Abstract
A series of benzamide and picolinamide derivatives containing dimethylamine side chain (4a–4c and 7a–7i) were synthesised and evaluated for acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity in vitro. Structure–activity relationship investigation revealed that the substituted position of dimethylamine side chain markedly influenced the inhibitory activity and selectivity against AChE and BChE. In addition, it seemed that the bioactivity of picolinamide amide derivatives was stronger than that of benzamide derivatives. Among them, compound 7a revealed the most potent AChE inhibitory activity (IC50: 2.49 ± 0.19 μM) and the highest selectivity against AChE over BChE (Ratio: 99.40). Enzyme kinetic study indicated that compound 7a show a mixed-type inhibition against AChE. The molecular docking study revealed that this compound can bind with both the catalytic site and the peripheral site of AChE.
Introduction
Alzheimer’s disease (AD), as a chronic and progressive neurodegenerative disorder characterised by memory loss, language impairment and intellectual ability degression, is one of most common diseases in the elderly populationCitation1–3. Although the precise aetiology of AD is not elucidated enough, AChE inhibitors remain the primary drugs for the therapy to increase acetylcholine level in brain to meliorate the symptomCitation4,Citation5.
In recent decades, many natural products or their derivatives were discovered as new potential remedy for the treatment of ADCitation6–9. Followed by these investigations, a series of natural products derivatives were synthesised and evaluated for AChE inhibitory activity in our laboratory. Among them, Mannich base derivatives of Flavokawain B with chalcone structure were found better AChE inhibitory activity than other compoundsCitation10. Afterwards, a series of chalcone nitrogen-containing derivatives were synthesised and revealed potent AChE inhibitory activityCitation11–13. All results suggested that two aromatic rings with a spacer were possible privilege structures for the design of AChE inhibitors.
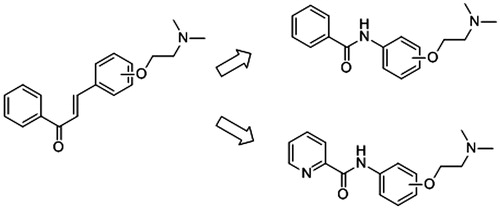
Here, we intent to explore whether the α,β-unsaturated carbonyl group linked two benzene rings in chalcone structure can be replaced by other structural units? And the substituted position of nitrogen-containing side chain can influence the inhibition activity against AChE? Thus, a series of benzamide and picolinamide derivatives were designed, synthesised and evaluated the biological activity of inhibiting AChE (). These derivatives are similar to chalcone derivatives in our previous investigations. In fact, recently, a lot of benzamide and picolinamide derivatives were investigated as anticancer agentsCitation14, DNA minor groove bindersCitation15, 11 b-hydroxysteroid dehydrogenase inhibitosCitation16 or metabotropic glutamate receptor 5 antagonistsCitation17, but few investigations on the bioactivity of them in inhibiting AChE or BChE were reportedCitation18. In order to study the possible inhibition profile and mechanism of new synthesised compounds, enzyme kinetic experiments and molecular docking studies were performed.
Materials and methods
Chemistry
All chemicals or reagents were of analytical reagent grade without further purification. The purity of compounds was checked by Shimadzu LC-20 A high-performance liquid chromatography. The melting points were measured on a WRS-lA melting point detector. Infrared spectra were obtained from Shimadzu Infinity-1 infrared spectrometer. 1H NMR spectra were obtained from a Bruker 400 MHz NMR spectra instrument in CDCl3 with TMS as the internal reference. Mass spectra were obtained from Finnigan LCQ Advantage MAX by electrospray ionisation (ESI-MS).
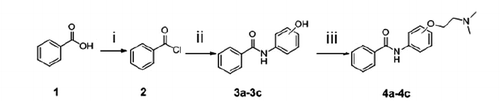
General procedure for the synthesis of 3a–3c
Benzoyl chloride (compound 2) was synthesised from benzamide (compound 1, 5 mmol) with excess oxalyl chloride (2.55 ml, 30 mmol) in CH2Cl2 (40 ml) containing N,N-dimethylformamide (DMF) as a catalytist. The mixture was refluxed for about 2 h until the disappearance of the benzoyl acid monitoring by TLC, and then, the mixture was cooled to room temperature. The redundant oxalyl chloride was evaporated under vacuum. The crude benzoyl chloride was used in the following reaction without further purification.
