Abstract
The protein glycation inhibitory activity of ethanolic extract of Lawsonia inermis (henna) plant tissues was evaluated in vitro using the model system of bovine serum albumin and glucose. Protein oxidation and glycation are posttranslational modifications that are implicated in the pathological development of many age-related disease processes. This study investigated the effects of Lawsonia inermis ethanolic extract and its components, on protein damage induced by a free radical generator in in vitro assay system. We found that alcoholic extract of Lawsonia inermis can effectively protect against protein damage and showed that its action is mainly due to Lawsone. In addition, the presence of gallic acid also plays an important role in the protective activity against protein oxidation and glycation. Two known compounds, namely, Lawsone and gallic acid previously isolated from this plant were subjected to glycation bioassay for the first time. It was found that the alcoholic extract, lawsone (1) and gallic acid (2) showed significant inhibition of Advanced Glycated End Products (AGEs) formation and exhibit 77.95%, 79.10% and 66.98% inhibition at a concentration of 1500 μg/mL, 1000 μg/mL and 1000 μM respectively. Lawsonia inermis, compounds 1 and 2 were found to be glycation inhibitors with IC50 82.06 ± 0.13 μg/mL, 67.42 ± 1.46 μM and 401.7 ± 6. 23 μM respectively. This is the first report on the glycation activity of these compounds and alcoholic extract of Lawsonia inermis.
Introduction
The accumulation of the reaction products of protein glycation (nonenzymatic reaction of proteins with glucose and other reducing sugars) in living organisms leads to structural and functional modifications of tissue proteins. Many studies have shown a significant role for glycation in the progress of normal ageing and the pathogenesis of age-related diseases, such as diabetes, atherosclerosis, end-stage renal disease, rheumatoid arthritis, and neurodegenerative diseases [Citation1]. The present study was undertaken to find out the glycation inhibitory activity of the alcoholic suspension of Lawsonia Inermis and its constituents.
Lawsonia inermis is a shrub of Asian origin, indigenous to Egypt, Arabia and India [Citation2]. It is cultivated in many tropical countries and warm temperate region as a hedge plant. In the West and the Middle East, the leaves are utilized locally for cosmetic purposes [Citation3]. It highlights hair especially as tint for hands, feet and nails Citation4–6 and is also employed as a deodorant. It is an excellent conditioning agent.and used as an ingredient in shampoos, hair dyes, conditioners and rinses. The dye is also used to stain leather and hides in various dye industries for commercial use.
Medicinally, L. inermis is used as an astringent, antihemorrhagic, intestinal antineoplastic, cardio-inhibitor, hypotensive, sedative, anti-inflammatory and antioxidant [Citation7,Citation8]. It has also been used as a folk remedy against amoebiasis, soothe fevers, headache, jaundice, hysteria, nervous disorder and leprosy Citation4, Citation9–12. Henna extracts show antibacterial, antifungal and ultraviolet light screening activity. It has exhibited antifertility activity in animals and may induce menstruation. In Malaysia, the leaf decoction is used after child birth and for beri-beri, rheumatism, skin disorders, stomach disorders and venereal disease [Citation10]. Some of the tribals are using this plant leaves in a lower concentration for body pain, skin infections to reduce the lesions after bee sting, allergy, infections, inflammations [Citation13]. L. inermis leaves are used as a remedy in skin diseases in the form of paste or decoction against boils burns, bruises and skin inflammation. Leaves in the form of paste are used as external application in head ache and rubbed over the soles of the burning feet. Decoction of the leaves is used as gargle in sore throat [Citation14]. Crude extract of L. inermis (Linn) leaves shows dose dependent analgesic, antipyretic effect in rats [Citation15]. Leaves are also useful to bring down the severity of many medical problems like dysentery, diseases of the spleen, lumbago, bronchitis and syphilitic eye infection [Citation16,Citation17]. Lawsonia shows anticancer against sarcoma 180 in mice and walker 256 carcinosarcoma.
