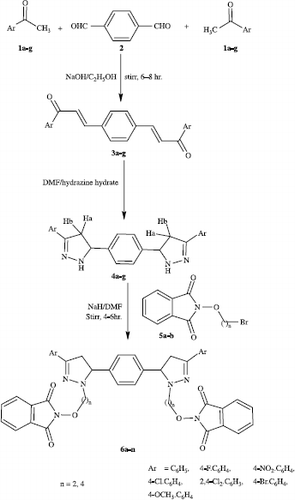
Abstract
A facile synthesis of 5,5′-(1,4-phenylene)bis(3-aryl-2-pyrazolines) 4a-g has been achieved by the cyclo-addition reaction of hydrazine hydrate with bis-substituted chalcones 3a-g, which in turn were prepared by the Clasien-Schmidt condensation of p-substituted acetophenones 1a-g with terephthaldehyde. Condensation of 4a-g with ω-bromoalkoxyphthalimides 5a-b afforded the titled compounds 6a-n, some of which exhibited significant antimalarial as well as antimicrobial activity.
Introduction
The pyrazoline nucleus is a ubiquitous feature of pharmacological interest and has been proven to be a fertile source of medicinal agents such as antifungal [Citation1], antibacterial [Citation2], antiamoebic [Citation3], antitumor [Citation4], etc. Many pyrazolines have been found to have activity as analgesic, anti-inflammatory and cyclooxygenase-II (COX-II) [Citation5,6,7] agents. Some new testosterone derivatives with fused substituted pyrazoline ring are recognized as 5-α reductase inhibitors [Citation8]. Pyrazoles are known to possess COX-I, COX-II and human lipooxygenase inhibitory activities [Citation9]. Many pyrazoline derivatives of chalcones have been synthesized [Citation10] and have exhibited antibacterial [Citation11] and cytotoxic [Citation12] activities. Pyrazoles constitute an important group of heterocyclic compounds and some of them possess a wide range of pharmacological properties such as antiproliferative [Citation13], antimicrobial [Citation14], antidepressant [Citation15], antihyperglycemic [Citation16], anticancer [Citation17] etc. Several derivatives of alkoxyphthalimide have been synthesized [Citation18,19] and reported to demonstrate a wide range of pharmacological activities i.e. anticancer [Citation20], antimalarial [Citation21], anticonvulsant [Citation22] etc. In view of these observation and in continuation of our work on pyrazoline based heterocycles, it was considered to synthesize new chemical entities incorporating the two active pharmacophores namely, pyrazoline and alkoxyphthalimide in a single molecular framework using chalcones of teraphthaldehyde and substituted acetophenones as basic building blocks.
Materials and methods
Chemistry
All the melting points were determined in open capillaries and are uncorrected. IR spectra were recorded on a Perkin-Elmer 1800 (FTIR) spectrometer and 1H NMR and 13C NMR spectra (CDCl3) were recorded on a DRX-300 (300 MHz) spectrometer using TMS as internal standard. The mass spectra were recorded on a Jeol SX-102 (FAB) mass spectrometer in which m-nitrobenzyl alcohol was used as matrix. The purity of synthesized compounds was checked by TLC using silica gel-G and spots were exposed by iodine vapor. All compounds gave satisfactory micro analytical results. In the present investigation the α-β unsaturated carbonyl compounds [Citation23] 3a-g [3,3′-(1,4-phenylene)bis[1-(phenyl/4-fluorophenyl/4-nitrophenyl/4-chlorophenyl/2,4-dichlorophenyl/4-bromophenyl/4-methoxyphenyl)prop-2-ene-1-one] and ω-bromoalkoxyphthalimides [Citation24] 5a-b (phthalimidoxyethyl bromide/phthalimidoxybutyl bromide) were prepared by reported methods.
Synthesis of 5,5′-(1, 4-phenylene)bis(3-phenyl-2-pyrazoline) 4a
A mixture of compound 3a (0.01 mol) and hydrazine hydrate (0.02 mol) in DMF was refluxed for 5 h. After cooling, the reaction mixture was poured on crushed ice and the separated solid was recrystallised from ethanol. Compounds 4b-g were similarly prepared using suitable reagents with minor modification of the reaction conditions.
