Abstract
A series of N-{2-[4-(substituted)piperazin-1-yl]-2-oxoethyl}acetamides were synthesized as prospective novel atypical antipsychotic agents. Microwave irradiation of acetyl glycine (I) with substituted piperazines in the presence of DCC in DMF for about 3-5 min gave the titled compounds (P:1-7). All the synthesized compounds were screened for their in vivo pharmacological activity in Swiss albino mice. D2 antagonism studies were performed using the climbing mouse assay model and 5-HT2A antagonism studies were performed using quipazine induced head twitches in mice. Among the synthesized compounds P4 was found to be the most active compound.
Introduction
Schizophrenia is a devastating psychiatric illness that affects approximately 1% of the world population irrespective of ethnic, economic or cultural boundaries [Citation1]. The disorder is characterized by the presence of positive symptoms such as hallucinations, disorganized thoughts, delusions and irrational fears and negative symptoms, including social withdrawal, diminished affect, poverty of speech, lack of energy and the inability to experience pleasure. In addition, schizophrenic patients may suffer cognitive deficits viz., impaired attention, verbal fluency, memory recall and executive function [Citation2].
Following the introduction of chlorpromazine for treatment of schizophrenia in the 1950s, a large number of neuroleptic (or typical antipsychotic) drugs were developed for the treatment of schizophrenia. All typical antipsychotic drugs are potent D2 dopamine receptor antagonists [Citation3,Citation4].
Although typical antipsychotic drugs are quite effective at reducing the positive symptoms of schizophrenia, they are ineffective towards negative and cognitive symptoms and cause serious side effects such as acute and tardive dystonia, acute and chronic akathisia, tardive dyskinesia, elevation of serum prolactin levels and neuroleptic malignant syndrome [Citation5]. Clozapine was the first drug discovered in 1958 whish was devoid of extra pyramidal side effects and cured both negative and cognitive symptoms apart from curing positive symptoms [Citation6]. This finding of the unequivocal antipsychotic efficacy in the absence of extrapyramidal symptoms led to the concept of ‘atypical’ antipsychotics.
The finding that clozapine is a potent serotonin 5-HT2A receptor antagonist, made researchers come up with molecules having both 5-HT2A and D2 antagonism. With an exception of quetiapine and amisulpride all other atypical antipsychotics such as olanzapine, risperidone, sertindole, iloperidone, ziprasidone, and aripiprazole are potent 5-HT2A and D2 antagonists [Citation7].
But these molecules are also not completely devoid of side effects. Side effects caused by atypical antipsychotics are a result of their significant binding affinity to numerous receptors other than required for atypical antipsychotic activity. Side effects associated with these drugs include weight gain (Serotonergic 5-HT2C and Histaminic H1 receptors blockade), postural or orthostatic hypotension, sedation, dizziness (α1-adrenergic blockade), somnolence (Histaminic H1 receptor blockade), seizures (Muscarinic receptor blockade), new-onset type2 diabetes mellitus, exacerbation of pre-existing type2 diabetes mellitus, hyperlipidemia (increase of triglycerides and leptin, a lipid regulatory hormone), atropine like side effects such as dry mouth, constipation, urinary retention (Muscarinic M1 receptor blockade), cardiac ventricular arrhythmias (prolongation of QTC interval due to the blockade of Ikr channels), myocarditis, insomnia, headache and other possible secondary cardiovascular complications [Citation8].
In the etiology of schizophrenia NMDA receptors hypofunction is also reported [Citation9,Citation10]. It is also reported that agonists at this site will alleviate the negative and cognitive symptoms. Direct agonists of the NMDA receptor, however, may not be feasible candidates in this regard, because of the propensity of such drugs to produce excessive excitation and seizures. Glycine is a positive allosteric modulator and obligatory co-agonist at the NMDA receptor [Citation11], hence the glycine site agonists, including glycine, D-cycloserine and D-serine, appear to be effective in reducing negative symptoms and cognitive impairment in patients with schizophrenia [Citation12,Citation13].
The poor penetration of the blood–brain barrier by glycine, and the partial agonistic properties of D-cycloserine, appears to make these agents less than optimal for providing pharmacological agonism of the glycine regulatory site on the NMDA receptor [Citation13]. Hence we increased the lipophilicity of glycine by attaching various substituted piperazines at carboxy terminal and prepared these novel compounds for the treatment of schizophrenia.