A number of components such as lawsone [Citation18], xanthones [Citation19,Citation20], isoplumbagin, phenolic glycosides, coumarins, gallic acid [Citation21], xanthones, quinoids, organo-chlorine compounds, β-sitosterol glucosides, flavonoids including luteolin and its 7-O-glycosides, fats, resinm, henna-tanin. and triterpenoids [Citation22,Citation23] have been isolated from various parts of the plant. The leaf was found to contain thirty-six components, which constituted 80.4% of the oil, were identified. The major components were ethyl hexadecanoate (24.4%), (E)-methyl cinnamate (11.4%), isocaryophyllene (8.1%), (E)-β-ionone (5.8%) and methyl linolenate (4.1%). The flower oil was found to contain among other secondary metabolites, (Z)-2-hexenol, linalool and β-ionone [Citation24]. The commercial essential oil comprised of mainly α-terpineol, terpinolene, δ-3-carene and γ-terpineol [Citation25]. Lawsonia leaves contains a coloring compound 2-OH-1, 4 naphthaquinone (Lawsone) 1 in higher concentration [Citation26] which has proved to have analgesic, genotoxiCIT000y [Citation27], anti inflammatory and antipyretic effects in rat models [Citation16] and also some inhibitory effect against Proteus and Staphylococcus aureus [Citation28]. Gallic acid slightly inhibits Streptococcus aureus. It contributes to significant inhibition of colon, esophageal, liver, lung, tongue and skin cancers. Based on these observations, compounds 1, 2 and alcoholic extract of the aerial parts of Lawsonia inermis were screened to determine the possible antiglycation activities.
Materials and methods
General experimental procedures
The mass spectra were recorded on a Jeol HX-110 instrument. The 1H and 13C NMR spectra were recorded in CDCl3 at 500, 400 and 125, 75 MHz, respectively, on a Bruker AM-500, 400 NMR spectrometer. The UV and IR spectra were recorded on Shimadzu UV-240 and JASCO A-320 spectrophotometers, respectively. Optical rotations were measured on a polatronic D Polarimeter. The purity of the compounds was checked on TLC (Si-gel, Merck PF254, 0.25 mm thickness). Melting points were determined in glass capillary tubes using a Buchi 535 and a Gallenkamp 30/MF-370 melting point apparatus. Bovine serum albumin (BSA) was purchased from Research Organics Cleveland, while others chemicals {glucose anhydrous, trichloroacetic acid sodium azide, dimethyl sulfoxide, sodium dihydrogen phosphate, sodium chloride, disodium hydrogen phosphate, potassium chloride, potassium dihydrogen phosphate, and sodium hydroxide} were purchased from Sigma Aldrich. Sodium phosphate buffer (pH 7.4), was prepared by mixing Na2HPO4 and NaH2PO4 (67 mM) containing sodium azide (3 mM), phosphate buffer saline (PBS) pH 10 was prepared by mixing NaCl (137 mM) + Na2HPO4 (8.1 mM) + KCl (2.68 mM) + KH2PO4 (1.47 mM). pH 10 was adjusted with NaOH (0.25 mM), while BSA (10 mg/mL) and anhydrous glucoses (50 mg/mL) solutions were prepared in sodium phosphate buffer.
Plant material
L. inermis leaves were collected in summer (April and May) locally and identified by a taxonomist, Mr. Abid Askari, PSO at the Botany Section of PCSIR Laboratories Complex, Karachi. After the species identification was done, Coimbatore were allowed to dry in open air in the shaded area for few weeks. Air dried L. inermis leaves were powdered mechanically. At first the dried leaf powder was tested for the presence of contamination before and after autoclaving.
Extraction and isolation
Air-dried powdered aerial parts of L. inermis (20 kg dry weight) were extracted with EtOH (100 L). The EtOH extract was concentrated to a gum (822 gm), dissolved in distd. water and extracted thoroughly with pet. ether (45 L). The pet. ether soluble portion was evaporated under reduced pressure to yield a gum (66.92 gm).