5,5′-(1,4-phenylene)bis[3-(4-phenyl)-2-pyrazoline] 4a
IR (KBr) cm− 1: 3048 (C-H str., ArH), 3405 (N–H str.), 1456 (C = C skeleton), 1602 (C = N str.), 1270 (C–N str.), 1124 (N-N str.); 1H NMR (CDCl3) δ: 7.18–7.45 (m, 14H, ArH, J (Hz) = 7.62, 7.95), 6.18 (s, 2H, NH), 4.74 (dd, 2H, CH), 3.42 (dd, 2H, CHbHa), 2.88 (dd, 2H, CHbHa) J (Hz) = 7.04, 8.52; 13C NMR δ: 41.33 (CH2), 71.87 (CH).
5′-(1,4-Phenylene)bis[3-(4-fluorophenyl)-2-pyrazoline] 4b
IR (KBr) cm− 1: 3076 (C–H str., Ar-H), 3418 (N–H str.), 1544 (C = C skeleton), 1608 (C = N str.), 1275 (C-N str.), 1133 (N–N str.), 1386 (C–F str.); 1H NMR (CDCl3) δ: 6.99–7.21 (m, 12H, Ar–H, J (Hz) = 7.95, 9.00, H–F = 7.34), 6.18 (s, 2H, NH), 4.74 (dd, 2H, CH), 3.43 (dd, 2H, CHbHa), 2.89 (dd, 2H, CHbHa), J (Hz) = 7.04, 8.52; 13C NMR δ: 41.33 (CH2), 71.87 (CH), 166.78 (C–F).
5,5′-(1,4-Phenylene)bis[3-(4-nitrophenyl)-2-pyrazoline] 4c
IR (KBr) cm− 1: 3067 (C–H str., Ar–H), 3411 (N–H str.), 1328–1540 (N–O str., NO2), 1280 (C–N str.); 1H NMR (CDCl3) δ: 7.18–8.17 (m,12H, ArH, J (Hz) = 7.95, 8.70), 6.18 (s, 2H, NH), 4.74 (dd, 2H, CH), 3.53 (dd, 2H, CHbHa), 2.99 (dd, 2H, CHbHa), J (Hz) = 7.04, 8.52; 13C NMR δ: 40.66 (CH2), 71.87 (CH), 148.08 (C–F).
5,5′-(1,4-Phenylene)bis[3-(4-chlorophenyl)-2-pyrazoline] 4d
IR (KBr) cm− 1: 3070 (C–H str., Ar–H), 3413 (N–H str.), 1535 (C = C str.), 744 (C–Cl str.); 1H NMR (CDCl3) δ: 7.18–7.66 (m,12H, ArH, J (Hz) = 7.95, 8.32), 6.18 (s, 2H, NH), 3.43 (dd, 2H, CHbHa), 2.89 (dd, 2H, CHbHa), J (Hz) = 7.04, 8.52; 13C NMR δ: 41.09 (CH2), 71.87 (CH).
5,5′-(1,4-Phenylene)bis[3-(2,4-dichlorophenyl)-2-pyrazoline] 4e
IR (KBr) cm− 1: 3078 (C–H str., Ar–H), 3421 (N–H str.), 1567 (C = C str.), 748 (C–Cl str.); 1H NMR (CDCl3) δ: 7.18–7.82 (m,10H, ArH, J (Hz) = 7.95, 8.50), 6.18 (s, 2H, NH), 3.53 (dd, 2H, CHbHa), 2.99 (dd, 2H, CHbHa), J (Hz) = 7.04, 8.52; 13C NMR δ: 41.30 (CH2), 71.67 (CH).
