Abstract
The present paper describes the synthesis, characterization and in vitro biological evaluation screening of different classes (ammoniacates, dioximates, carboxylates, semi- and thiosemicarbazidates) of Co(II), Co(III), Cu(II), Ni(II), Mn(II), Zn(II) and Fe(III) complexes. Schiff bases were obtained from the reaction of some salicyl aldehydes with, respectively, furoylhydrazine, benzoylhydrazine, semicarbazide, thiosemicarbazide and S-methylthiosemicarbazide to give tridentate ligands containing ONO, ONS or ONN as donor atoms. The synthetic metal complexes are of various geometrical and electronic structures, thermodynamic and thermal stabilities, and magnetic and conductance properties. All complexes, except those of Cu, are octahedral. Some Cu, Co and Mn compounds have a dimeric or a polymeric structure. The composition and structure of complexes were analysed by elemental analysis, IR and 1H NMR and 13C NMR spectroscopies, and magnetochemical, thermoanalytical and molar conductance measurements. All ligands and metal complexes were tested as inhibitors of human leukemia (HL-60) cells growth, and the most potent, the Cu(II) complexes, have been also tested for their in vitro antibacterial and antifungal activities. Structure-activity relationships were carried out.
Introduction
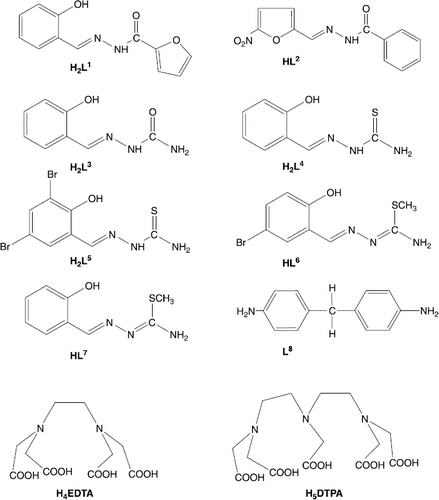
Twenty-eight years after the first approval of cisplatin in the clinic against a number of cancer diseases, cisplatin and related compounds continue to be among the most efficient anticancer drugs used so far. Efforts are now focused to develop novel platinum- and non-platinum-based antitumor drugs to improve clinical effectiveness, to reduce general toxicity and to broaden the spectrum of activity [Citation1]. DNA is a main target for the therapeutic treatment of various disorders and diseases. It can interact with many biomolecules and synthetic compounds including organometallic compounds and metal complexes. Therefore, investigations of the interaction of some ligands with transition metals can further provide and/or improve our understanding about rational metal based inhibitors design [Citation2–7]. We have started a program directed toward the synthesis of different classes of anticancer, antibacterial and antifungal agents designed with complexes of a transition metal and an organic ligand [Citation8–12]. Thiosemicarbazones and their transition metal (Cu and Co) complexes demonstrated potent cytotoxic activities against a series of murine and human tumor cells in culture [Citation13–15]. In continuation of this approach, the present paper describes the chemical synthesis, characterization and biological in vitro evaluation of different classes (ammoniacates, dioximates, carboxylates, semi- and thiosemicarbazidates) of Co(II), Co(III), Cu(II), Ni(II), Mn(II), Zn(II) and Fe(III) complexes. Schiff bases H2L1 - HL7 obtained from interaction of salicyl aldehydes and appropriate amine were used as tridentate ligands containing ONO, ONS or ONN as donor atoms (). Diamine L8, H4EDTA and H5DTPA were also used as ligands. The composition and structure of synthesized complexes were analysed by elemental analysis, IR and NMR spectroscopies, magnetochemical, thermoanalytical and molar conductance measurements. All complexes were tested as inhibitors of human leukemia (HL-60) cells growth. The most potent, the Cu(II) complexes, have been also tested for their in vitro antibacterial activity against Staphylococcus aureus (Wood-46, Smith, 209-P), Staphylococcus saprophyticus, Streptococcus faecalis, Escherichia coli (O-111), Salmonella typhimurium, Salmonella enteritidis, Klebsiella pneumoniaie, Pseudomonas aeruginosa, Proteus vulgaris and Proteus mirabilis and antifungal activity against laboratory stems Aspergillus niger, Aspergillus fumigatus, Candida albicans and Penicillium.
Materials and methods
General
All commercially available reagents and chemicals were of analytical- or reagent-grade purity and used as received. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at room temperature with a Bruker DRX 400 spectrometer. All chemical shifts (1H, 13C) are given in ppm versus SiMe4 using DMSO–d6 as solvent. IR spectra were recorded on a Specord M80 and are reported in cm− 1. Classic methods were applied for C, H, N and Br elemental analyses, which were performed at Academy of Sciences of Moldova (Institute of Chemistry). The complexes were analysed for their metal contents by EDTA titration [Citation16].TG/DT combined analyses were carried out using a SETARAM 92-1600 instrument. Each sample was deposited in a platinum crucible, which was heated (60°C h− 1) in a current of air to allow evacuation of the products resulting from decomposition. Magnetic measurements were carried out on solid complexes using the Gouy's method [Citation17].
Synthesis of Schiff's bases (H2L1-HL7)
General procedure with H2L1
A hot solution (50°C) of salicylic aldehyde (10 mmol) in ethanol (25 mL) was added to a magnetically stirred solution of furoylhydrazine (10 mmol) in warm ethanol and the mixture was refluxed for 30 min. After completion of the reaction, the mixture was cooled and the solid residue was filtered, washed with cold ethanol, then with diethyl ether, and dried. Crystallization from ethanol gave H2L1. The same method was applied for the synthesis of HL2 - HL7 by using corresponding benzoylhydrazine and 5-nitrofurfural (HL2), salicylic aldehyde and semicarbazide (H2L3), salicylic aldehyde and thiosemicarbazide (H2L4), 3,5-dibromosalicylic aldehyde and thiosemicarbazide (H2L5), 5-bromosalicylic aldehyde and S-methylthiosemicarbazide (HL6), salicylic aldehyde and S-methylthiosemicarbazide (HL7).