The remaining aqueous layer was acidified with acetic acid to pH 3, and then extracted with CHCl3. The remaining aqueous acidic layer was made alkaline with NH4OH to pH 12 and extracted with CHCl3 (40 L). The CHCl3 soluble portion was dried as a crude mixture (74.96 gm), which was chromatographed on a si-gel column (Merck, 70–230 mesh, 2015.01 gm). Elution of this column with 96% CHCl3-MeOH (15 L) yielded an impure mixture (11.02 gm, Fr.20–26, each fraction 500 ml each) containing compounds 1 and 2. This mixture was chromatographed on a SiO2 gel column (2.5 cm × 70 cm, Merck, 70–230 mesh, 322.11 gm) which was first eluted with CHCl3. and then with 5:95 MeOH: CHCl3. Fractions from 55–90 (500 ml each), 3.96 gm obtained with 5:95 MeOH: CHCl3 (3 L) were again subjected to CC over silica gel (70–230 mesh size, 99.24 gm). The column (1.5 cm × 50 cm) was initially eluted with CHCl3-MeOH (96:4, 9 L) to afford eighteen fractions. These were combined and further purified by repeated TLC plates (Merck, PF 254, 0.5 mm) using CHCl3: MeOH (92:8) to afford 1 (18.13 mg, 9.0 × 10− 5% yield with Rf = 0.32). Fractions 90–96 obtained on elution with 80:20, 3.5 L, CHCl3-MeOH contain 2, which was purified by preparative tlc plates using 85:15 CHCl3-MeOH (20 mg, 1.0 × 10− 4% yield with Rf = 0.47).
Lawsone (1): Yellow crystals, M. P. 191–193°C, EIMS: m/z 174.15, UV/vis (MeOH), IR (CHCl3), 1H NMR (500 MHz, CDCl3): δ 13C NMR (125 MHz, CD3OD) δ: Citation29–33.
Gallic acid (2): White powder, EIMS: m/z 170.12, UV/vis (MeOH), IR (CHCl3), 1H NMR (500 MHz, CDCl3): δ: 13C NMR (125 MHz, CD3OD) δ Citation29–33.
In vitro glycation inhibition assay
Sample preparation
Samples were prepared in DMSO for plant extracts (1 mg /mL), for pure compounds (2 mM).
Non-enzymatic glycation of protein
According to the method of Vinson and Howard, bovine serum albumin (BSA) (10 mg/ml) in 40 μL phosphate buffer, the reaction mixture was mixed with an aqueous solution of test sample. Six concentrations were prepared for Lawsonia inermis alcoholic extract, its components lawsone and gallic acid. After incubating at 37°C for two weeks, the fluorescent reaction products were assayed on a fluoresence spectrophotometer with an exCIT000ation wavelength of 370 nm and an emission wavelength of 340 nm. The data were expressed in terms of percentage inhibition, calculated from a control measurement of the flouresence intensity of the reaction mixture with no test sample.
Methodology
In 96-well plate assays, each well contain 60 μL reaction mixtures (20 μL BSA (10 mg/mL+20 μL of glucose anhydrous (50 mg/mL)+20 μL test sample) [Citation34]. Glycated control contain 20 μL BSA+20 μL glucose+20 μL sodium phosphate buffer (pH 7.4, 67 mM), while blank control contains 20 μL BSA and 40 μL sodium phosphate buffer. Reaction mixture was incubated at 37°C for 7-days [Citation35]. After incubation, 6 μL (100%) of TCA was added in each well and centrifuged (15,000 rpm) for 4 minutes at 4°C [Citation36]. After centrifugation, the pellets were rewashed with 60 μL (10%) of TCA [Citation36]. The supernatant containing glucose, inhibitor and interfering substance was removed and pellet contains AGE-BSA were dissolved in 60 μL PBS [Citation28]. Assessment of fluorescence spectrum (ex. 370 nm), and change in fluorescence intensity (ex. 370 nm to em. 440 nm) based on AGEs were monitored by using a spectrofluorimeter (RF-1500, Shimadzu, Japan) [Citation1]. % Inhibition was calculated through the following formula.
