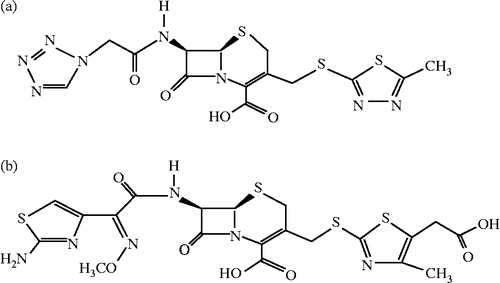
Abstract
Tyrosinase (EC 1.14.18.1) catalyzes both the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones that form brown or black pigments. In the present paper, the effects of Cefazolin and Cefodizime on the activity of mushroom tyrosniase have been studied. The results showed that the Cephalosporin antibacterial drugs (Cefazolin and Cefodizime) could inhibit both monophenolase activity and diphenolase activity of the enzyme. For the monophenolase activity, Both Cefazolin and Cefodizime could lengthen the lag time and decrease the steady-state activities, and the IC50 values were estimated as 7.0 mM and 0.13 mM for monophenolase activity, respectively. For the diphenolase activity, the inhibitory capacity of Cefodizime was obviously stronger than that of Cefazolin, and the IC50 values were estimated as 0.02 mM and 0.21 mM, respectively. Kinetic analyses showed that inhibition by both compounds was reversible and their mechanisms were competitive and mixed-type, respectively. Their inhibition constants were also determined and compared. The research may offer a lead for designing and synthesizing novel and effective tyrosinase inhibitors and also under the application field of Cephalosporins.
| Abbreviations: | ||
| DMSO, | = | Dimethylsulfoxide |
| L-DOPA, | = | L-3,4-dihydroxyphenylalanine |
| Tyr, | = | L-Tyrosine |
| IC50, | = | the inhibitor concentrations leading to 50% activity lost |
Introduction
Tyrosinase (1.14.18.1), widely distributed in nature, is a metalloenzyme oxidase which catalyzes two distinct reactions of melanin synthesis—the hydroxylation of monophenol and the oxidation of o-diphenol to the corresponding o-quinone [Citation1]. It is a key enzyme in melanin biosynthesis, involved in determining the color of mammalian skin and hair. Its abnormal expression is responsible for the various dermatological disorders, such as melasama age spots, actinic damages. It also contributes to neuromelanin formation in the human brain and the neurodegeneration associated with Parkinson′s disease [Citation2]. Tyrosinase inhibitors, therefore, should be clinically useful for the treatments of some dermatological disorders associated with melanin hyperpigmentation [Citation3] and also important in cosmetics for whitening and depigmentation after sunburn [Citation4,Citation5]. The inhibition of tyrosinase has been the subject of many studies Citation6–10. Some drugs have inhibitory effects on the enzyme activity. For example, Captopril is used to treat the disease of hypertension and heart failure and Methimazole is a kind of antithyroid drug. Both Captopril and Methimazole are tyrosinase inhibitors [Citation11,Citation12].
An important group of tyrosinase inhibitors are constituted by compounds structurally analogous to phenolic substrate. These compounds generally show competitive inhibition toward these substrates, although such inhibition may vary depending on the enzyme source and the substrate used [Citation13,Citation14]. Phloridzin [Citation15], cinnamic acid and its derivatives [Citation16], 4-vinylbenzaldehyde and 4-vinylbenzoic acid [Citation17] have been described as reversible inhibitors of this enzyme and 3,5-dihydroxyphenyl decanoate [Citation18] was irreversible one. In our continuing investigation of tyrosinase inhibitors, twenty-two of Cephalosporins antibacterial drugs were tested for the inhibitory effects, Cefodizime and Cefazolin were found to have more potent inhibitory effects on mushroom tyrosinase. The aim of this present work is, therefore, to carry out the inhibitory kinetic studies of Cefazolin and Cefodizime on the activities of the enzyme, to evaluate the kinetic parameters and inhibition constants characterizing the system and investigate the mechanism. These effects have not been reported previously in the literature. Studies have emphasized their uses in developing preparations for the prevention and/or treatment of hyperpigmentation. It is suggested that Cefodizime and Cefazolin can be used in the creams to avoid cutaneous hyperpigmentation. In addition, these date may provide the basis for developing novel tyrosinase inhibitors.