Salicilydenfuroylhydrazone (H2L1)
Yield: 65%. IR (KBr): 3650 (m, OH), 3058 (m, NH), 1630 (m, C = O), 1586 (w, C = N), 1535 (m, NNH). 1H NMR (DMSO–d6): 12.28 (s, 1H, NNH), 8.74 (s, 1H, HC = N), 8.40 (s, 1H, OH), 7.88, 7.81, 6.75 and 6.74 (m, 4H, phenyl), 7.80, 7.27 and 6.74 (m, 3H, furan). 13C NMR (DMSO–d6): 152.4 (C = O), 150.5 (HC = N), 152.1 (C–OH), 135.9, 135.4, 121.2, 116.5 and 115.7 (phenyl), 151.9, 146.8, 114.5 and 114.5 (furan). Elemental analysis found for C12H10N2O3: C, 62.4; H, 4.3; N, 12.4; calculated: C, 62.6; H, 4.4; N, 12.1%.
5-Nitrofurfuroylidenbenzoylhydrazone (HL2)
Yield: 67%. IR (KBr): 3058 (m, NH), 1625 (m, C = O), 1585 (w, C = N), 1535 (m, NNH). 1H NMR (DMSO–d6): 12.26 (s, 1H, NNH), 8.41 (s, 1H, HC = N); 7.94, 7.92, 7.58, 7.56 and 7.64 (m, 5H, phenyl); 7.54 and 7.28 (d, 2H, J = 3 Hz, furan). 13C NMR (DMSO–d6): 165.8 (C = O), 151.8 (HC = N), 135.5, 127.7, 127.3, 128.6, 128.3 and 132.2 (phenyl), 163.4, 151.9, 115.4 and 114.7 (furan). Elemental analysis found for C12H9N3O4: C, 55.9; H, 3.3; N, 16.2; calculated: C, 55.6; H, 3.5; N, 16.2%.
Salicylidensemicarbazone (H2L3)
Yield: 87%. IR (KBr): 3600 (m OH), 3058 (m, NH), 1630 (m, C = O), 1586 (w, C = N), 1535 (m, NNH). 1H NMR (DMSO–d6): 11.20 (s, 1H, NNH), 9.98 (s, 1H, OH), 8.35 (s, 1H, HC = N), 7.93 and 8.02 (1s 2H, NH2), 8.20, 7.21, 6.85 and 6.80 (m, 4H, phenyl). 13C NMR (DMSO–d6): 197.6 (C = O), 154.4 (HC = N), 140.6 (C–OH), 116.2, 132.1, 121.4, 126.7 and 118.0 (phenyl). Elemental analysis found for C8H9N3O2: C, 59.3; H, 4.9; N, 23.4; calculated: C, 59.6; H, 5.1; N, 23.5%.
Salicylidenthiosemicarbazone (H2L4)
Yield: 75%. IR (KBr): 3600 (m, OH), 3058 (m, NH), 1560 (s, C = S), 1586 (w, C = N), 1535 (m, NNH). 1H NMR (DMSO–d6): 11.39 (s, 1H, NNH), 9.88 (s, 1H, OH), 8.37 (s, 1H, HC = N), 7.93 and 7.91 (2s, 2H, NH2), 8.20, 7.21, 6.85 and 6.80 (m, 4H, phenyl). 13C NMR (DMSO–d6): 177.6 (C = S), 156.4 (HC = N), 139.6 (C–OH); 116.0, 131.1, 120.4, 126.7 and 118.9 (phenyl). Elemental analysis found for C8H9N3OS: C, 49.4; H, 4.9; N, 21.3; calculated: C, 49.2; H, 4.9; N, 21.5%.
3,5-Dibromosalicylidenthiosemicarbazone (H2L5)
Yield: 72%. IR (KBr): 3650 (m, OH), 3058 (m, NH), 1560 (s, C = S), 1586 (w, C = N), 1535 (m, NNH), 1H NMR (DMSO–d6): 11.45 (s, 1H, NNH), 10.55 (s, 1H, OH), 8.29 (s, 1H, HC = N), 8.10 and 8.01 (2s, 2H, NH2), 8.20 and 7.56, (2s, 2H, phenyl). 13C NMR (DMSO–d6): 178.5 (C = S), 155.4 (HC = N), 150.2 (C–OH), 118.1, 137.5, 111.2, 130.8 and 123.0 (phenyl). Elemental analysis found for C8H7Br2N3OS: C, 27.0; H, 2.1; N, 11.8; Br, 44.3; S, 9.3%; calculated: C, 27.2; H, 2.0; N, 11.9; Br, 45.3; S, 9.1%.
5-Bromosalicyliden-S-methylthiosemicarbazone (HL6)
Yield: 58%. IR (KBr): 3600 (m, OH), 3058 (m, NH), 1658 (s, C-S), 1586 (w, C = N). 1H NMR (DMSO–d6): 9.88 (s, 1H, OH), 8.34 (s, 1H, HC = N), 8.60 (s, 2H, NH2), 8.20, 7.34 and 6.80 (m, 3H, phenyl); 2.10 (s, 3H, CH3). 13C NMR (DMSO–d6): 160.6 (C-S), 154.2 (HC = N), 137.2 (C–OH), 118.0, 133.2, 111.4, 128.7 and 122.9 (phenyl), 7.9 (S–CH3). Elemental analysis found for C9H10BrN3OS: C, 37.4; H, 3.6; N, 14.5; calculated: C, 37.5; H, 3.5; N, 14.6%.