5,5′-(1,4-Phenylene)bis[3-(4-bromophenyl)-2-pyrazoline] 4f
IR (KBr) cm− 1: 3063 (C–H str., Ar–H), 3407 (N–H str.), 1524 (C = C str.), 576 (C–Br str.); 1H NMR (CDCl3) δ: 7.18–7.47 (m, 12H, ArH, J (Hz) = 7.95, 8.50), 6.18 (s, 2H, NH), 3.45 (dd, 2H, CHbHa), 2.91 (dd, 2H, CHbHa), J (Hz) = 7.04, 8.52; 13C NMR δ: 41.91 (CH2), 71.87 (CH), 123.79 (C-Br).
5,5′-(1,4-Phenylene)bis[3-(4-methoxyphenyl)-2-pyrazoline] 4g
IR (KBr) cm− 1: 3053 (C–H str., Ar–H), 3410 (N–H str.), 1028 (C–O str.); 1H NMR (CDCl3) δ: 7.18–7.49 (m, 12H, ArH, J (Hz) = 7.95, 8.74), 6.18 (s, 2H, NH), 3.45 (dd, 2H, CHbHa), 2.92 (dd, 2H, CHbHa), J (Hz) = 7.04, 8.52; 13C NMR δ: 41.12 (CH2), 71.87 (CH), 160.80 (C–O), 55.20 (O-CH3).
Synthesis of 5,5′-(1,4-phenylene)bis(1-N-ethoxyphthalimido-3-phenyl-2-pyrazoline) 6a
Compound 4a (0.01 mol) was dissolved in DMF and sodium hydride (0.02 mol) was added to it portionwise with constant stirring at 5°C for 1 h. ω-Bromoethoxyphthalimide 5a (0.02 mol) was then added to the above mixture with constant stirring on a magnetic stirrer for 1–2 h and further stirred for 4–6 h. Excess of solvent was removed in vacuo. After cooling, a brownish coloured solid was obtained which was recrystallised from ethanol. Compounds 6b-n were similarly prepared using appropriate reactants with an appropriate reflux time. Physical and analytical data for 6a–6n are given in .
Table I. Physical and Analytical data for compounds.
6a
IR (KBr) cm− 1: 3040 (C–H str., Ar–H), 1728 (C = O str., CO–N–CO), 1616 (C = N str.), 1220 (C–N str.), 1107 (N–N str.), 1370 (N–O str.), 2830 (C–H str., CH2); 1H NMR (CDCl3) δ: 7.08–7.81 (m, 22H, ArH, J (Hz) = 6.63, 7.95, 8.32), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.51 (t, 4H, OCH2, J (Hz) = 12.00), 3.08 (t, 4H, NCH2, J (Hz) = 15.00), 13C NMR δ: 166.00 (C = O), 67.30 (CH), 70.77 (OCH2), 55.48 (NCH2), 45.60 (CH2); MS m/z: 744 [M]+, 667, 598, 590, 570, 452, 396, 348, 292, 174, 77.
5,5′-(1,4-Phenylene)bis[1-N-ethoxyphthalimido-3-(4-fluorophenyl)-2-pyrazoline] 6c
IR (KBr) cm− 1: 3073 (C–H str., Ar–H), 1734 (C = O str., CO–N–CO), 1621 (C = N str.), 1223 (C–N str.), 1115 (N–N str.), 1381 (N–O str.), 2842 (C–H str., CH2), 1387 (C–F str.); 1H NMR (CDCl3) δ: 6.96–7.81 (m, 20H, ArH, J (Hz) = 7.95, 9.00, H–F = 8.66), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.51 (t, 4H, OCH2, J (Hz) = 12.00), 3.08 (t, 4H, NCH2, J (Hz) = 15.00), 13C NMR δ: 166.74 (C–F), 166.00(C = O), 67.30 (CH), 70.77 (OCH2), 55.48 (NCH2), 45.80 (CH2); MS m/z: 780 [M]+, 685, 634, 606, 590, 488, 432, 348, 292, 190, 174, 146, 95.
