Abstract
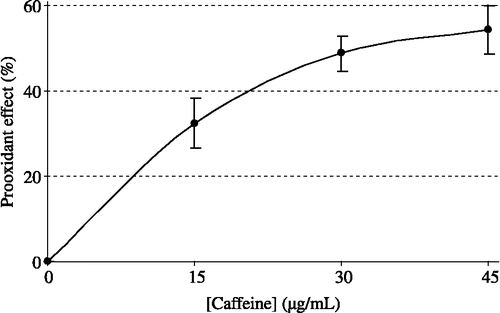
Caffeine is a methylxanthine compound that acts as a stimulant in humans. It is the most widely consumed behaviourally active substance in the western world and it affects calcium influx and release in living cells. Caffeine is present in various substances such as tea, coffee and some medications. This article is focused on the impact of caffeine on oxidation. Caffeine promoted the peroxidation of linoleic acid emulsions used by 32.5, 48.9, and 54.3%, respectively, at 15, 30 and 45 μg/mL concentrations. Standard antioxidants such as α-tocopherol and trolox, a water-soluble analogue of tocopherol, inhibited 76.2 and 93.2% peroxidation of linoleic acid emulsion at 45 μg/mL concentration. Also, PC50 value (caffeine concentration that produced a 50% increase in linoleic acid peroxidation) of caffeine was found to be 185 μM.
Introduction
Caffeine (1,3,7-trimethylxanthine) () is a member of the methylxanthine family of drugs, and is present in coffee, tea and medicinal products. It is probably the most widely consumed psychoactive substance known to man. The amount of caffeine in food items ranges from 25–120 μg/mL of coffee, to 16–333 μg/mL of tea and 8–16 μg/mL for colas [Citation1]. Caffeine in doses of 150–250 mg produces a sense of well being, alertness, allays fatigue, improves motor performance and increases motor activity [Citation2]. Also, it interacts with neurotransmitters in the brain and promotes higher functions such as vigilance and attention [Citation3,Citation4].
Historically, caffeine has been the subject of extensive research, and studies have been conducted in various species to determine the impact of caffeine on various biochemical and physiological processes. Based on the studies conducted to date it is clear that in addition to its well-established psychoactive effects, it also impacts upon the endocrine, cardiovascular, respiratory, renal and gastrointestinal systems [Citation5]. Various hypotheses, such as blocking of adenosine receptors, mobilization of intracellular calcium, and inhibition of phosphodiesterases and binding of caffeine to benzodiazepine receptors have been postulated as the possible mechanisms of action of caffeine at the cellular level [Citation1]. There is often intense debate and controversy over the general impact of caffeine on human well-being which has probably been greatest in the field of cardiology Citation6-9. In addition, evidence is now accumulating to indicate that caffeine and its methylxanthine metabolites can affect the functioning of the immune system, but hitherto caffeine has escaped the scrutiny of a consolidated review dealing with its ability to have an impact upon the immune system [Citation10].
Oxidation processes are very important for living organism. The uncontrolled production of reactive oxygen species (ROS) and insufficient antioxidant protection result in the onset of many diseases and accelerate ageing. ROS include free radicals such as superoxide anion radicals (), hydroxyl radicals (OH√) and non free-radical species such as H2O2 and singlet oxygen (1O2), which are various forms of activated oxygen Citation11-13. There is a balance between the generation of ROS and inactivation of ROS by the antioxidant system in organisms. When there is an imbalance between ROS and antioxidant defence mechanisms, ROS may lead to oxidative modification in cellular membranes or intracellular molecules Citation14-15. In addition, under pathological conditions or oxidative stress, ROS are overproduced and result in peroxidation of membrane lipids, leading to the accumulation of lipid peroxides. The aim of this article is to demonstrate the prooxidant effects of caffeine in the linoleic acid emulsion system.
Material and methods
Chemicals
Caffeine, linoleic acid, α-tocopherol, and polyoxyethylenesorbitan monolaurate (Tween-20) were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). Trolox was purchased from Fluka, ammonium thiocyanate from Merck and all other chemicals used were analytical grade and obtained from either Sigma-Aldrich or Merck.