Experimental
Chemistry
Melting points were determined in open capillaries using Büchi 530 melting point apparatus without correction. The reactions were monitored and the purity of the compounds checked by ascending thin layer chromatography (TLC) using silica gel coated aluminium plates (Merck 60 F254, 0.25 mm) and the spots were visualized under ultra violet light at 254 and 366 nm. The microwave assisted procedures were carried out in a LG microwave oven specially designed for organic synthesis operating at a maximum power of 1000 W. Infra red (IR) spectra were recorded in KBr pellets on Jasco IR Report-100 or Schimadzu IR Prestige-21 FT-IR spectrophotometer (cm− 1). 1H-NMR spectra were obtained from Bruker DRX300 spectrometer using tetramethylsilane (TMS) as internal standard [chemical shifts in δ, parts per million (ppm)], mass spectra on a VG-70-S mass spectrometer and elemental analysis (C, H, N) on a Perkin Elmer 2400 CHN elemental analyzer.
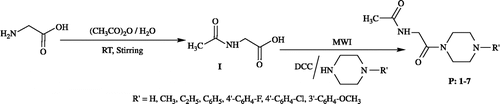
The synthetic route to the required compounds is outlined in . For the synthesis of the titled compounds, acetyl glycine required as starting material was prepared by acetylating glycine at room temperature. Microwave irradiation of acetyl glycine with substituted piperazines in the presence of DCC in DMF for about 3–5 min gave the corresponding piperazinyl derivatives (P: 1-7).
General procedure for synthesis of the compounds
(Acetylamino)acetic acid or acetyl glycine (I)
Literature procedure [Citation14] was prepared according to the % Yield: 84% (19.6 g); Melting Point: 206°C (Lit. M. P. 207-208°C [Citation14]).
N-{2-[4-(substituted)piperazin-1-yl]-2-oxoethyl}acetamide (P: 1-7)
A mixture of 0.0085 mol of acetyl glycine (I), 0.01 mol of substituted piperazine, 1.94 g (0.0094 mol) of DCC and 3 mL of DMF was taken in a 100 mL Erlenmeyer flask and subjected to microwave irradiation at 80% power output (800 watt) for about 4 min. Once the reaction showed completion on TLC (9: 1 chloroform, methanol as mobile phase), the reaction mixture was poured into ice-water mixture and the precipitated solid was filtered, dried and recrystallized using suitable solvent to afford pure P: 1-7.
N-(2-oxo-2-piperazin-1-ylethyl)acetamide (P1)
% Yield: 86% (1.35 g); Melting Point: 216-217°C; Recrystallization solvent: ethanol. Molecular Weight: 185; IR (KBr) cm− 1: 3360 (N-H stretch); 2850, 2735 (aliphatic C-H stretch); 1642 (C = O stretch); 1250 (aliphatic C-N stretch). 1H NMR (CDCl3) (δ) ppm: 1.78 (s, 1H, NH); 2.12 (s, 3H, COCH3); 2.45-2.63 (t, 4H, N4(CH2)2); 3.10-3.21 (t, 4H, N1(CH2)2); 4.62(s, 2H, COCH2NH); 5.35 (s, 1H, NH); 6.35 (s, 1H, COCH2NH). Mass (FAB, M+): Calculated: 185.1128; Found: 185.219.
N-[2-(4-methylpiperazin-1-yl)-2-oxo-ethyl]acetamide (P2)
% Yield: 75% (0.80 g); Melting Point: 190-192°C; Recrystallization solvent: ethanol. Molecular Weight: 199; IR (KBr) cm− 1: 3345 (N-H stretch); 2890, 2751, 1372 (aliphatic C-H stretch); 1721, (C = O stretch); 1154 (aliphatic C-N stretch). 1H NMR (CDCl3) (δ) ppm: δ 2.07 (s, 3H, COCH3); δ 4.54 (s, 2H, COCH2NH); δ 2.59-2.74 (t, 4H, N4(CH2)2); δ 3.06-3.29 (t, 4H, N1(CH2)2); δ 2.37 (s, 3H, CH3). Mass (FAB, M+): Calculated: 185.1128; Found: 185.1643.