Materials and methods
Tyrosinase (EC 1.14.18.1) from mushroom was the product of Sigma (St. Louis, MO, USA). The specific activity of the enzyme is 6680 U/mg. Dimethylsulfoxide (DMSO), L-Tyrosine (Tyr) and L-3,4-dihydroxyphenylalanine (L-DOPA) were purchased from Aldrich (St. Louis, MO, USA). Cefazolin and Cefodizime were purchased from National Institute for the Control of Pharmaceutical and Biological Products. The purities of the two compounds were 99.3% and 88.6%, respectively. They have been purified by P-HPLC before doing kinetic experiments. All other reagents were local analytical grade. Re-distilled and ion-free water was used.
The monophenolase activity assay was performed by the method as previously reported by Chen et al [Citation19]. In this investigation, Tyr was used as the substrate. The reaction media was 3 mL of 50 mM Na2HPO4-NaH2PO4 buffer (pH 6.8) contained 1.0 mM Tyr and 0.1 mL of the enzyme (containing 100 μg). The reaction was done at 30°C and started by addition of the enzyme. Enzyme activity was monitored spectrophotometrically by following the increasing absorbance at 475 nm accompanying the oxidation of the substrates with a molar absorption coefficient of 3700 (M− 1·cm− 1) each 5 sec for 10 min [Citation20]. The diphenolase activity assay was performed as previously reported using L-DOPA as substrate [Citation21]. The reaction media (3mL) contained 0.5 mM L-DOPA and 20 μg of the enzyme in 50 mM Na2HPO4-NaH2PO4 buffer (pH 6.8). Enzyme activity was determined by following the increasing in optical density at 475 nm accompanying the oxidation of L-DOPA to dopachrome. The reaction was carried out at a constant temperature of 30oC.
The inhibitory effects of inhibitors on enzyme activity were determined as follows: The inhibitor was first dissolved in DMSO and used for the experiment at 30 times dilution. The final concentration of DMSO in the test solution is 3.3%. In this method, 0.1 mL of DMSO solution containing different concentrations of the inhibitors was first mixed with 2.8 mL of substrate solution, then, a portion of 100 μL of enzyme solution was added into this mixture and the residual activity was determined. Controls, without inhibitor but containing 3.3% DMSO, were routinely carried out. The extent of inhibition by the addition of the sample was expressed as the concentration necessary for 50% inhibition (IC50). The value of IC50 was defined as the inhibitor concentrations leading to the initial rate lost by 50% for the diphenolase or leading to the steady-state rate lost by 50% for the monophenolase. The inhibition type of Cefazolin or Cefodizime on the diphenolase was determined by the Lineweaver-Burk plots and the inhibition constants were determined from secondary plots of the apparent Km/Vmax or 1/Vmax versus the concentration of the inhibitor. A Beckman UV-650 spectrophotometer was used for absorbance and kinetic measurements.
Results
Effects of Cefodizime and Cefazolin on the monophenolase activity of mushroom tyrosinase
The effects of Cefazolin and Cefodizime (see ) at different concentrations on the oxidation of L-tyrosine by mushroom tyrosinase were first studied. In the presence of different concentrations of Cefazolin, the kinetic courses of the oxidation of the substrate are shown in . When the monophenolase activity of mushroom tyrosinase was assayed using L-tyrosine as substrate, a lag period, characteristic of monophenolase activity, was observed simultaneously with the appearance of the first stable product, dopachrome. The system reached a constant rate after the lag period, which was estimated by extrapolation of the linear portion of the product accumulation curve to the abscissa. After the reaction system reached the steady state, the curve of product increased linearly with increasing reaction time. And the slope of the line denoted the steady-state rate. The lag time and the steady-state rate were be influenced by Cefazolin as shown in , respectively. The lag time prolonged to a great extent with increasing the inhibitor concentration, meanwhile, the steady state rate descended distinctly. When the concentration of Cefazolin increased to 10.0 mM, the lag time was enlarged about 4 times and the steady state rate descended by 62.4%, indicating that the inhibitory effects of Cefazolin on monophenolase were concentration-dependent. The IC50 value for the monophenolase activity inhibited by Cefazolin was determined to be 7.0 mM.