Prooxidant effect determination in linoleic acid emulsion
The prooxidant effect of caffeine was determined according to the ferric thiocyanate method using oxidation of linoleic acid emulsion as described by Gülçin [Citation16,Citation17]. For preparation of stock solutions, 10 mg caffeine or 10 mg trolox dissolved in 10 mL distilled water were prepared freshly for each experiment. Ten mg of α-tocopherol was dissolved in 10 mL of ethanol. Different concentrations of caffeine (from 15 μg/mL–45 μg/mL) in 2.5 mL of sodium phosphate buffer (0.04 M, pH 7.0) were added to 2.5 mL of linoleic acid emulsion in sodium phosphate buffer (0.04 M, pH 7.0). The linoleic acid emulsion was prepared by mixing and homogenising 15.5 μL of linoleic acid, 17.5 mg of Tween-20 as emulsifier, and 5 mL phosphate buffer (pH 7.0). The control was composed of 2.5 mL of linoleic acid emulsion and 2.5 mL of sodium phosphate buffer (0.04 M, pH 7.0). The mixed solution (5 mL) was incubated at 37°C in a polyethylene flask. During the linoleic acid peroxidation, peroxides are formed that leads to oxidation of Fe2 + to Fe3 + . The latter ions form a complex with ammonium thiocyanate and this complex has a maximum absorbance at 500 nm. The peroxide level was determined by reading the absorbance at 500 nm in a spectrophotometer (Shimadzu, UV-1208 UV-VIS Spectrophotometer, Japan) after reaction with 0.1 mL FeCl2 (3.5%) and 0.1 mL thiocyanate (NH4SCN, 30%) at intervals during incubation. This step was repeated every 5 h. The percentage of inhibition or activation was calculated relative to the control at each interval over a 30 h period. High absorbance indicated high linoleic acid emulsion peroxidation. For preparation of the FeCl2 solution, 0.281 g FeCl2.3/4H2O was dissolved in 9.8 mL HCl (37.5%), and the volume adjusted to100 mL with distilled water. For thiocyanate, 30 g of NH4SCN was dissolved in 70 mL of distilled water.
Calculation of prooxidant and antioxidant effects
The percentage prooxidant effect of caffeine on linoleic acid emulsion peroxidation was calculated by the following equation:
where AC is the absorbance of a control reaction which contains only linoleic acid emulsion and sodium phosphate buffer and AS is the absorbance in the presence of different concentrations of caffeine [Citation18,Citation19].
On the other hand, the percentage inhibition of lipid peroxidation in linoleic acid emulsion by α-tocopherol and trolox was calculated by the following formula:
in here AC is the absorbance of control reaction which contains only linoleic acid emulsion and sodium phosphate buffer and AS is the absorbance in the presence of standard compounds such as α-tocopherol and trolox Citation20-22.
Determination of PC50 value for caffeine
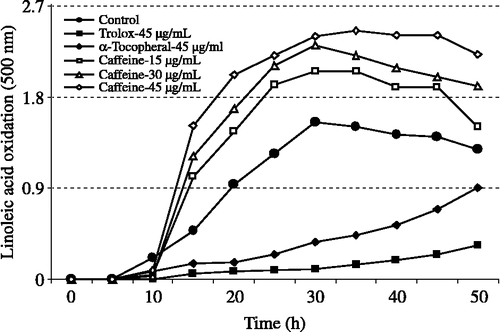
As can be seen from , the oxidation of linoleic acid emulsion in the control mixture reached a maximum at 30 h reaction. For determination of the PC50 value of caffeine (caffeine concentration that produced 50% linoleic acid prooxidation), the effect of three different caffeine concentrations (15, 30 and 45 μg/mL) were investigated. The prooxidant effect of caffeine at different concentrations was measured at 30 h reaction. As can be seen from , the PC50 value was obtained after prooxidant activity was plotted vs. caffeine concentrations (r2:08968) and then calculated from the graphs using the formula given below:
Statistical analysis
The experimental results were performed in triplicate. The data were recorded as mean ± standard deviation and analysed by SPSS (version 11.5 for Windows 2000, SPSS Inc.). One-way analysis of variance was performed by ANOVA procedures.
Results and discussion
The ferric thiocyanate method measures the amount of peroxide, the primary product of lipid peroxidation, produced during the initial stages of oxidation. During linoleic acid peroxidation, peroxides are formed and these peroxides react with ferrous ions (Fe+2) to form ferric ions (Fe+3). The latter ions form a complex with thiocyanate ions (SCN− ) which has a maximum absorbance at 500 nm. For comparison, α-tocopherol and trolox were used as standard antioxidants in the linoleic acid system and exhibited effective antioxidant activity in this system. The prooxidant effect of different concentrations of caffeine (15–45 μg/mL) on peroxidation of linoleic acid emulsions are shown in and were found to be 32.5, 48.9 and 54.3%. On the other hand, α-tocopherol and trolox at a concentration of 45 μg/mL had 76.2 and 93.2% antioxidant effect on the same emulsion system. There was a significant difference (p < 0.05) between the effects of 15 μg/mL and 45 μg/mL caffeine. However, the difference was not statistically significant between 15 μg/mL and 30 μg/mL or 30 μg/mL and 45 μg/mL. In addition, as can be seen in , the PC50 (caffeine concentration that produced 50% linoleic acid prooxidation) value of caffeine was calculated as 185 μM. Consequently, these results clearly indicate that caffeine has an effective prooxidant activity in the peroxidation of linoleic acid emulsions.
Lipid peroxidation is an important chemical change, which lowers the nutritional quality of food and pharmaceutical products. The primary and secondary products of lipid oxidation are detrimental to health [Citation23]. Lipid peroxidation involves a series of free radical-mediated chain reaction processes and is associated with several types of biological damage. The role of free radicals and ROS is becoming increasingly recognized in the pathogenesis of many human diseases, including cancer, aging and atherosclerosis. In the body, excess production of free radicals induces lipid cell membranes to produce lipid peroxides and ROS, which leads to many biological changes, such as DNA damage, aging, heart disease and cancer [Citation15,Citation22,Citation24].