N-[2-(4-ethylpiperazin-1-yl)-2-oxo-ethyl]acetamide (P3)
% Yield: 89% (1.50 g); Melting Point: 208-210°C; Recrystallization solvent: ethanol. Molecular Weight: 213; IR (KBr) cm− 1: 3349 (N-H stretch); 2858, 2752 (aliphatic C-H stretch); 1645 (C = O stretch); 1252 (aliphatic C-N stretch). 1H NMR (CDCl3) (δ) ppm: 1.28 (t, 3H, CH2CH3); 2.02 (s, 3H, COCH3); 2.35 (q, 2H, CH2CH3); 2.45-2.60 (t, 4H, N4(CH2)2); 3.12-3.26 (t, 4H, N1(CH2)2); 4.45 (s, 2H, COCH2NH); 6.27 (s, 1H, COCH2NH). Mass (FAB, M+) Calculated: 213.1627; Found: 213.1577.
N-{2-[4-(4-chlorophenyl)piperazin-1-yl]-2-oxoethyl}acetamide (P4)
% Yield: 69% (0.75 g); Melting Point: 188-189°C; Recrystallization solvent: ethanol. Molecular Weight: 295.5; IR (KBr) cm− 1: 3360 (N-H stretch); 3072, 3030 (aromatic C-H stretch); 2850, 2735, 1365 (aliphatic C-H stretch); 1558, 1497 (aromatic C = C stretch); 1720, (C = O stretch); 1150 (aliphatic C-N stretch); 710 (C-Cl stretch). 1H NMR (CDCl3) (δ) ppm: δ 2.04 (s, 3H, COCH3); δ 4.35 (s, 2H, COCH2NH); δ 2.55-2.68 (t, 4H, N4(CH2)2); δ 3.06-3.24 (t, 4H, N1(CH2)2); δ 6.53-7.39 (m, 4H, Ar-H). Mass (FAB, M+) Calculated: 295.1117; Found: 295.1182. Mass (FAB, M++2) Calculated: 295.1324; Found: 297.1293.
N-{2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl}acetamide (P5)
% Yield: 74% (0.74 g); Melting Point: 214-216°C; Recrystallization solvent: methanol. Molecular Weight: 279; IR (KBr) cm− 1: 3355 (N-H stretch); 3062, 3037 (aromatic C-H stretch); 2878, 2744 (aliphatic C-H stretch); 1646 (C = O stretch); 1559, 1507 (aromatic C = C stretch); 1256 (aliphatic C-N stretch); 1110 (C-F stretch). 1H NMR (CDCl3) (δ) ppm: 2.12 (s, 3H, COCH3); 2.48-2.73 (t, 4H, N4(CH2)2); 3.14-3.36 (t, 4H, N1(CH2)2); 4.44 (s, 2H, COCH2NH); 6.11 (s, 1H, COCH2NH); 6.57-6.91 (m, 4H, Ar-H). Mass (FAB, M+) Calculated: 279.1428; Found: 279.1328.
N-[2-oxo-2-(4-phenylpiperazin-1-yl)ethyl]acetamide (P6)
% Yield: 88% (1.52 g); Melting Point: 185-186°C; Recrystallization solvent: ethanol. Molecular Weight: 261; IR (KBr) cm− 1: 3358 (N-H stretch); 3071, 3030 (aromatic C-H stretch); 2859, 27526 (aliphatic C-H stretch); 1644 (C = O stretch); 1545, 1477 (aromatic C = C stretch); 1266 (aliphatic C-N stretch). 1H NMR (CDCl3) (δ) ppm: 2.06 (s, 3H, COCH3); 2.57-2.66 (t, 4H, N4(CH2)2); 3.11-3.31 (t, 4H, N1(CH2)2); 4.38 (s, 2H, COCH2NH); 6.08 (s, 1H, COCH2NH); 6.59-7.38 (m, 5H, Ar-H). Mass (FAB, M+): Calculated: 261.1525; Found: 261.1768.
N-{2-[4-(3-methoxyphenyl)piperazin-1-yl]-2-oxoethyl}acetamide (P7)
% Yield: 67% (0.74 g); Melting Point: 192-194°C; Recrystallization solvent: methanol. Molecular Weight: 291; IR (KBr) cm− 1: 3354 (N-H stretch); 2881, 2764 (aliphatic C-H stretch); 1647 (C = O stretch); 1556, 1494 (aromatic C = C stretch); 1263 (aliphatic C-N stretch); 1104 (aliphatic C-O stretch). 1H NMR (CDCl3) (δ) ppm: 2.15 (s, 3H, COCH3); 2.39-2.53 (t, 4H, N4(CH2)2); 3.10-3.31 (t, 4H, N1(CH2)2); 3.73 (s, 3H, OCH3); 4.56 (s, 2H, COCH2NH); 6.12 (s, 1H, COCH2NH); 6.18-6.99 (m, 4H, Ar-H). Mass (FAB, M+): Calculated: 291.1619; Found: 291.1996.