Salicyliden-S-methylthiosemicarbazone (HL7)
Yield: 65%. IR (KBr): 3600 (m, OH), 3058 (m, NH), 1586 (w, C = N). 1H NMR (DMSO–d6): 9.80 (s, 1H, OH), 8.30 (s, 1H, HC = N), 8.40 (s, 2H, NH2), 8.11, 7.21, 6.90 and 6.80 (m, 4H, phenyl), 2.11 (s, 3H, CH3). 13C NMR (DMSO–d6): 160.0 (C-S), 155.2 (HC = N), 137.0 (C–OH), 118.0, 130.2, 120.4, 131.7, 115.9 and 118.4 (phenyl), 7.9 (S–CH3). Elemental analysis found for C9H11N3OS: C, 51.7; H, 5.2; N, 20.3; calculated: C, 51.6; H, 5.3; N, 20.1%.
Bis(4-aminophenyl)methane (L8)
This ligand was commercially available.
Syntheses of metal complexes (Table I)
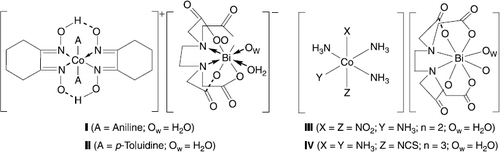
The reagents for the synthesis of I and II were Bi(HEDTA) · 2H2O, obtained according to a published method [Citation18], BaCO3 and the two sulphates [Co(NxH)2(An)2]2SO4·5H2O and [Co(NxH)2(p-Tol)2]2SO4·5H2O. The dioximates were prepared by reaction of CoSO4, NxH (1,2-cyclohexanedionedioxime) and the aromatic amine aniline (An) or para-toluidine (p-Tol) in a molar ratio of 1:2:3 in the presence of oxygen. The compounds I and II are crystalline substances stable in air, soluble in water, poorly soluble in alcohols, and insoluble in acetone and diethyl ether.
Trans-[co(nxh)2(an)2]2[bi(edta)(h2o)]2 · 7h2o (I)
The complex Bi(HEDTA) · 2H2O (1.068 g, 2 mmol) was dissolved in water (50 mL) upon heating. BaCO3 (0.197 g, 1 mmol) was added and the mixture heated with stirring for 1 h to give Ba(BiEDTA)2 solution, then [Co(NxH)2(An)2]2SO4 · 5H2O (0.577 g, 1 mmol) (NxH2 = nioxime = 1,2-cyclohexanedione dioxime) in water (20 mL) was added. BaSO4 was filtered off, and the clear solution was allowed to stand at room temperature. Transparent and brownish crystals of I were separated by filtration, washed with ethanol (2–5 mL) and diethyl ether (2–3 mL), and dried in air up to the constant weight. Yield: 65%. Water content: Exp. (TGA) 7.5%, Calc. 7.33%.
Trans-[co(nxh)2(p-tol)2][bi(edta)] · 4h2o (Ii)
Red-brown crystals of complex II were obtained as above by reaction of [Co(NxH)2(p-Tol)2]2SO4·5H2O and Ba(BiEDTA)2. Yield: 52%. Water content: Exp. (TGA) 6.5%, Calc. 6.41%.
Trans-[co(nh3)4(no2)2][bi(edta)(h2o)] · 2h2o (Iii)
A solution containing trans-[Co(NH3)4(NO2)2]2SO4 (0.534 g, 1 mmol) dissolved in a minimum of hot water was added with vigorous stirring to an aqueous solution of Ba[Bi(EDTA)]2 (prepared from 1.068 g, 2 mmol, of Bi(HEDTA)·2H2O as described in literature [Citation18]) and BaCO3 (0.197 g, 1 mmol). After removal of precipitated BaSO4, the solution was allowed to stand overnight. The resulting yellow crystalline product was collected by filtration, washed with ethanol and then dried in air. This complex is soluble in water but insoluble in alcohols, acetone, or diethyl ether. It can be recrystallized from water solution without change in composition. Yield: 80%.
[Co(nh3)5ncs][bi(edta)]2 · 4h2o (Iv)
The complex IV was prepared by reaction of Ba[Bi(EDTA)]2 and [Co(NH3)5NCS]SO4·2H2O in aqueous solution. Bi(HEDTA) · 2H2O (1.068 g, 2 mmol) was dissolved in hot water (80 mL) and BaCO3 (0.197 g, 1 mmol) was added. After complete dissolution, a solution of [Co(NH3)5NCS]SO4 ·2H2O (0.334 g, 1 mmol) of water (20 mL) was added with stirring. The precipitated BaSO4 was removed by filtration. Ethanol was added and the solution was allowed to stand for 24 h. [Co(NH3)5NCS][Bi(EDTA)]2 · 4H2O crystallized as orange needles from a clear solution. The compound was recrystallized from hot water and dried in air. Yield: 72%.
[Co2(-h2o)(-ccl3coo)2(ccl3coo)2(h2o)4]·h2o (V), [Mn2(-h2o)(-ccl3coo)2 (Ccl3coo)2(h2o)4] ·H2o (Vi) And [Zn(cf3coo)2(h2o)2] · 2H2o (Vii)
Non-symmetric Co(II), Mn(II) trichloroacetate complexes were prepared as described in the literature [Citation19,20].
[Fe(l6)(h2o)3]so4• Or ·2H2o (Viii)
This complex was prepared according to the literature [Citation21].
Compounds Ix-xiii
The complexes of type M(HL1)2 · nH2O (M = Mn2 + , Co2 + , Ni2 + , Zn2 + and Cu2 + ; n = 0 – 3) were synthesised from metal acetates and H2L1 in the presence of ammonium hydroxide solution (pH = 8) [Citation22,23].
[Co(l7)(h2o)3]2so4 · 2h2o (Xiv) And [Cu(l2)2] · 2H2o) (Xv)
These complexes were obtained as described in the literature [Citation21].
{[Cu(hl3)(h2o)bi(edta)(h2o)] · 4h2o}2 (Xvi)
A solution of [Cu(HL3)(H2O)]2SO4 · 2H2O (0.521 g, 0.8 mmol), prepared as described in literature [Citation24], in distilled water (50 mL) was added to a solution of Ba[Bi(EDTA)]2 (0.8 mmol) obtained upon reacting ?i(?EDTA) · 2H2O (0.854 g, 1.6 mmol) with BaCO3 (0.158 g, 0.8 mmol) in water (25 mL). BaSO4 was filtered off and the resulting solution, after heating for half an hour on a water bath, was filtered hot and left for two days. The light-green crystals were collected by filtration, washed with water and ethanol prior to be dried. Yield: 75%.