Figure 2. Progress curves for the inhibition of monophenolase activity of mushroom tyosinase by Cefazolin. (a) The concentrations of Cefazolin for curves 1–6 were 0, 2.0, 4.0, 6.0, 8.0 and 10.0 mM, respectively. (b) Relationship of the steady-state rate with the concentrations of Cefazolin. (c) Relationship of the lag time with the concentrations of Cefazolin.
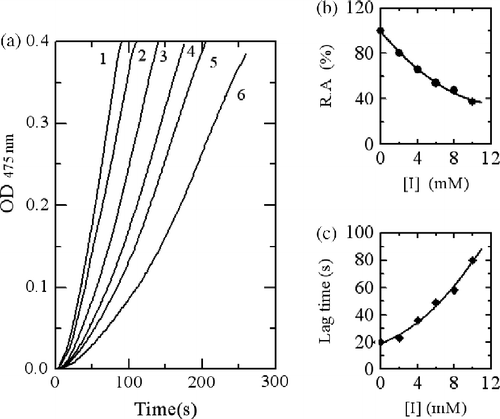
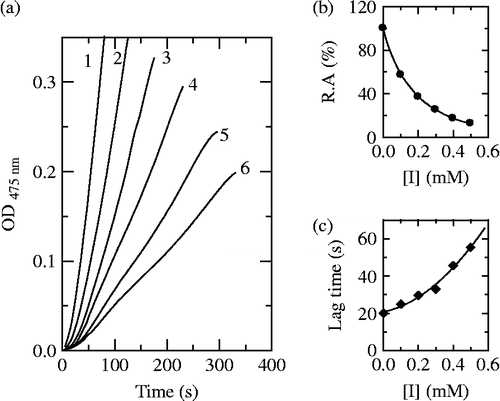
When using Cefodizime as inhibitor, the kinetic courses of the oxidation of the substrate are shown in . The results showed that the lag time and the steady-state rate of the enzyme for catalyzing the hydroxylation of L-tyrosine were also influenced as shown in , respectively. With increasing inhibitor concentration, the lag time changed from 20 seconds in its absence to 55.4 seconds in the presence of 0.5 mM. The steady state rate of monophenolase activity decreased by 87.4% at 0.5 mM of Cefodizime. The inhibitory effects of Cefodizime on monophenolase activity were also concentration-dependent. The IC50 value was determined to be 0.13 mM.
Figure 3. Progress curves for the inhibition of monophenolase activity of mushroom tyosinase by Cefodizime. (a) The concentrations of Cefodizime for curves 1–6 were 0, 0.1, 0.2, 0.3, 0.4 and 0.5 mM, respectively. (b) Relationship of the steady-state rate with the concentrations of Cefodizime. (c) Relationship of the lag time with the concentrations of Cefodizime.
Effect of Cefazolin and Cefodizime on the diphenolase activity of mushroom tyrosinase
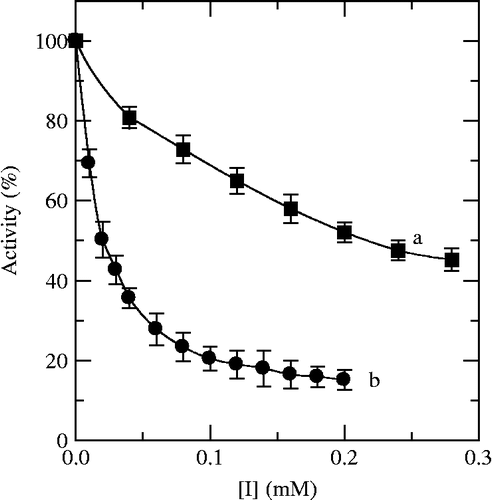
The assay to the diphenolase activity used L-DOPA as substrate. The progress curve of the enzyme reaction was a straight line passing through the origin without lag period. The formation of the product was in proportion to reaction time. The value of the slope of line indicated the diphenolase activity. Cefazolin and Cefodizime were tested for the effect on the oxidation of L-DOPA by mushroom tyrosinase. The result showed that both Cefazolin and Cefodizime can inhibit the diphenolase activity as shown in . With increasing the concentration of inhibitors, the diphenolase activity of mushroom tyrosinase decreased with concentration-dependence. From , the inhibitor concentration leading to 50% activity loss (IC50) of Cefazolin and Cefodizime were estimated to be 0.21 mM and 0.02 mM, respectively.