5,5′-(1,4-Phenylene)bis[1-N-ethoxyphthalimido-3-(4-nitrophenyl)-2-pyrazoline] 6e
IR (KBr) cm− 1: 3069 (C–H str., Ar–H), 1732 (C = O str., CO–N–CO), 1332-1546 (N–O str., NO2), 2840 (C–H str., CH2), 1221 (C–N str.); 1H NMR (CDCl3) δ: 7.32–8.18 (m, 20H, ArH, J (Hz) = 6.63, 7.95, 8.70), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.51 (t, 4H, OCH2, J (Hz) = 12.00), 2.97 (t, 4H, NCH2, J (Hz) = 15.00), 13C NMR δ: 166.00 (C = O), 67.30 (CH), 70.77 (OCH2), 55.48 (NCH2), 45.13 (CH2); MS m/z: 834 [M]+, 712, 688, 660, 590, 542, 486, 348, 292, 244, 146, 122.
5,5′-(1,4-Phenylene)bis[1-N-ethoxyphthalimido-3-(4-chlorophenyl)-2-pyrazoline] 6g
IR (KBr) cm− 1: 3067 (C–H str., Ar–H), 1733 (C = O str., CO–N–CO), 2840 (C–H str., CH2), 1379 (N–O str.), 744 (C–Cl str.); 1H NMR (CDCl3) δ: 7.10–7.80 (m, 20H, ArH, J (Hz) = 7.95, 8.32), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.52 (t, 4H, OCH2, J (Hz) = 12.00), 3.08 (t, 4H, NCH2, J (Hz) = 15.00), 13C NMR δ: 166.00 (C = O), 67.30 (CH), 70.77 (OCH2), 55.48 (NCH2), 45.56 (CH2); MS m/z: 817 [M + 4]+, 813 [M]+, 704, 702, 667, 639, 591, 521, 348, 292, 226, 222, 174, 146, 113, 111.
5,5′-(1,4-Phenylene)bis[1-N-butoxyphthalimido-3-(2,4-dichlorophenyl)-2-pyrazoline] 6j
IR(KBr) cm− 1: 3076 (C–H str., Ar-H), 1736 (C = O str., CO–N–CO), 1381 (N–O str.), 749 (C–Cl str.); 1H NMR (CDCl3) δ:7.18–7.86 (m,18H, ArH, J (Hz) = 6.63, 7.95, 8.50), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.56 (t, 4H, OCH2, J (Hz) = 10.30), 1.77–1.80 (m, 8H, NCH2(CH2)2 CH2O, J(Hz) = 6.00), 3.25 (t, 4H, NCH2, J (Hz) = 14.00), 13C NMR δ: 165.30 (C = O), 67.30 (CH), 70.77 (OCH2), 55.48 (NCH2), 45.13 (CH2), 26.78 (NCH2CH2), 28.11 (OCH2CH2); MS m/z: 946 [M+8]+, 938 [M]+, 797, 792, 793, 736, 648, 646, 534, 404, 298, 292, 290, 202, 149, 146, 145.
5,5′-(1,4-Phenylene)bis[1-N-butoxyphthalimido-3-(4-bromophenyl)-2-pyrazoline] 6l
IR (KBr) cm− 1: 3063 (C–H str., Ar-H), 1731 (C = O str., CO–N–CO), 1379 (N–O str.), 574 (C–Br str.); 1H NMR (CDCl3) δ:7.18–7.86 (m, 20H, ArH, J (Hz) = 7.95, 8.50), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.56 (t, 4H, OCH2, J (Hz) = 10.30), 1.76–7.80 (m, 8H, NCH2(CH2)2CH2O, J (Hz) = 6.00), 3.24 (t, 4H, NCH2, J (Hz) = 14.00), 13C NMR δ: 165.35 (C = O), 69.88 (CH), 75.60 (OCH2), 57.00 (NCH2), 43.25 (CH2), 26.78 (NCH2CH2), 28.11 (OCH2CH2); MS m/z: 960[M + 2]+, 958 [M]+, 812, 804, 803, 756, 666, 648, 554, 404, 312, 310, 292, 202, 156, 155, 146.