[Cu(hl3)(h2o)]2[bi(dtpa)] · 10h2o (Xvii)
In a 100 mL container, [Cu(HL3)(H2O)]2SO4 · 2H2O (0.521 g, 0.8 mmol) was dissolved in deionized water (50 mL). Separately, BiH2DTPA · 2H2O (0.508 g, 8 mmol) [Citation18,24] was dissolved in distilled water (25 mL) under stirring and heating, and BaCO3 (0.158 g, 8 mmol) was gradually added to this clear solution. The second solution was then added dropwise to the fist one. BaSO4 was filtered off and the resulting solution was left for crystallization at room temperature. The light-green crystalline powder was collected by filtration, washed with water and ethanol prior to be dried. Yield: 85%.
[Cu(l8)2]so4 (Xviii)
This complex was prepared as previously described [Citation25].
[Cu(hl4)(h2o)][bi(edta)] · h2o (Xix)
A solution containing [Cu(HL4)(H2O)]2SO4 · 3H2O (0.137 g, 0.2 mmol), prepared by reacting equimolar quantities of CuSO4·5H2O and H2L4 in ethanol, in distilled water (150 mL) was added to a solution of Ba[Bi(EDTA)]2 (0.2 mmol) obtained upon reacting Bi(HEDTA) · 2H2O [Citation18] (0.214 g, 0.4 mmol) with BaCO3 (0.0395 g, 0.2 mmol) in water (25 mL). BaSO4 was filtered off and the resulting green-bluish substance was collected by filtration, washed with water and ethanol prior to be dried. Yield: 52%.
[Cu(hl4)h2o]2[bidtpa] ·6H2o (Xx)
A solution containing [Cu(HL4)(H2O)]2SO4·3H2O (0.1366 g (0.2 mmol), prepared by reacting equimolar quantities of CuSO4 · 5H2O and salicylidenthiosemicarbazone (H2L4) in ethanol, in distilled water (150 mL) was added to a solution of Ba[Bi(DTPA)] (0.2 mmol), which was obtained upon reacting BiH2DTPA · 2H2O [Citation26] (0.127 g, 0.2 mmol) with BaCO3 (0.039 g, 0.2 mmol) in water (25 mL). BaSO4 was filtered off and the resulting green powder was collected by filtration, washed with water and ethanol prior to be dried. Yield: 46%.
[Cu(hl5)cl] (Xxi)
To a solution of Cu(II) chloride (10 mmol) in ethanol (30 mL), heated (50–55°C) and mixed continuously with a magnetic agitator, was added a solution of 3,5-dibromosalicyliden-thiosemicarbazone (H2L5) (10 mmol) in ethanol (120 mL) and the mixture was heated for 30–40 min. After cooling, the small green crystals formed from the reaction mixture were filtered on glass filter, washed with ethanol and diethyl ether, and dried in air. Yield: 81%.
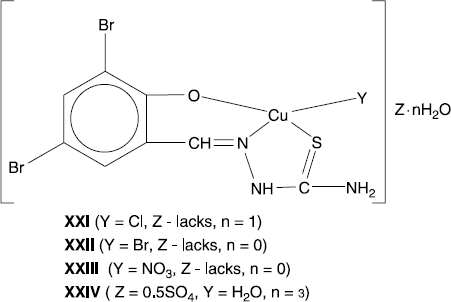
[Cu(hl5)br] (Xxii), [Cu(hl5)no3] (Xxiii) And [Cu(hl5)(h2o)]2so4 (Xxiv)
According to the method reported above for XXI and using Cu(II) bromide, Cu(II) nitrate trihydrate or Cu(II) sulphate pentahydrate with 3,5-dibromosalicylic aldehyde thiosemicarbazone as initial substances in the 1:1 molar ratio, we synthesized XXII, XXIII and XXIV, respectively. Yields are 85% for XXII, 79% for XXIII and 80% for XXIV.
[Cu(hl4)no3] (Xxv), [Cu(hl4)(thio)]2so4. H2o (Xxvi) And (Cu(hl4)(h2o)(2so4 (Xxvii)
These complexes were prepared as previously described [Citation25].
Antileukemia bioassay (Table II)
Cell culture
Human promyelocytic leukemia cells HL-60 (ATCC, Rockville, MD, USA) were routinely grown in suspension in 90% RPMI-1640 (Sigma, Saint Louis, USA) containing L-glutamine (2 nM), antibiotics (100 IU penicillin/mL, 100 μg streptomycin/mL) and supplemented with 10% (v/v) foetal bovine serum (FBS), in a 5% CO2 humidified atmosphere at 37°C. Cells were currently maintained twice a week by diluting the cells in RPMI 1640 medium containing 10% FBS.
Cell proliferation assay
The cell proliferation assay for complexes I-XXVII and ligands was performed using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)2-(4-sulfophenyl)-2H-tetrazolium (MTS) (Cell Titer 96 Aqueous, Promega, USA), which allowed us to measure the number of viable cells. In brief, triplicate cultures of 10,000 cells in a total of 100 μL medium in 96-well microtiter plates (Becton Dickinson and Company, Lincoln Park, NJ, USA) were incubated at 37°C, 5% CO2. Compounds were dissolved in ethanol to prepare the stock solution of 1 × 10− 2 M. These compounds and doxorubicin (Novapharm, Toronto, Canada), as a positive control, were diluted at multiple concentrations with culture media, added to each well and incubated for 3 days. Following each treatment, MTS (20 μL) was added to each well and the mixture incubated for 4 h. MTS is converted to water-soluble colored formazan by dehydrogenase enzymes present in metabolically active cells. Subsequently, the plates were read at 490 nm using a microplate reader (Molecular Devices, Sunnyvale, CA).