Pharmacology
Experimentation protocol on mice was approved by Institutional Animal Ethics Committee of the Birla Institute of Technology & Science, Pilani (Protocol No. IAEC/RES/11/2, 17.09.07).
Swiss albino
mice (25-30 g) of either sex obtained from Hissar Agricultural University, Hissar, Haryana, India were housed under normal laboratory conditions (12 hr light-dark cycle) with free access to food and water. The experimental sessions were conducted during the light phase of the cycle between 9 a.m. and 3 p. m. in a diffusely illuminated room maintained at 25 ± 1°C and a relative humidity of 55 ± 5%. All the animals were used on more than one occasion, but never more than thrice, with 14 day recovery periods between drug treatments as per the literature protocol [Citation16]. A group of six mice were used for each compound per the dose indicated.
D2 receptor antagonism studies in nigrostriatal pathway (climbing mouse assay)
Drugs
Apomorphine hydrochloride (1 mg/kg); Risperidone (0.6 mg/kg); new chemical entities (P: 1-7) (10 mg/kg).
Apomorphine hydrochloride solution (as per the base calculations) was prepared in distilled water containing 0.1% w/v sodium metabisulphite and was injected subcutaneously 1 h before testing.
Risperidone and new chemical entities were prepared as suspensions in 0.25% w/v sodium carboxymethylcellulose in distilled water and were injected intraperitoneally (i.p.), 30 min before testing.
Inhibition or reversal of Apomorphine induced cage-climbing behavior in mice by a test molecule is an indication of mesolimbic dopaminergic D2 receptor antagonism [Citation15]. During the experimentation, mice were placed individually in separate aluminium cages, measuring 20 × 15 × 15 cm3, with walls lined with 1 cm2 aluminium wire mesh (diameter 2 mm). They were placed in the above cages 30 min for adaptation before the experiment. Groups of mice were administered with either the test molecule (10 mg/kg) or vehicle or Risperidone intraperitonially 1 h prior to the apomorphine challenge (1 mg/kg, subcutaneous (s.c.)). Mice were then observed for the climbing behavior after 10, 20 and 30 min and the scoring was done as below.
“0”, when all the four feet were placed on the cage floor,
“1”, when three feet were placed on the cage floor,
“2”, when two feet were placed on the cage floor,
“3”, when one foot was placed on the cage floor, and
“4”, when all the four feet were off the cage floor.
5-HT2A Receptor antagonism studies (Quipazine induced head twitches)
Drugs
Quipazine maleate (5 mg/kg); Risperidone (0.6 mg/kg); new chemical entities ((P: 1-7)) (10 mg/kg).
Quipazine maleate solution (as per the base calculations) was prepared in distilled water containing 0.1% w/v sodium metabisulphite and was injected intraperitoneally (i.p.), 30 min before testing. Risperidone and new chemical entities were prepared as suspension in 0.25% w/v sodium carboxymethylcellulose in distilled water and were also injected intraperitoneally (i.p.), 30 min before testing.
Inhibition or reversal of Quipazine induced head-twitches in mice by the test molecule is an indication of central serotonergic 5-HT2A receptor antagonism [Citation16]. During the experimentation, mice were placed individually in separate plastic translucent cages, measuring 20 × 15 × 15 cm3. They were placed in the above cages 30 min for adaptation before the experiment. Groups of mice were intraperitoneally administered with either the test molecule (10 mg/kg) or vehicle or Risperidone 1 h prior to the Quipazine maleate challenge (5 mg/kg, intraperitoneal (i.p.)). The head twitches were then counted between 30 and 40 min. The percentage inhibition or reversal of head twitches was calculated by the difference from the count of treated subjects to the count of control animals and referring it to count of control group set to 100%.