Results and discussion
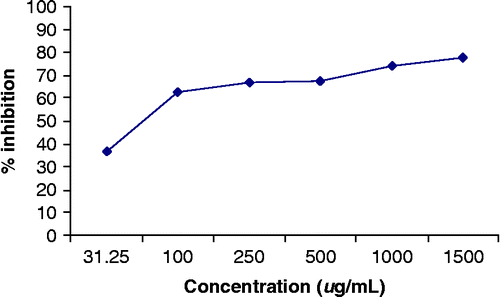
In this study we tested Lawsonia inermis plant tissue and its two constituents Lawsone (1) and gallic acid (2) for their inhibitory activity on protein glycation. Lawsone (1) was isolated as a yellow crystalline compound from the alcoholic extract of leaves of Lawsonia inermis by column and thin layer chromatography. Compound 1 (C10H6O3) was identified by comparison of its data with those reported earlier [Citation20]. 1H-NMR and 13C-NMR of compound 1 were the same as reported previously Citation29–33. Compound 2(C7H6O5) was isolated as white powder from alcoholic extract of the same plant. It was identified by comparison of its data with those reported earlier, which was originally isolated from Onosma hispidum. The structures were established by spectroscopic studies Citation29–33, Citation37–46. shows the effect of Lawsonia inermis extract and its components on Advance Glycated ends Product (AGEs) formation after incubation for 2 weeks. L. inermis alcoholic extract inhibited the reaction 77.95%, 74.28%, 67.32%, 66.62%, 62.62% and 36.67%, significantly at concentrations of 1500, 1000, 500, 250, 100 and 31.25 μg/mL respectively and sustained dose dependency. Its IC50 value was 82.06 ± 0.13 μg/mL ().
Table I. In vitro quantitative inhibition of glycation by compounds 1, 2 and an alcoholic extract of Lawsonia inermis.
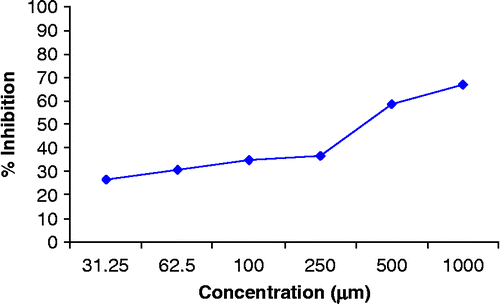
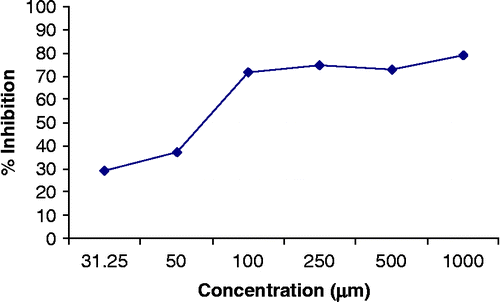
Compound 1 inhibited the reaction 79.1%, 72.9%, 74.7%, 72.0%, 37.25% and 29.6%, () significantly at concentrations of 1000, 500, 250, 100, 50 and 31.25 μM/ml respectively and sustained dose dependency. Its IC50 value was 67.42 ± 1.46 μM. Compound 2 inhibited the reaction 66.98%, 58.83%, 36.51%, 34.88%, 30.62% and 26.87%, significantly at concentrations of 1000, 500, 250, 100, 62.5 and 31.25 μM/ml respectively () and sustained dose dependency. Its IC50 value was 401.7 ± 6.23 μM.
The most active compound was lawsone. Its IC50 value was 67.42 ± 1.46 μM. and approched that of Rutin at 41.9 ± 2.3 μM. L. inermis (leaves) was the second most bioactive extract followed by gallic acid. The IC50 values of these were above 67 μM.
Lawsonia inermis, compound 1 and 2 exhibited in vitro glycation activity with IC50 82.06 ± 0.13 μM, 67.42 ± 1.46 μM and 401.7 ± 6. 23 μM respectively. Rutin (IC50 41.9 ± 2.3 μM) was used as a positive control [Citation1]. The antioxidant activities of Lawsonia inermis and compounds 1 and 2 were earlier reported through pharmacological methods. The glycation inhibitory activity was significantly correlated with the antioxidative potency of the extracts. There is growing interest in natural products with combined anti-glycation and antioxidant properties as they may have reduced toxiCIT000y. The positive glycation inhibitory and antioxidant activity of this plant might suggest a possible role in targeting ageing and diabetic complications.