Antibacterial bioassay (Table III)
The antibacterial activity of complexes XXI-XXIV and also of their prototype Furaciline was determined under liquid nutritive environment [2% of peptonate bullion (pH 7.0)] using successive dilutions method [Citation11,12]. Staphylococcus aureus (Wood-46, Smith, 209-P), Staphylococcus saprophyticus, Streptococcus faecalis, Escherichia coli (O-111), Salmonella typhimurium, Salmonella enteritidis, Klebsiella pneumoniaie, Pseudomonas aeruginosa, Proteus vulgaris and Proteus mirabilis standard stems were used as reference culture for in vitro experiment. The dissolution of studied substances in dimethylformamide, microorganisms' cultivation, suspension obtaining, determination of minimal inhibition concentration (MIC) and minimal bactericide concentration (MBC) were carried out according to the method previously reported [Citation27].
Antifungal bioassay (Table IV)
Antimycotic properties of the complexes XXI-XXIV were investigated in vitro on laboratory stems: Aspergillus niger, Aspergillus fumigatusi, Candida albicans and Penicillium. The activity was determined in liquid Sabouroud nutritive environment (pH 6.8). The inoculates were prepared from fungi stems which were harvested during 3–7 days. Their concentration in suspension is (2–4) × 106 colonies forming units/mh. Sowings for levures and micelles were incubated at 37°C during 7 and 14 days, respectively.
Results and discussion
Chemistry
The heterometallic bismuth(III) complexes containing cobalt(III) dioximates (I and II) or ammoniacates (III and IV) are of cation-anion structure (). The complex I is an unique example, which differs structurally from other Bi(EDTA)− complexes by formation of a bridge between the cationic and anionic moieties through an oxime oxygen atom. The influence of the minor changes in the cationic moiety on the anionic sub-lattice is the subject of a special interest in this class of compounds. The heterometallic complexes I-IV have been obtained by an exchange reaction of barium EDTA bismuthate(III) with corresponding cobalt(III) dioximate/ ammoniacate sulphates. After removal of barium sulphate the resulting solution contains cobalt(III) nioximate/ammoniacate complex cation and bismuth(III) EDTA complex anion in molar ratio 1:1. Initial complex dioximates were prepared by passing an intense flow of air through a mixture of cobalt(II) sulphate heptahydrate, 1,2-cyclohexanedionedioxime, and corresponding amine in methanol–water solution. Complexes I-IV are brown crystalline substances readily soluble in water or dimethylsulfoxide and insoluble in acetone or diethyl ether. The complexes are stable to storage in air under normal conditions and can be recrystallized from water without change of their composition.
The 1H NMR spectra of diamagnetic complexes I and II are similar. The following signals are characteristic for the coordinated p-toluidine: 2.24 ppm for the CH3 group, 5.48 ppm for the NH2 group and two doublets at 6.49 and 7.02 ppm (JHH = 7.7 Hz) for the aromatic hydrogen atoms. A confirmation of the trans-configuration of complex cations in I and II in solution is provided by the observation of a large singlet (ΔH/2 ≈ 20 Hz) at 17.7 ppm corresponding for two symmetric hydrogen bonds [Citation28]. These data are in concordance with X-ray analysis in solid state [Citation29]. The signals from CH2-groups of nioxime ligands were observed at 2.54 and 1.51 ppm (1.50 ppm for complex II) as strong singlets. The singlet signal at 3.26 ppm was identified as that of ethylene protons of EDTA ligand. The NCH2CH2N component of EDTA in the domain of fast exchange and the signal from this group is a singlet. The four acetate methylene protons give rise to an AB quartet system JAB = 15.9 Hz for I, and 15.6 Hz for II in the range 3.68–3.73 ppm.
The thermogravimetric investigation of I-IV in air (5 °C/min) revealed four major steps corresponding to dehydration, deamination, ligand pyrolysis and formation of inorganic residue. The dehydration step occurs in a wide range of temperature, from 50°C up to 160–170°C. Deamination (removal of aniline or p-toluidine) begins at 180–195°C, but it is overlapped by the strong exothermic effect that begins near 220°C and finishes around 480–490°C. The investigations of pyrolysis products were carried out using X-ray powder diffraction. X-ray powder diffraction patterns of all four residues are identical. The final products were identified as the sellinite-type phase with a minor mixture of the cobalt (II, III) oxide Co3O4 (∼5%). X-ray diffraction data of the sellinite-type phase were indexed on the basis of a body-centred cubic structure with a = 10.190(2)Å. In accordance with literature [Citation30] this is the Bi26 − xCoxO40 − δ-phase with the metal (Bi:Co) ratio ≈ 1:1.
The complexes V and VI, (μ-aqua)di(μ-tricloracetato)-tri(aqua)cobalt(II)-aqua-bis(triclor-acetato)cobalt(II) and (μ-aqua)di(μ-tricloracetato)-tri(aqua)manganese(II)-aqua-bis(triclor-acetato)manganese(II), have an asymetric structure including two nonequivalent metal atoms (Co or Mn) up bridged by one water molecule and two μ-tricloroacetate anions [Citation19,20]. Main IR features are in good agreement with the proposed structure. The effective magnetic moments of V and VI, 4.8 B.M. for cobalt(II) and 5.5 B.M. for manganese(II), are characteristic for an octahedral environment of central atoms, but the values are a little lower than those for a theoretical high spin state. This fact confirms a weak antiferromagnetic interaction in dimeric bimetallic complexes V and VI. The zinc trifluoracetate complex VII is diamagnetic in 3d10 ground electronic state.