Because of biological variation in caffeine metabolism, consumption of caffeine-containing food and beverages can yield varying plasma concentrations among individuals in a population. For example, in adults the caffeine half-life is reduced in smokers compared to non-smokers. De Leon and co-workers reported plasma caffeine concentrations 2- to 3-fold higher in non-smokers than smokers for each level of caffeine intake [Citation25]. On the other hand, the caffeine half-life is approximately doubled in women taking oral contraceptives [Citation26,Citation27]. Also, because caffeine is such a widely consumed drug, study participants may have residual levels of caffeine and/or metabolites in their plasma, prior to the experiment. These difficulties may contribute to inconsistencies in studies investigating the effects of caffeine consumption [Citation10]. It has been stated that plasma levels reach 20–50 μM following ingestion of 2–3 cups of coffee (where 1 cup of coffee contains 75–150 mg caffeine) [Citation28]. Horrigan and co-workers made similar observations, using HPLC analysis of human blood samples [Citation29].
Conclusion
According to data obtained from the present study, caffeine was found to be an effective prooxidant in a linoleic acid emulsion assay. Based on the discussion above, the usage of caffeine should be restricted in lipid-containing food or pharmaceutical products. This restriction is required for nutritional quality and prolonging the shelf life of food or pharmaceuticals.
References
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 1999; 51: 83–126
- Tripathi KD. Bronchial asthma. Essentials of medical pharmacologyFourth ed., KD Tripathi. Jaypee, New Delhi 1999; 222–238
- Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell Mol Life Sci 2004; 61: 857–872
- Dixit A, Vaney N, Tandon OP. Effect of caffeine on central auditory pathways: An evoked potential study. Hearing Res 2006; 220: 61–66
- Aruna AS. Caffeine: Fact and fallacy1sted. Global Publishing Network, New Orleans 1997
- Reville W. Caffeine risk interview may be a storm in a teacup, Irish Times 21-12-2000.
- Anonymous L. Coffee and the heart. Harvard Heart Lett 2002; 12: 3–4
- James JE. Critical review of dietary caffeine and blood pressure: A relationship that should be taken seriously. Psychosom Med 2004; 66: 63–71
- Ziegelstein RC. Wake up and smell the caffeine. Am J Cardiol 2004; 94: 981–982
- Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: Friend or foe?. Pharmacol Therapeut 2006; 111: 877–892
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press, Oxford 1989; 23–30
- Gülçin İ, Büyükokuroglu ME, Oktay M, Küfrevioglu OI. On the in vitro antioxidant properties of melatonin. J Pineal Res 2002; 33: 167–171
- Gülçin İ, Oktay M, Küfrevioğlu OI, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L). Ach J Ethnopharmacol 2002; 79: 325–329
- Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harng jyur (Chrysanthemum morifolium Ramat). Lebensm Wiss Technol 1999; 32: 269–277
- Büyükokuroğlu ME, Gülçin İ, Oktay M, Küfrevioğlu Öİ. In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 2001; 44: 491–495
- Gülçin İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nut 2005; 56: 491–499
- Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-D-glucopyranosyl)-hederagenin. Phytother Res 2006; 20: 130–134
- Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Screening of antioxidant and antiradical activity of monodesmosides and crude extract from Leontice smirnowii Tuber. Phytomedicine 2006; 13: 343–351
- Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006; 217: 213–220
- Gülçin İ, Beydemir Ş, Alici HA, Elmastaş M, Büyükokuroğlu ME. In vitro antioxidant properties of morphine. Pharmacol Res 2004; 49: 59–66
- Gülçin İ, Elias R, Gepdiremen A, Boyer L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 2006; 223: 759–767
- Gülçin İ. Antioxidant and antiradical activities of L-carnitine. Life Sci 2006; 78: 803–811
- Gülçin İ, Alici HA, Cesur M. Determination of invitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005; 53: 281–285
- Gülçin İ, Berashvili D, Gepdiremen A. Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J Ethnopharmacol 2005; 101: 287–293
- De Leon J, Diaz FJ, Rogers T. A pilot study of plasma caffeine concentrations in a US sample of smoker and nonsmoker volunteers. Prog Neuro-Psychoph 2003; 27: 165–171
- Patwardhan RV, Desmond PV, Johnson RF, Schenker S. Impaired elimination of caffeine by oral contraceptive steroids. J Lab Clin Med 1980; 95: 603–608
- Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur J Clin Pharmacol 1985; 28: 425–428
- Fredholm BB. Trends Pharmacol Sci 1980; 1: 129–132
- Horrigan LA, Diamond M, Connor TJ, Kelly JP. Caffeine inhibits monocyte and neutrophil chemotaxis at concentrations relevant to normal human consumption. Trinity College Dublin, Ireland 2003; 49–54, Proceedings of the International Cytokine Society Annual Meeting,