Acknowledgements
The authors wish to thank the Ministry of Science and Technology, Government of Pakistan for providing the financial support for the current study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- YK Hye, and K Kyong. (2003). Protein glycation and antioxidative activity of some plant extracts in vitro. J Agric Food Chem 51:1586–1591.
- D Amelio, and FS Botanicals. A phytocosmetic desk reference. Boca Raton: CRC Press; (1999). p 126–135.
- HC Ong, and J Norzalina. (1997). 70:10–14. Malay Herbal Medicine in Gemencheh, Negri, Sembilan, Malaysia. Fitoterapia
- RWJ Keay. Trees in Nigeria. Oxford: Clarendon Press; (1989). p 50.
- DJ Mabberley. The plant book. London: Cambridge University Press; (1990) 318.
- HH EI-Kamali, and SA Khalid. (1996). 68:301–366. The Most Common Herbal Remedies in Central Sudan. Fitoterapia
- BH Ali, AK Bashir, and MO Tanira. (1995). Anti-inflammatory, antipyretic, and analgesic effects of Lawsonia inermis L. (henna) in rats. Pharmacology 51 (6):356–363.
- BR Mikhaeil, FA Badria, GT Maatooq, and MM Amer. (2004). Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch [C] 59 (7–8):468–476.
- Bep-Oliver-Bever. Medicinal plants in tropical west Africa. London: Cambridge University Press; (1986). p 53.
- JM Dalziel. The useful plants of west tropical Africa. London: Crown Agents; (1937). p 40–41.
- HM Burkill. (1995). The Useful Plants of West Tropical Africa. Families J-L, Royal Botanic Gardens 3:562–564.
- A KI-Merzabani, AA EI-Hazer, MA Attia, AK Al Duweini, and AM Ghazal. (1976). Screening system for Egyptian plants with potential anti-tumor activity. Planta Med 36:150–155.
- A Zargari. Medicinal plants. Vol.2 Tehran University Publication; (1991). p 353–358.
- UN Dhur. Chopra's indigenous drugs of India, 2nd ed. Calcutta: Dhur UN and sons Pvt. Ltd; New Delhi, (1958). p 48–55.
- WC Evans. Trease and evans pharmacognosy. 4th ed. London: WB Saunders Co. Ltd.. (1996). p248.
- PK Warrier, VP Nambiar, and C Ramankutty. editors. Indian medicinal plants, compoundium of 500 species. Chennai: Orient Longmann Pvt. Ltd.. (1995). p 303–306.
- JA Duke. Handbook of medicinal herbs. Haemolytic activity and nephrotoxiCIT000y of 2-hydroxy-1,4-naphthoquinone in rats. Boca Raton: CRC Press; (1985). p 274.
- DK Bhardway, R Murari, TR Seshadri, and R Singh. (1976). Lacoumarin from Lawsonia inermis. Phytochemistry 15:1789–1792.
- DK Bhardway, TR Seshadri, and R Singh. (1977). Xanthones from Lawsonia inermis. Phytochemistry 16:1616–1617.
- DK Bhardway, RK Jain, BC Jain, and CK Mehta. (1978). 1-Hydroxy-3,7-dimethoxy-6-acetoxyxanthone, A new xanthone from Lawsonia inermis. Phytochemistry 17:1440–1441.
- RH Stanley, and AF Gilbert. Hair dyes and hair- dyeing chemistry and technique. London: Great Britain Whitefriars press Ltd; (1939). p 72–77.
- G Sarita, A Mohammed, AM Sarwar, N Masatake, and S Tatsuko. (1994). Isolation and characterization of a dihydroxysterol from Lawsonia inermis. Nat Prod Lett 4:195–201.
- G Handa, A Kapil, S Sharma, and J Singh. (1977). Lawnermis acid: A new anticomplementary triterpenoid from Lawsonia inermis seeds. Indian J Chem VB (36B):252–256.
- KC Wong, and YE Teng. (1995). Volatile components of Lawsonia inermis L Flowers. J Essent oil Res 7:425–428.