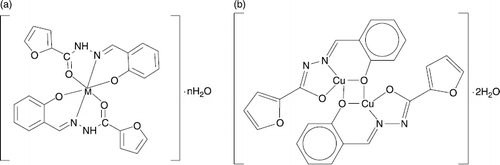
The complexes IX-XIII of type [M(HL1)2] · nH2O (M = Mn2 + , Co2 + , Ni2 + , Zn2 + and Cu2 + ; n = 0 – 3) were synthesised from metal acetates and H2L1 in the presence of ammonia (pH = 8). Physico-chemical properties and composition of these metal complexes are shown in and their structures are represented in . The magnetic moment of 4.9 B.M. is indicative of three unpaired electrons for cobalt (II) compound X with pronounced spin orbital interaction in an octahedral environment. The nickel(II) complex XI showed a μeff of 3.1 B.M., which corresponds to two unpaired electrons per nickel(II) ion for a six-coordinated configuration.
Table I. Physical and analytical data of the metal complexes I-XXVII.
The manganese(II) compound IX with an effective magnetic moment of 5.9 B.M. is an example of 5 electrons in a high state of octahedral coordination. The zinc(II) complex XII is diamagnetic. The thermal stability and total decomposition temperature (t) of the metal complexes IX-XII is influenced by the nature of the central atom according to the following relation: t (Co) ≥ t (Ni) ≥ t (Zn) t > (Mn). For complex XIII, a comparison of the IR spectra of the Shiff's base H2L1 [Citation31–32] to their metal chelates indicated that H2L1 is coordinated to the metal atom mainly in a deprotonated way acting in a tridentate ONO manner including a phenolic oxygen, an azomethine nitrogen and an amidic oxygen, then forming five and six atom rings with the central metal atom. In IR, a band that appears at 1560 cm− 1 due to the azomethine group was shifted to lower frequency by 28 cm− 1 indicating the participation of azomethine nitrogen in the complexation. A new band appearing at 460 cm− 1 was assigned to ν(M-O) [Citation33] whereas the absence of the band at 1635 cm− 1 demonstrated that the oxygen has formed a coordinative bond with metal ions in an enolic form. A weak band at 400 cm− 1 was assigned to ν(M-N). The room temperature magnetic moment of the solid copper(II) complex XIII, 1.6 B.M., demonstrates the anti-ferromagnetic spin-spin interaction through a dimeric complex association.
The heterobimetalic (Bi,Cu) complexes containing the salicylic aldehyde semicarbazone (XVI or XVII) or salicylic aldehyde thiosemicarbazone (XIX or XX) were prepared by reacting [Cu(HL3)(H2O)]2SO4• or ·2H2O or [Cu(HL4)(H2O)]2SO4• or ·3H2O with solutions of Ba[Bi(EDTA)]2 or Ba[Bi(DTPA)].
The coordinative compounds XXI-XXIV (figure 4) have been prepared in 79–85% yields by a reaction between hydrate of copper(II) chloride, bromide, nitrate or sulphate with 3,5-dibromosalicylic aldehyde thiosemicarbazone (H2L5) in the 1:1 molar ratio. The mechanism of the given reaction is connected with the addition of this ligand, which has the role of tridentate ONS ligand, to copper(II) ion. Chloride, bromide, nitrate or water occupies the fourth place in the inner coordination sphere. At the same time, the deprotonation of the phenol takes place in the reaction mixture. Complexes XXI-XXIV are stable in contact with air, poorly soluble in water and alcohol, soluble in dimethylformamide and dimethylsulfoxide, practically insoluble in diethyl ether. The composition and each structure have been determined from the elemental analysis, IR spectroscopy, magnetochemistry and thermogravimetry methods. By determining the molar electric conductibility in dimethylformamide, it was established that XXI-XXIII are non-electrolytes (![]() = 4–9 Ohm− 1 cm2 mol− 1) whereas XXIV was a triple electrolyte (
= 4–9 Ohm− 1 cm2 mol− 1) whereas XXIV was a triple electrolyte (![]() = 149 Ohm− 1. cm2. mol− 1). According to the magnetochemical research of complexes at room temperature (293 K), the calculated values of their effective magnetic moment are close to the spin value for an uncoupled electron and represent 1.93 (XXI), 1.85 (XXII), 1.99 (XXIII) and 2.09 (XXIV) B.M. This fact allows us to suppose that the studied substances have a monomeric structure [Citation17,34,35].
= 149 Ohm− 1. cm2. mol− 1). According to the magnetochemical research of complexes at room temperature (293 K), the calculated values of their effective magnetic moment are close to the spin value for an uncoupled electron and represent 1.93 (XXI), 1.85 (XXII), 1.99 (XXIII) and 2.09 (XXIV) B.M. This fact allows us to suppose that the studied substances have a monomeric structure [Citation17,34,35].
The comparative analysis of the IR spectra of the synthesized compounds and the ligand (3,5-dibromosalicylic aldehyde thiosemicarbazone) was made in order to determine the coordination mode of azomethine with copper(II) ion. It was established that the thiosemicarbazone in XXI-XXIV behaves as a monodeprotonated tridentate ONS ligand, connected to the central ion by a deprotonated phenolic oxygen atom, azomethine nitrogen and sulphur, forming metalocycles of five and six members. This fact finds the explanation in the disappearing of the δ(OH) absorption band in the IR spectra, which can be observed in the free thiosemicarbazone in the range 1245–1240 cm− 1. In compounds XXI-XXIV and their structural analogues, ν(C = N) absorption band is shifted by 35–30 cm− 1 to a smaller frequency [in starting thiosemicarbazone, ν(C = N) is observed in the range of 1620–1610 cm− 1]. The mentioned coordination mode of 3,5-dibromosalicylic aldehyde thiosemicarbazone is supported by the appearance of a series of new absorption bands in the range 630–300 cm− 1, bands that according to the published data are detected as ν(Cu-O), ν(Cu-N) and ν(Cu-S). Besides this, the confrontation of the absorption bands maxima, determined according to those described in [Citation36], proves that the nitrate group in compound XXIII is coordinated to a central ion and behaves as a monodentate ligand in the interior coordination sphere [the intervals of the main oscillation frequencies coincide (ν1(A1) = 1295–1250; ν2(A1) = 1035–970; ν4(B1) = 1530–1480; ν6(B2) = 800–780 cm− 1) and two weak absorption bands appear at 1780 and 1720 cm− 1]. However, the sulphate-ion in XXIV is placed in the exterior sphere. In fact, a single absorption band characteristic for this non-coordinated anion is observed in the range 1110–1120 cm− 1.