5,5′-(1,4-Phenylene)bis[1-N-butoxyphthalimido-3-(4-methoxyphenyl)-2-pyrazoline] 6n
IR (KBr) cm− 1: 3065 (C–H str., Ar–H), 1728 (C = O str., CO–N–CO), 1375 (N–O str.), 1060 (O–CH3 str.); 1H NMR (CDCl3) δ:6.94–7.83 (m, 20H, ArH, J (Hz) = 6.63, 7.95, 8.74), 4.20 (dd, 2H, CH), 3.48 (dd, 2H, CHbCHa), 2.90 (dd, 2H, CHbCHa), (J (Hz) = 11.75, 14.00), 4.56 (t, 4H, OCH2, J (Hz) = 10.30), 1.77-7.80 (m, 8H, NCH2(CH2)2 CH2O, J (Hz) = 6.00), 3.30 (t, 4H, NCH2, J (Hz) = 14.00), 13C NMR δ: 165.00 (C = O), 69.87 (CH), 74.60 (OCH2), 57.00 (NCH2), 43.25 (CH2), 26.78 (NCH2CH2), 28.11 (OCH2CH2); MS m/z: 860 [M]+, 753, 714, 658, 646, 568, 456, 404, 292, 214, 202, 107.
Antimalarial activity
A simple in vitro, one-step, one-pot, microtiter-plate based, high-throughput antimalarial drug screening method [Citation25] was used based on the fact that the red colour of here- PFHRP II (Plasmodium falciparum histidine- rich protein II) complex changes to green on dissociation of the bound hence by a candidate antimalarial drug. This method involves the use of a mixture of hence and a recombinantly produced HRP II to which a candidate drug is added and the change in colour following a short incubation period is monitored.
Antimicrobial activity
All the newly synthesized compounds were also tested in vitro for their ability to inhibit the growth of four bacterial strains viz. Klebseilla pneumoniae, Proteus mirabilis, Bacilus subtilis and Escherichia coli using 50 μg/mL concentrations in DMF by the cup or well method [Citation26]. Antimicrobial activity was measured as a function of zone of inhibition (mm). Results were compared to the standard ciprofloxacin by measuring zone of inhibition using disc diffusion method [Citation27].
Result and discussion
Chemistry
5,5′-(1,4-Phenylene)bis(3-phenyl-2-pyrazoline) 4a was prepared by the cyclisation of an α-β unsaturated carbonyl compound 3a with hydrazine hydrate in DMF (Scheme ). In order to obtain the titled compound 6a, ω-bromoethoxyphthalimide 5a was condensed with 4a, sodium hydride in DMF being used to replace the acidic hydrogen present on the nitrogen. Confirmations of the formation of 6a from 4a were obtained through spectral and analytical data. Disappearance of N–H str. (3405 cm− 1) in IR and singlet for N–H proton (δ 6.18) in NMR which were present in 4a and appearance of carbonyl absorption band at 1728 cm− 1 of CO–N–CO, N–O str. at 1370 cm− 1 and two triplets at δ 4.51 and 3.08 for OCH2 and NCH2 respectively in the 1H NMR spectra indicated formation of the titled compound 6a. Compounds 6c, 6e, 6g, 6i, 6k and 6m were similarly prepared. Compounds 6b, 6d, 6f, 6h, 6j, 6r and 6n were synthesized using ω-bromo-butoxyphthalimide.
Antimalarial activity
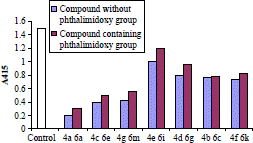
Using the present assay seven pyrazoline derivatives were tested. It was observed that phthalimidoxy-containing derivatives are more active than the parent pyrazoline analogues (without phthalimidoxy). Unsubstituted aryl derivative were observed to be the least potent, the methoxy and nitro derivatives possess intermediate activity, and the halogenated derivatives to be the most potent; of the four halogen derivatives studied, the 2,4-dichloro and 4-chloro-derivatives seemed to be slightly more effective than the fluoro- and bromo derivatives. Graphical representation of comparative antimalarial activity for the synthesized compounds is depicted in .