The mechanism of inhibition of mushroom tyrosinase by Cefazolin and Cefodizime
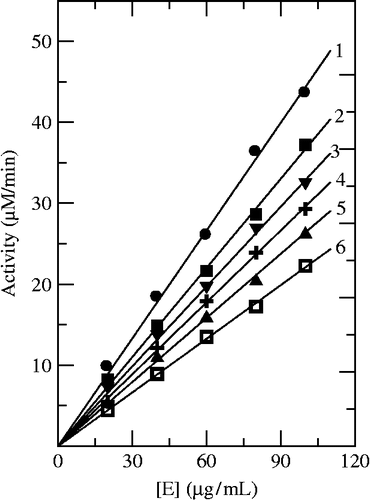
Using Cefazolin and Cefodizime as inhibitors, we studied their inhibition mechanism on the enzyme for oxidation of L-DOPA. Both inhibitors had the same behavior. shows the relationship of enzyme activity with the enzyme concentration in the presence of different concentrations of Cefazolin. The plots of the remaining enzyme activity versus the concentrations of enzyme in the presence of different concentrations of Cefazolin gave a family of straight lines, which all passed through the origin. Increasing the inhibitor concentration resulted in the lowering of the slope of the line, indicating that the inhibition by Cefazolin was reversible. The presence of inhibitor did not reduce the amount of enzyme, but just resulted in the inhibition of enzyme activity. Both Cefazolin and Cefodizime were reversible inhibitors of mushroom tyrosinase for oxidation of L-DOPA.
Inhibition of the diphenolase activity of mushroom tyrosinase by Cefazolin follows a competitive mechanism
The kinetic behavior of mushroom tyrosinase during the oxidation of L-DOPA was studied. Under the conditions employed in the present investigation, the oxidation reaction of L-DOPA by mushroom tyrosinase follows Michaelis-Menten kinetics. In the presence of Cefazolin, Lineweaver-Burk plots of the enzyme are shown in . the results showed that Cefazolin was a competitive inhibitor since increasing the concentration of Cefazolin resulted in a family of lines with a common intercept on the ordinate but with different slopes. The inhibition constant for the inhibitor binding the free enzyme, KI, was obtained from the secondary plot (inset in ) and summarized in .
Figure 6. Determination of the inhibitory type and inhibition constants of Cefazolin on mushroom tyrosinase. (a) Lineweaver-Burk plots of the diphenolase activity of mushroom tyrosinase inhibited by cefazolin. The concentrations of Cefazolin for curves 1–6 were 0, 0.04, 0.08, 0.12, 0.16 and 0.20 mM, respectively. (b) The plot of Km app. verus the concentration of Cefazolin to determine the inhibition constant.
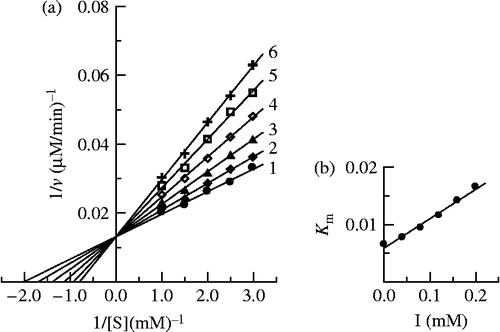
Table I. Inhibition constants of Cefodizime and Cefazolin with mushroom tyrosinase.
Inhibition of the diphenolase activity of mushroom tyrosinase by Cefodizime follows a competitive-uncompetitive mixed-type mechanism
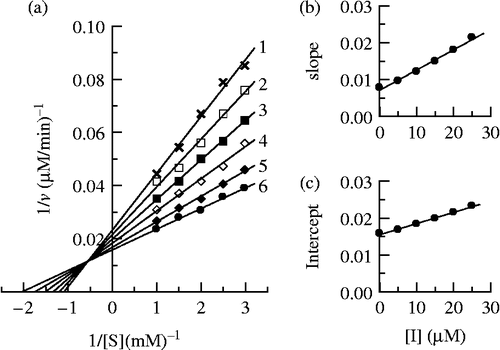
Using Cefodizime as inhibitor, the Lineweaver-Burk plot was showed in . The results illustrated in showed that Cefodizime was a mixed-type inhibitor since increasing its concentration resulted in a family of lines with different slope and intercept, but they were intersected one another in the second quadrant. This behavior is observed that the inhibitor can bind not only with free enzyme, but also with the enzyme-substrate complex, and their equilibrium constants are different. The inhibition constant for the inhibitor binding with the free enzyme (E), KI, was obtained from a plot of the slopes of the straight lines versus the inhibitor concentrations (). The inhibition constant for the inhibitor binding with the enzyme-substrate complex (ES), KIS, was obtained from a plot of the intercepts of the straight lines versus the inhibitor concentrations (). The values obtained are summarized in .