Thermal analysis of complexes XXI-XXIV showed that their thermolysis occurs in steps. An endothermic effect, which corresponds to the breaking of the crystallization water molecules (dehydration) of compounds XXII and XXIV in the temperature range of 75–96°C. In the case of XXIV, the process of deaquation occurs with endothermic effect at 155°C, but at 470 (XXI), 460 (XXII), 425 (XXIII) and 430 (XXIV)oC, the complete thermooxidative destruction of the coordinated thiosemicarbazone with exothermic effect takes place.
Antileukemia activity
All 27 compounds were tested as inhibitors of HL-60 cells proliferation. These human promyelocytic leukemia cells were incubated for three days in the presence of synthetic compounds (ligands and complexes) and the number of viable cells was measured using the MTS assay. The results are expressed as the percentage of cell growth inhibition at three concentrations. The ligands have insignificant inhibitor activity (data not shown), but some metal complexes selectively act in this biological process (). The nature, electronic structure and coordination number of the central atom, the geometric configuration of metal complexes and the nature of the ligands (donor atoms) appear to modulate the cell proliferation. Among all the compounds tested, the most indicative are copper(II) complexes XIX, XX, XXIV, XXVI and XXVII and cobalt(II) complex V, which efficiently inhibited the HL-60 cell proliferation at 1 μM.
Table II. Antiproliferative activity of complexes I–XXVII on human leukemia (HL-60) cells at three concentrations.
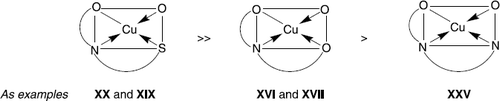
The quite essential activity of copper complexes may be a consequence of Jahn-Teller distortion effect, which takes place along the axe dz2 of copper(II) [Citation37]. The activity of copper complexes is influenced also by the nature of the ligand donor atoms that are present in coordination polyhedra. In fact, complexes with coordinated ligand containing a sulphur donor atom (ONS) are essentially more active than complexes including inner sphere oxygen (ONO) or nitrogen (ONN) (figure 5).
In the case where a sulphur donor atom is blocked by a CH3 group (as for ONN ligands HL6 and HL7), the biological activity of copper complex became insignificant as illustrated by a comparison of XX and XXV. If copper is capsulated in a dimeric complex as for XIII () or polynuclear as for XVI, the cell growth inhibition dramatically change and became minimal. In the series of complexes [Cu(HL5)Y] (XXI-XXIV) having the same metal (copper) and tridentate ONS ligand H2L5, the second inner sphere ligand Y (), influence the biological activity in the following order: H2O (XXIV) > Cl− (XXI) > Br− (XXII) ≅ NO3− (XXIII). In this case, the presence of an inner coordination sphere labile ligand, as a molecule of water, raises considerably (till 60%) the antiproliferative activity. Only one complex with a metal atom other than copper gave a significant inhibition of cell proliferation. Thus, the cobalt complex V inhibited 40% of cell growth at 1 μM. For the cobalt(II) and cobalt(III) octahedral complexes V and X with coordination number 6, the thermodynamic stability is also important as well as in the case of copper(II) complexes XXI-XXIV. In solutions, the most labile cobalt (II) complex V (Co2 + has the electronic configuration 3d7 with an inner coordination sphere lifetime of the ligand 10− 4 to 10− 5 s) is more active in comparison with the inert cobalt(III) complex X (Co3 + has the electronic configuration 3d6 with a lifetime 104–105 s) [Citation38,39].
Antibacterial activity
Four copper(II) complexes have been screened for their in vitro antibacterial and antifungal activity. Experimental results, obtained from the study of antimicrobial activity of compounds XXI-XXIV, are given in . As can be seen, they display bacteriostatic activity towards gram-positive and gram-negative bacteria in 0.018–2000 μg/mL concentration. Pseudomonas aeruginosa is an exception, for which MIC is 300–4000 μg/mL). For comparison, we also presented the antimicrobial data characteristic for Furaciline, a bactericide used in medical practice. The experimental data prove that complexes XXI-XXIV display an antimicrobial activity of 16–1052 times higher towards staphylococci and streptococci than Furaciline and outruns by 16–517 times its bacteriostatic activity towards majority of gram-negative microorganisms. At the same time the mentioned compounds are of 4–9 times more active towards gram-positive bacteria and not less of 130 times – towards gram-negative microorganisms than their structural analogue (after MIC).
Table III. Minimum inhibitor concentration (MIC) and minimum bactericide concentration (MBC) in μg/mL for complexes XXI-XXIV in comparison with Furaciline.
Antifungal activity
The experimental data obtained from the study of antimycotic properties of selected compounds XXI-XXIV are given in . They also display selective activity towards investigated fungi stems in the concentration range of 18.7–300 μg/mL. In order to make a comparison, data regarding the activity of Nistatine, an antifungal agent used in medicine for mycose treatment, are also given in . The data show that the synthesized complexes display an antimycotic activity of 6.4–1.1 times higher towards majority fungi than Nistatine. The properties found for synthesized and studied coordinative compounds are of interest from the view point of the growth of the arsenal of antimicrobial and antimycotic remedies.
Table IV. Antimicotic activity (MIC / MBC) in μg/mL for complexes XXI-XXIV in comparison with Nistatine.
Conclusion
Metal complexes have been efficiently elaborated by reacting a series of 3d metal with different organic ligands. They have various geometrical and electronic structure, thermodynamic and thermal stabilities, magnetic and conductance properties. All complexes are octahedral except those of Cu which are of square planar or pyramidal geometry. Some copper, cobalt and manganese compounds have dimeric or polymeric structure. From our investigations we have deducted that there are three most indicative criteria for future synthesis of biological active coordination compounds from the view point of the inhibitors of HL-60 cells proliferation:
Use of copper (II) planar or pyramidal complexes;
Presence of sulphur donor atom in the ligand composition;
Use of ONS - tridentate ligands.