- VJ Reichling, M Harkenthal, H Brandt, and C Bayerl. (1999). Temporare HennaTaottoos. Deut Apoth Ztg 33:35–41.
- K Bhuvaneswari, S Gnana Poongothai, A Kuruvilla, and B Appala Raju. (2002). Inhibitory concentrations of lawsonia innermis dry powder for urinary pathogens. Indian Journal of Pharmacology 34:260–263.
- D Kirkland, and D Marzin. (2003). An assessment of the genotoxiCIT000y of 2-hydroxy-1, 4-naphthoquinone, the natural dye ingredient of Henn. Mutat Res 537 (2):183–199.
- DK Pandy, H Cahandra, AR Sexena, and SN Dixit. (1982). Indian Drugs 19:459.
- Y Yan, HW Guo, WP Jian, KE Yang, SL Tong, CG Fang, Q Li, and X Chang. (2004). Catalytic Conversion of 2-naphthol to 2-hydroxy-1,4-naphthoquinone under mild conditions. Chinese Journal of Chemistry 22:487–491.
- R Morris, J Nagarkatti, J Heaton, and C Lane. Aldrich catalog handbook of fine chemical 1996–1997. Aldrich Chemical Co., Inc.; (1996). p 829.
- YX Zhao, and XY Sun. Spectrum analysis and structure identifying of organic compounds (Chinese). ed. I Chinese Science and Technology University Press; (June 1992) p 315–325.
- CJ Pouchert. The Aldrich library of infrared spectra. ed. III. Aldrich Chemical Company, Inc, (1981) p 897A.
- RM Silverstein, GC Bassler, and TC Morrill. Spectrometric identification of organic compounds. ed. 3 John Wiley; (1977) p 65.
- N Takako, Y Takako, T Katsutoshi, S Seui, and RJ Lekh. (2002). J Agric Food Chem 50:2418–2422.
- Y Fumio, A Toshiaki, Y Yoshihiro, and N Hiroyuki. (2000). Antioxidative and anti- glycation activity of Garcinol from Garcinia indica fruit rind. J Agric Food Chem 48:180–185.
- M Nobuyasu, A Tadashi, S Chihiro, K Hiroyuki, O Mitsuharu, H Junichi, and U Makoto. (2002). Screening system for the Maillard reaction inhibitors from natural product extracts. J Health Sci 48 (6):520–526.
- Atta-ur-Rahman, N Sultana, S Jahan, and MI Choudhary. (2005). Studies on the constituents of Commiphora mukul. Z Naturforsch 60b:1202–1206.
- Atta-ur-Rahman, N Sultana, S Jahan, and MI Choudhary. (1998). Phytochemical studies on Skimmia laureola. Nat Prod Lett 12 (3):223.
- K Fatima, and N Sultana. (2003). Studies on bioassay directed antifungal activity of medicinal plants of Skimmia laureola, Calotropis procera and P. pterocarpum. J Chem Soc Pak 25:328.
- Atta-ur-Rahman, N Sultana, MI Choudhary, PM Shah, and MR Khan. (1998). Isolation and structural studies on the chemical constituents of Skimmia laureola. J Nat Prod 61 (6):713.
- Atta-ur-Rahman. Nuclear magnetic resonance spectroscopy, basic principles. New York: Springer-Verlag; (1986) 227.
- Atta-ur-Rahman. One-and two-dimentional NMR spectroscopy. Amsterdam: Elsevier Science Publishers; (1989) p 406.
- N Sultana, Atta-ur-Rahman, and A Khalid. (2006). New natural cholinesterase inhibiting and calcium channel blocking quinoline alkaloids. J Enz Inhib Med Chem 21 (6):703.
- N Sultana, Atta-ur-Rahman, A Khalid. A new fatty ester and a new triterpene from Skimmia laureola Nat Prod Res 2008., (in press).
- N Sultana, Atta-ur-Rahman, and TH Khan. (2005). Tyrosinase inhibitor fatty ester and a quinoline alkaloid from Skimmia laureola. Z Naturforsch B 60:1186.
- Atta-ur-Rahman, and N Sultana. (1997). Phytochemical studies on Adhatoda vasica. Nat Prod Lett 10:249.