Figure 7. Determination of the inhibitory type and inhibition constants of cefodizime on mushroom tyrosinase. (a) Lineweaver-Burk plots of the diphenolase activity of mushroom tyrosinase inhibited by cefodizime. The concentrations of cefodizime for curves 1–6 were 0, 0.005, 0.01, 0.015, 0.02 and 0.025 mM respectively. (b) The plot of the slope versus the concentration of cefodizime. (c) The plot of the intercept versus the concentration of cefodizime.
Discussion
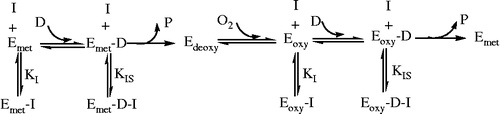
Tyrosinase is composed of four subunits and contains two binuclear coppers in its active sites per tetramer [Citation22]. In the process of catalysis, tyrosinase has three existing forms, Emet, Eoxy and Edeoxy [Citation23]. Both the Emet form and Eoxy form can catalyze the diphenol substrate (D); the Eoxy form can also catalyze the monophenol substrate (M) but the Emet form cannot. The Edeoxy form can combine with oxygen. If an inhibitor binds only with the free enzyme molecule, Emet and Eoxy form, to become Emet-I and Eoxy-I, respectively, it will be a competitive inhibitor for the diphenolase. If an inhibitor binds only with the enzyme–substrate complex (Emet-D form and Eoxy-D form) to become Emet-D-I and Eoxy-D-I, respectively, it will be an uncompetitive inhibitor for the diphenolase. This can be written as . From the results, it can be presumed that Cefazolin can only combine with free enzymes (Emet and Eoxy) and display a competitive mechanism. In contrast, Cefodizime can not only combine with free enzymes (Emet and Eoxy) but also combine with enzyme–substrate complexes (Emet-D or Eoxy-D), which displays a competitive and uncompetitive mixed type mechanism.
Both Cefazolin and Cefodizime are antibacterial drugs, which have been abroad used in prophylaxis and/or treatment of bacteria infection. In our investigation of tyrosinase inhibitors, they were found to have inhibitory effects on tyrosinase. These effects have not been reported previously in the literature. In the paper, we took L-DOPA as substrate for the diphenolase activity and L-Tyrosine for the monophenolase activity of the enzyme. The effects of Cefazolin and Cefodizime on the diphenolase activity and the monophenolase activity of tyrosinase were first studied. The results showed that both Cefazolin and Cefodizime could inhibit the diphenolase activity and the monophenolase activity of tyrosinase. For the monophenolase activity, both compounds could not only lengthen the lag period but also decrease the steady-state rate. As shown in , the IC50 values of Cefazolin and Cefodizime for the monophenolase activity were obviously discrepant. The former was about fifty-four times as larger as the latter, indicating that the inhibitory intensity of Cefodizime was stronger than that of Cefazolin on the monophenolase activities of the enzyme. From , With increasing inhibitor concentration, the lag time changed from 20 seconds in the absence of Cefodizime to 55.4 seconds in the presence of 0.5 mM Cefodizime. When Cefodizime concentration was 0.5 mM, the steady state rate of monophenolase activity descended by 87.4% (). However, if the lag time wanted to prolong to 55.4 seconds, the concentration of Cefazolin must reach to 7.2 mM, and at this concentration the steady state rate of monophenolase activity only descended by 52.0% (). For diphenolase activity, the inhibition of both compounds on the enzyme was displayed as reversible courses. But their inhibition types were very different. Cefazolin was competitive inhibitor and Cefodizime was competitive-uncompetitive one. The inhibitory intensity was also obviously discrepant. The IC50 value of Cefazolin for the diphenolase activity was about ten times as larger as that of Cefodizime (), indicating that the inhibitory intensity of Cefodizime was stronger than Cefazolin for the diphenolase.
