested compounds and indicates their ability to scavenge free radicals. It seems that the tris(2-aminoethyl)amine (TREN) derivative, was the most potent in interacting with DPPH in agreement with its LOX inhibitory activity.
In superoxide anion radical-scavenging tests where non enzymatic superoxide anion radicals were generated, the tested compounds exhibited high scavenging activity in the order 4>2>1>3>6 () whereas compound 5 shows very low activity. Compounds 1, 2, 4 with the highest scavenging activity present higher lipophilicity values. However, superoxide anion radical scavenging activity does not proceed in parallel with lipophilicity. Lipophilicity is an important physicochemical parameter for the kinetics of biologically active compounds. Antioxidants of hydrophilic or hydrophobic character are expected to act as radical scavengers in the aqueous phase or as chain breaking antioxidants in biological membranes, respectively. The lipophilicity of most of these coumarins was determined from RPTLC and expressed as RM values. This is considered to be a reliable, fast, and convenient method for expressing lipophilicity [Citation6]. From our results it can be concluded that the RM values could not be used as a successful relative measure of the overall lipophilic/hydrophilic balance of these molecules as this was indicated by the clog P-theoretically calculated values [Citation27]- which express their theoretical lipophilicity in the standard octanol-water system. We could attribute this to the different nature of the hydrophilic and lipophilic phases in the two systems. Our attempt to correlate lipophilicity as clog P or RM with the reducing ability of the tested compounds was unsuccessful.
We tried to linearly correlate the expressions of anti-inflammatory, antioxidant and free radical scavenging activity for the tested compounds. None of these correlations were sufficiently satisfactory due to the small number of the compounds in the study. Attempts to correlate these expressions of activity with RM values in a linear or non-linear regression analysis gave not exceedingly sharp correlations. Unfortunately the number of compounds is insufficient to calculate a combination of all the effects.
From our results, it can be concluded, that lipophilicity is not the main property responsible for the anti-inflammatory/antioxidant activity of the investigated coumarins.
The above compounds especially 1, 3, 4 and 5 constitute an interesting template for the evaluation of new synthetic lipoxygenase inhibitors and may be helpful for the design of new therapeutic tools against inflammation. In general, structural features seems to affect the biological activities.
Acknowledgements
We would like to thank Dr C. Hansch and Biobyte Corp. 201 West 4th Str., Suite 204, Claremont CA California, 91711, USA for free access to the C-QSAR program and MEBGAL for the kind offer of carrageenin.
References
- Fylaktakidou K, Hadjipavlou-Litina D, Litinas K, Nicolaides D. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activity. Curr Pharm Des 2004; 30: 3813–3833
- Egan D, Kennedy RO, Moran E, Cox D, Prosser E, Thornes D. The pharmacology, metabolism, analysis and applications of coumarin and coumarin related compounds. Drug Metab Rev 1990; 22: 503–529
- Murray RDH, Mendez I, Brawn SA. The natural coumarins. Wiley, New York 1982
- Paya M, Halliwell B, Hoult JRS. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem Pharmacol 1992; 44: 205–214
- Kontogiorgis C, Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidants agents. J Enzyme Inhib Med Chem 2003; 18: 63–69
- Kontogiorgis C, Hadjipavlou-Litina D. Synthesis and anti-inflammatory activity of coumarin derivatives. J Med Chem 2005; 48: 6400–6408
- Kimura Y, Okuda H, Arichi S, Baba K, Kozawa M. Inhibition of the formation of 5-hydroxy-6,8,11,14-eicosatetranoic acid from arachidonic acid in polymorphonuclear leukocytes by various coumarins. Biochim Biophys Acta 1985; 834: 224–229
- Yoshimoto T, Furukawa M, Yamamoto S, Horie T, Kohno W. Flavonoids: potent inhibitors of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun 1983; 116: 612–618
- Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr Med Chem 2005; 12(8)887–916
- Tegtmeier M, Legrum W. 7-amino-coumarins as substrates of cytochrome P450-isozymes. Arch Pharm 1998; 331: 143–148
- Katerinopoulos HE. The coumarin moiety as chromophore of fluorescent ion indicators in biological systems. Curr Pharm Des 2004; 10: 3835–3852
- Dakanali M, Roussakis E, Kay AR, Katerinopoulos HE. Synthesis and photophysical properties of a fluorescent TREN-type ligand incorporating the coumarin chromophore and its zinc complex. Tetrahedron Lett 2005; 46: 4193–4196
- , Katerinopoulos HE, Foukaraki E, Liepouri F, Akoumianaki A, Deligeorgiev TG. Synthesis and fluorescent spectra of potential fluorescent ion indicators. 220th Amer Chem Soc Meeting, Washington DC, August 2000, MEDI 273.
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985; 260: 3440–3450
- Ellis GA, Blake DR. Why are non-steroidal anti-inflammatory drugs so variable in their efficacy? A description of ion trapping. Ann Rheum Dis 1993; 241–243
- Day RO, Graham GG, Williams KM, Brooks PM. Variability in response to NSAIDs. Fact or fiction?. Drugs 1988; 643–651
- Brooks PM, Day RO. Non steroidal anti-inflammatory drugs—differences and similarities. N Eng J Med 1988; 1716–1725
- Shanbhag VR, Crider MA, Gohkale R, Harpalani A, Dick RM. Ester amide and prodrugs of ibuprofen and naproxen. Synthesis, anti-inflammatory activity and gastrointestinal toxicity. J Pharm Sci 1992; 81: 149–154
- Candan F. Effect of Rhus coribum L. (Aracardiaceae) on superoxide radical scavenging and xanthine oxidase activity. J Enzyme Inhib Med Chem 2003; 18: 59–62
- Shen TY. Non steroidal anti-inflammatory agents. Burger's medicinal chemistry, ME Wolf. John Wiley and Sons, New York 1980; 1217–1219
- Winter CA. Non-steroidal anti-inflammatory drugs, S Grattini, MNG Dukes. Excepta Medica, Amsterdam 1965; 190
- Kuroda T, Suzuki F, Tamura T, Ohmori K, Hosoe H. A synthesis and potent anti-inflammatory activity of 4-hydroxy-2-(H)-oxo-1-phenyl-1,8-naphthylyridine-3-carboxamides. J Med Chem 1992; 53: 1130–1136
- Taraborewala IB, Kauffman JM. Synthesis and structure activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine alkanoic acids and 4-(-2)carboxy-phenylaminobenzene alkanoic. J Pharm Sci 1990; 79: 173–178
- Muller K. 5-lipoxygenase and 12-lipoxygenase: Attractive targets for the development of novel antipsoriatic drugs, reviews and trends. Arch Pharm 1994; 327: 3–19
- Pontiki E, Hadjipavlou-Litina D. Review in quantitative structure activity relationships on lipoxygenase inhibitors. Mini Rev Med Chem 2003; 3: 487–499
- Hadjipavlou-Litina D, Pontiki E. Quantitative structure activity relationships on lipoxygenase inhibitors. Internet Electron J Mol Des 2002; 1: 134–141
- , Biobyte Corp., 201 West 4th Str., Suite 204 Claremont CA, California 91711 USA.