Results and discussion
Synthesis and characterization
Microwave irradiation of the known [Citation14] acetyl glycine with substituted piperazines in the presence of DCC in DMF for about 3-5 min gave the corresponding piperazinyl derivatives (P: 1-7). The yields obtained varied from 67–89%. It was observed that the reaction was simple and accelerated many fold when carried out in a microwave environment rather than by the conventional method. IR spectral analysis of the final compounds (P: 1-7) showed strong peaks at ∼3345 cm− 1 and ∼1642 cm− 1, due to N-H stretch and C = O stretch respectively. In 1H-NMR, methyl protons of amide functionality were observed as a singlet around δ 2.05-2.17; methylene protons of glycine functionality were seen at δ 4.54 as a singlet; methylene protons (cyclic) adjacent to N1 nitrogen of piperazine showed a triplet in the range of δ 3.06-3.29 whereas methylene protons (cyclic) adjacent to N4 nitrogen of piperazine showed triplet in the range of δ 2.57-3.74. Elemental (CHN) analysis indicated that the calculated and observed values were within the acceptable limits ( ± 0.4%).
Pharmacological investigation and discussion
Percentage inhibition (expressed as average (μ) ± standard error of the mean (S.E.M.)), in antagonizing dopamine D2 receptors, is calculated at 10th, 20th and 30th min after injecting Apomorphine hydrochloride and the results obtained are detailed in as per the literature protocol [Citation15]. The results clearly indicate that the novel compounds have the capability of antagonizing mesolimbic dopaminergic D2 receptors with % inhibition varying between 55% and 95% at the dose level studied, which is a secondary consequence of the cage climbing behaviour. A maximum of 95% inhibition was observed with P1, while a minimum of 55% inhibition was observed with P3. The results () also clearly indicate that all the new chemical entities have the capability of antagonizing central serotonergic 5-HT2A receptors, which is evident from the reduction in number of head twitches. % Inhibition was found to vary between 29% and 71% at the dose level studied as per the literature protocol [Citation16]. A maximum inhibition of 71% was observed with P2, while a minimum of 29% inhibition was observed with P3.
Table I. Results of D2 and 5-HT2A antagonism studies of titled compounds.
The overall results for the novel compounds are summarized in . Compound P4 is the most active one among the synthesized compounds with 5-HT2A/D2 ratio of 0.853 whereas the standard drug risperidone exhibited a 5-HT2A/D2 ratio of 1.0989.
We conclude from our investigation that P4 might be promising for development as a novel atypical antipsychotic agent. Further studies with respect to the side effect profile are in progress in our laboratory.
Acknowledgements
Sincere thanks are due to UGC, New Delhi, for providing financial assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- A Jablensky. The 100-year epidemiology of schizophrenia. Schizophr Res 1997;28:111–125.
- KT Mueser, SR McGurk. Schizophrenia. Lancet 2004;363:2063–2072.
- P Seeman, T Lee. Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 1975;188 (4194):1217–1219.
- I Creese, DR Burt, SH Snyder. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976;192 (4238):481–483.
- S Miyamoto, GE Duncan, CE Marx, JA Lieberman. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 2005;10:79–104.
- R Matz, W Rick, D Oh, H Thompson, S Gershon. Clozapine – a potential antipsychotic agent without extrapyramidal manifestations. Psychopharmacol Bull 1975;11 (1):14–19.
- J Horacek, V Bubenikova-Valesova, M Kopecek, T Palenicek, C Dockery, P Mohr, C Hoschl. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 2006;20 (5):389–409.
- CA Tamminga. The promise of new drugs for schizophrenia treatment. Can J Psychiatry 1997;42 (3):265–273.
- DC Goff, JT Coyle. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001;158:1367–1377.
- GE Duncan, S Zorn, JA Lieberman. Mechanisms of typical and atypical antipsychotic drug action in relation to dopamine and NMDA receptor hypofunction hypotheses of schizophrenia. Mol Psychiatry 1999;4:418–428.
- PD Leeson, LL Iversen. The glycine site on the NMDA receptor: Structure–activity relationships and therapeutic potential. J Med Chem 1994;37:4053–4067.
- DC Goff, G Tsai, DS Manoach, JT Coyle. Dose-finding trial of D cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry 1995;152:1213–1215.
- DC Javitt, I Zylberman, SR Zukin, U Heresco-Levy, JP Lindenmayer. Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry 1994;151:1234–1236.
- SF Brain, JH Antony, WG Peter, PWG Smith, RT Austin. Vogels text book of practical organic chemistry. 5th ed. 1989. p 1155.
- B Costall, RJ Naylor, V Nohria. Climbing behaviour induced by Apomorphine in mice: A potential model for detection of neuroleptic activity. Eur J Pharmacol 1978;50:39–50.
- JB Malick, E Doren, A Barnett. Quipazine induced head twitch in mice. Pharmacol Biochem Behav 1977;6:325–329.