Antimicrobial activity
Most of the compounds were inactive against Bacilus subtilis, whereas all the compounds exhibited low to moderate activity against other strains of bacteria as compared to the standard drug (). Compound (6i) was found to be highly active against K. pneumoniae and the activity of (6k) was comparable to that of the standard; others were moderate active. In the case of E. coli the compounds were moderate to highly active as compared to the standard ciprofloxacin.
Table II. Antibacterial activity of compounds *†.
It can be concluded from the above results that the antibacterial activities of the compounds depend on the carbon chain length of the alkoxyphthalimide group and the presence of substitution on the phenyl group. It was observed for many compounds that as the length of alkyl side chain increased, inhibitory activity decreased.
Acknowledgements
The authors are thankful to the Head, Department of Chemistry, M. L. Sukhadia University, Udaipur (Rajasthan) for providing laboratory facilities, the Director, CDRI, Lucknow for providing spectral and analytical data and Dr. Dinkar Sahal, Senior Research Scientist, Malaria Research Laboratory, New Delhi for providing antimalarial activity results. Two authors (SO and DB) are thankful to CSIR and UGC, New Delhi for providing the necessary financial assistance respectively.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- OS Moustafa, and RA Ahmad. (2003). Synthesis and antimicrobial activity of some new cyanopyridines, isoxazoles, pyrazoles and pyrimidine bearing sulfonamide moiety. Phosphorus, Sulfur and Silicon 178:475–484.
- MM Ghorab, ZH Ismail, SM Abdel-Gawad, and A Abdel-Aziem. (2004). Antimicrobial activity of amino acid, imidazole and sulfonamide derivatives of pyrazolo[3,4-d]pyrimidine. Heteroatom Chem 15:57–62.
- M Abid, and A Azam. (2005). 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines: Synthesis and in vitro antiamoebic activities. Eur J Med Chem 40:935–942.
- IM Abdou, AM Saleh, and HF Zohdi. (2004). Synthesis and antitumor activity of 5-trifluoromethyl-2,4-dihydropyrazol-3-one nucleosides. Molecules 9:109–116.
- M Sukuroglu, BC Ergun, S Unlu, MF Sehin, E Kupeli, E Yesilada, and E Bengolu. (2005). Synthesis, analgesic and anti-inflammatory activities of [6-(3,5-dimethyl-4-chloropyrazole-1-yl)-3(2H)-pyridazinon-2-yl]acetamides. Arch Pharm Res 28:509–517.
- A Kumar, S Archana, Sharma, N Malik, P Sharma, K Kaushik, KK Saxena, VK Srivastava, RS Verma, H Sharma, SC Gupta, and RC Agarwal. (2004). Synthesis and anti-inflammatory, analgesic and COX-II inhibitory activities of indolyl pyrazolines. Ind J Chem 43B:1532–1536.
- AA Bekhit, HMA Ashour, and AA Guemei. (2005). Novel pyrazole derivatives as potential promising anti-inflammatory and antimicrobial agents. Arch Pharm (Weinheim) 338:167–174.
- A Amr, GES El, NA Abdel-Latif, and MM Abdlla. (2006). Synthesis of some new testosterone derivatives fused with substituted pyrazoline ring as promising 5-α reductase inhibitors. Acta Pharm 56:1203–1218.
- C Cusan, M Spalluto, M Prato, M Adams, A Bodensieck, R Bauer, A Tubaro, P Bernardi, and T Da Ros. (2005). Synthesis and biological evaluation of a new class of acyl derivatives of 3-amino-1-phenyl-4,5-dihydro-1H-pyrazol-5-ones as potential dual cyclooxygenase (COX-1 & COX-2) and human lipoxygenase (5-LOX) inhibitors. Farmaco 60:327–332.