Tyrosinase has two distinct catalytic functions: the hydroxylation of monophenol and the oxidation of o-diphenol. This can be written as . L-DOPA is an important neurotransmitter substance. Here, we report the effect of Cefazolin and Cefodizime on the hydroxylation of Tyr and the oxidation of L-DOPA by tyrosinase. For the Cefazolin, the IC50 value of the monophenolase activity was about thirty-three times as larger as diphenolase activity, the diphenolase activity descended by 50% when the concentration of Cefazolin was 0.21 mM. At this concentration, the monophenolase activity was almost not changed, which indicating that Cefazolin can inhibited conversion of an o-diphenol to the corresponding o-quinone but not inhibited conversion of tyrosine to 3,4-Dihydroxyphenylalanine at 0.21 mM. For Cefodizime, the IC50 value of the monophenolase activity was about six times as larger as diphenolase activity. It is suggested, therefore, that Cefazolin and Cefodizime might be used as an adjunctive therapy for treatment of hypopigmentation-related disorders, as well as L-DOPA-related disorders such as Parkinson′s disease.
Acknowledgements
The present investigation was supported by Grant 30570408 of the Natural Science Foundation of China, Grant 2007N0051 of the Science and Technology Foundation of Fujian Province and by the Program for Innovative Research Team in Science and Technology in Fujian Province University.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- DA Robb. In: R Lontie, editor. Copper proteins and copper enzymes. Boca Raton, FL: CRC Press; (1984). p 207–241.
- Y Xu, AH Stokes, WM Freeman, SC Kumer, BA Vogt, and KE Vrana. (1997). Mol Brain Res 45:159–162.
- A Palumbo, M D'Ischia, G Misuraco, and G Proto. (1991). Biochim Biophys Acta 1073:85–90.
- K Maeda, and M Fukuda. (1991). J Soc Cosmet Chem 42:361–368.
- Y Ohyama, and Y mishima. (1990). Fragrance J 6:53–57.
- C Robit, C Rouch, and F Cadet. (1997). Food Chem 59:355–360.
- JC Espín, and HJ Wichers. (1999). J Agric Food Chem 47:2638–2644.
- LP Xie, QX Chen, H Huang, HZ Wang, and RQ Zhang. (2003). Biochem (Moscow) 68:487–491.
- V Kahn, N Ben-Shalom, and V Zakin. (1997). J Agric Food Chem 45:4460–4465.
- KK Song, QX Chen, Q Wang, L Qiu, and H Huang. (2005). J Enz Inhib Med Chem 20 (3):239–243.
- JC Espin, and HJ Wichers. (2001). Biochim Biophys Acta 1544:289–300.
- A Andrawis, and V Khan. (1986). Biochem J 235:91–96.
- AM Mayer, and E Harel. In: PF Fox, editor. Food enzymology. London, UK: Elsevier; (1991). p 373–398.
- V Khan, and A Andrawis. (1985). Phytochemistry 24:905–908.
- Q Wang, L Qiu, XR Chen, KK Song, Y Shi, and QX Chen. (2007). Bioorgan Med Chem 15:1568–1571.
- Y Shi, QX Chen, Q Wang, KK Song, and L Qiu. (2005). Food Chem 92:707–712.
- KK Song, QX Chen, Q Wang, L Qiu, and H Huang. (2005). J Enz Inhib Med Chem 20 (3):239–244.
- L Qiu, QX Chen, Q Wang, H Huang, and KK Song. (2005). Bioorgan Med Chem 13:6206–6211.
- QX Chen, KK Song, Q Wang, and H Huang. (2003). J Enz Inhib Med Chem 18:491–496.
- M Jiménez, S Chazarra, J Escribano, J Cabanes, and F García-Carmona. (2001). J Agric Food Chem 49:4060–4063.
- QX Chen, and I Kubo. (2002). J Agric Food Chem 50:4108–4112.
- JN Ridríguez-López, LG Fenoll, and PA García-Ruiz. (2000). Biochem-US 39:10497–10506.
- A Sanchez-Ferrer, JN Rodriguez-Lopez, and F Garcia-Canovas. (1995). Biochim Biophys Acta 1247:1–11.