Our future investigations will be directed on the synthesis of copper(II) complexes with tridentate ONS containing ligands.
Acknowledgements
The authors wish to thank the CHUL Research Center at Québec City (Canada) and State University of Medicine and Pharmacy of Chisinau (Moldova) for their help in carrying out biological studies. We also thank the AUF (grant funding project 6301-PS-323) and FQRNT (grant funding project 115825) for financial support.
References
- M Galanski, VB Arion, MA Jakupec, and BK Keppler. (2003). Current Pharm Design 9:2078.
- ZH Chohan, H Pervez, A Rauf, KM Khan, and CT Supuran. (2006). J Enz INHIB Med Chem 21–193. (and articles cited herein)
- ZH Chohan, M Arif, Z Shafiq, M Yaqub, and CT Supuran. (2006). J Enz INHIB Med Chem 21:95.
- ZH Chohan, MU Hassan, KM Khan, and CT Supuran. (2005). J Enz INHIB Med Chem 20:183.
- ZH Chohan, CT Supuran, and A Scozafava. (2004). J Enz INHIB Med Chem 19:79.
- ZH Chohan, A Scozafava, and CT Supuran. (2003). J Enz INHIB Med Chem 18:259.
- PK Panchal, HM Parekh, PB Pansuriya, and MN Patel. (2006). J Enz Inhib Med Chem 21–203. (and articles cited herein)
- V Stavila, RL Davidovich, and A Gulea. (2006). Whitmire KH. Coord Chem Rev 250:2782.
- A Gulea, D Poirier, J Roy, V Ţapcov, and V Stavila. (2005). Patent of invention MD 2786. BOPI 6–28.
- A Gulea, D Poirier, J Roy, L Popovschi, and V Tapcov. (2005). Patent of invention MD 285. BOPI 9–24.
- NS Samusi, VI Prisacari, VI Ţapcov, SA Buraciov, and AP Gulya. (2004). Pharm Chem J 38:373.
- AP Gulya, NS Samusi, VI Prisacari, VI Ţapcov, SA Buraciov, SN Spinu, NP Begenari, D Poirier, and J Roy. (2007). Pharm Chem J 41:114.
- IH Hall, CB Lackey, TD Kistler, RW DurhamJr, EM Jouad, M Khan, XD Thanh, S Djebbar-Sid, O Benali-Baitich, and GM Bouet. (2000). Pharmazie 55:937.
- M Baldini, M Belicchi-Ferrari, F Bisceglie, PP Dall'aglio, G Pelosi, S Pinelli, and P Tarasconi. (2004). Inorg Chem 43:7170.
- FM Belicchi, F Bisceglie, G Pelosi, P Tarasconi, R Albertini, PP Dall'Aglio, S Pinelli, A Bergamo, and G Sava. (2004). J Inorg Biochem 8:30.
- G Schwarenbach, and H Flaschka. Die komplexometrische titration. Ferdinand Euke Verlag Stuttgart; (1965).
- S Russell, and Drago. Physical methods in chemistry. 2 W.B. Saunders Company (1977).
- SP Summers, KA Abboud, SR Farrah, and GJ Palenik. (1994). Inorg Chem 3:88.
- SG Shova, AP Gulya, GV Novitski, and MD Mazus. (1993). Proceeding of The XI-th International Conference” Physical Methods in Coordination Chemistry”. Kishinev 162.
- SG Shova, LG Turyatke, GV Novitskii, MD Mazus, and AP Gulya. (1996). Russian J Coord Chem 22:485.
- .
- V Tapcov, V Prisacari, M Birca, and A Gulea. (2002). Anale Ştiinţifice ale Universităţii de Stat din Moldova. Seria, Ştiinţe chimico-biologice, Chişinău. 358.
- A Gulea, D Poirier, J Roy, and V Tapcov. (2006). Patent of invention MD submitted
- AV Gerasimenko, RL Davidovich, IG Bulimestru, AP Gulea, and SW Ng. (2005). Acta Cryst E61:m1816.
- M Birca, V Tapcov, and A Gulea. XXVIII-a Conferinţa Naţională de Chimie. Călimăneşti-Căciulata. Vâlcea. România, Rezumate P., (2004) 133.
- MW Brechbiel, OA Gansow, CG Pippin, RD Rogers, and RP Planalp. (1996). Inorg Chem 35:6343.
- GN Pershin. (1971). Meditsina (Moscow) 357.
- AP Gulya, YA Simonov, and OA Bologa. (1988). Koordinatsionnaya Khimiya 14–201.
- V Stavila, A Gulea, S Shova, YA Simonov, and P Petrenko. (2004). Inorg Chim Acta 357:2060.
- Nl Rangavitta, TN Guru Row, and TN Rao. (1994). Eur J Solid State Inorg Chem 31–409.
- M Mohan, NS Gupta, and A Kumar. (1987). Kumar M. Inorg Chim Acta 135:167.
- M Mohan, A Kumar, and MK Kumar. (1987). Inorg Chim Acta 136:65.
- LJ Bellamy. The infrared spectra of complex molecules. John Wiley, New York, (1971) .
- N Samusi, V Prisacari, V Ţapcov, and S Buraciov. (1997). Patent of invention MD 678. BOPI 2–47.
- NS Samusi, VI Prisacari, VI Ţapcov, SA Buraciov, and AP Gulya. (2004). Pharm Chem J 38:373.
- K Nakamoto. Infrared spectra of inorganic and coordination compounds. Willey Interscience, New York, (1986) .
- I Bersuker. The Jahn – Teller effect. Cambridge University Press, , (2006) .
- F Riblet, G Novitchi, L Helm, A Gulea, and A Merbach. (2006). Chimia 60:224.
- G Novitchi, A Gulea, F Riblet, L Helm, R Scopelliti, and AE Merbach. (2004). Magnetic Res in Chemistry 42:801.