- A Solankee, and J Patel. (2004). Synthesis of chalcones, pyrazolines, aminopyrimidines and pyrimidinethiones as antibacterial agents. Ind J Chem 43B:1580–1584.
- D Azarifar, and S Maseud. (2002). Synthesis and characterization of new 3,5-dinaphthyl substituted 2-pyrazoline and study of their antimicrobial activity. Molecules 7:885–895.
- BA Bhat, KL Dhar, SC Puri, AK Saxena, M Shanmugavel, and GN Qazi. (2005). Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorg Med Chem Lett 15:3177–3180.
- S Chimichi, M Boccalini, MMM Hassan, G Viola, F Dall'Acqua, and M Curini. (2006). Synthesis, structural determination and photo-antiproliferative activity of new 3-pyrazolyl or isooxazolyl substituted 4-hydroxy-2(1H)-quinolinones. Tetrahedron 62:90–96.
- FE Goda, AR Maarouf, and ER El-Bendary. (2003). Synthesis and antimicrobial evaluation of new isoxazole and pyrazole derivatives. Saudi Pharm J 11 (3):111–117.
- AA Bilgin, E Palaska, and R Sunal. (1993). Studies on the synthesis and antidepressant activity of some 1-thiocarbamoyl-3,5-diphenyl-2-pyrazolines. Arzneim-Forsch/Drug Res 43:1041.
- A Sharon, R Pratap, P Tiwari, A Srivastava, PR Maulik, and VJ Ram. (2005). Synthesis and in vitro antihyperglycemic activity of 5-(1H-pyrazol-3-yl)methyl-1H-tetrazoles. Bioorg Med Chem Lett 15:2115–2117.
- VK Tandon, DB Yadav, AK Charurvedi, and PK Shukla. (2005). Synthesis of 1,4-(naphthoquinono[3,2-c]-1H-pyrazoles and their 1,4-naphthohydroquinone derivatives as antifungal, antibacterial and anticancer agents. Bioorg Med Chem Lett 15:3288–3291.
- R Sharma, DP Nagda, and GL Talesara. (2006). Synthesis of various isoniazidothiazolidinone and their imidoxy derivatives of potential biological interest. ARKIVOC i:1.
- N Dixit, DP Nagda, S Ojha, and GL Talesara. (2006). Synthesis, characterization and bio-assay of alkoxyphthalimide derivatives of some bis-[(2-thiazol-2′-yl-imino)-5-arylidene-4-thiazolidino]-3,4,3′,4′-biphenyls. AFINIDAD 63 (523):223–228.
- S Bihzad, B Al-Zaid, and IO Edafiogho. (2002). Phthalimidooxy compound (E-49) as a potential anticancer agents. Seventh Annual Health Science Poster Day 181.
- B Singh, D Mehta, LK Baregama, and GL Talesara. (2004). Synthesis and biological evaluation of 7-N-(n-alkoxyphthlimido)-2-hydroxy-4-aryl-6-aryliminothiazolidino[2,3-b]pyrimidines and related compounds. Ind J Chem 43B:1306.
- IO Edafiogho, KR Scott, JA Moore, VA Farrar, and JM Nicholson. (1991). Synthesis and anticonvulsant activity of imidoxy compounds. J Med Chem 34:387.
- A Levai. (2005). Synthesis of chlorinated 3,5-diaryl-2-pyrazolines by the reaction of chlorochalcones with hydrazines. ARKIVOC ix:344–352.
- GL Talesara. Studies on amino-oxy and related compounds. Ph.D. Thesis. (1984): 72.
- D Sahal, R Kannan, and VS Chauhan. (2003). Applying malaria parasites heme detoxification system for screening potential antimalarial drugs. Analyt Biochem 312:258–260.
- GJ Collee, GA Fraser, PB Marmion, and A Sinmons. Practical medicinal microbiology. 14th edn. Vol. II. Edinburg: Churchill Livingstone; (1996), 163. .
- PS Bisen, and K Verma. (1996). Handbook of Microbiology. 1st edn. New Delhi: CBS Publishers and distributors; p. 30.