The benzoyl chloride was dissolved in acetonitrile (20 ml) and then para-aminophenol, meta-aminophenol or ortho-aminophenol dissolving in toluene (1 ml, 11 mmol) was drop-wise added to the acetonitrile solution in an ice bath, respectively. The mixture was refluxed for 6–10 h until benzoyl chloride disappeared. After the solvent was removed under reduced pressure, the crude product was added into 30% sodium hydroxide solution and filtered, 10% HCl was applied to adjust pH of the solution to 3–4, followed by the production of the precipitation. Then light grey solid compound 3a, 3b and 3c were gained with yield of 80–90%.
General method for synthesis of 4a–4c
Compound 4a–4c was synthesised from compound 3a–3c (0.3208 g, 1.5 mmol) and 2-dimethylaminoethyl chloride hydrochloride (0.6729 g, 4.5 mmol) and purified using silica-gel column chromatography with methanol/dichloromethane (1:5 5 ∼ 70, v/v) as elution.
Spectrum data of compounds 4a–4c for the characterisation are outlined in Supplement data.
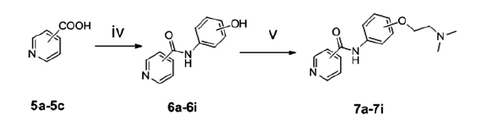
General procedure for the synthesis of 6a–6i
Hydroxybenzotriazole (HOBt) and dicyclohexylcarbodiimide (DCC) was added into an anhydrous toluene solution (15 ml) containing compound 5a, 5b or 5c (5 mmol, 1.0 equiv) at 0 °C, and the mixture was stirred for 45 min. Then, a series of aminophenol (2-aminophenol, 3- aminophenol and 4-aminophenol) (5.5 mmol, 1.1 equiv) were added to the reaction solution, respectively. The reaction mixture was stirred at room temperature for 10–12 h until compound 5a, 5b or 5c disappeared. Then, the reaction mixture was filtered and the toluene was evaporated under reduced pressure. The residue was dissolved in 10% NaOH and filtered, and then, 15% HCl was added to adjust pH of the solution to 3–4, followed by the production of precipitation. The desired products phenylcinnamide (compounds 6a–6i) were gained from the solution by filtration with a good yield of 70 ∼ 75%.
General procedure for the synthesis of 7a–7i
Compounds 7a–7i were synthesised from compound 6a–6i (1.5 mmol) and 2-dimethylaminoethyl chloride hydrochloride (4.5 mmol) with the general procedure and purified using silica-gel column chromatography with methanol/dichloromethane as elution to gain the desired products.
Spectrum data of compounds 7a–7i for the characterisation are outlined in Supplement data.
AChE and BChE inhibition assay
AChE/BChE activity assays were carried out by Ellman method with slight modificationCitation19. Each compound was dissolved in Tween 80 and diluted with water to various concentrations immediately before use. The assay solution which contained 40 μL AChE/BChE, 100 μL acetylthiocholine iodide/S-butyrylthiocholine iodide, 2.76 ml Na2HPO4/NaH2PO4 buffer (pH 8.0, 0.1 M), and 100 μL tested compound solution with different concentrations were incubated at 30 °C for 25 min. Then, the reaction was terminated with 100 μL 20% sodium dodecylsulphate (SDS), then 100 μL 10 mM 5, 5′-Dithiobis-(2-nitrobenzamide) (DTNB) as chromogenic agent was added into the mixture. The absorbance of each assay mixture was measured at 412 nm by UV spectroscopy, which showed a linear relationship with the activity of AChE/BChE. The IC50 values were calculated by Bliss method and expressed as Mean ± SD of the replicates.
Kinetic studies
Kinetic characterisation of AChE/BChE was performed by a modified method previous reportedCitation20. Compound 7a was pre-incubated with the enzyme at 30 °C, then 100 µL acetylthiocholine iodide including five concentrations was added into the assay mixture and kinetic characterisation was conducted spectrometrically at 412 nm. Additionally, the parallel control experiment was made without compound 7a in the mixture.
Molecular docking
Molecular modelling was performed by Molecular Operating Environment (MOE) software package. The X-ray crystallographic structures of AChE (PDB code: 1EVE)Citation21 and BChE (PDB code: 1P0I)Citation22 were gained from protein data bank. 3 D structure of compound 7a was established, and docked into the active site of the protein after energy being minimised. The dock scoring in MOE software was done by ASE scoring function.
Log p measurement
Octanol–water partition coefficients of compounds 4a–4c, 7a∼7i were measured by the shake flask method described previouslyCitation23. The aqueous phase was replaced by phosphate buffer solution (PBS, pH = 7.4). The assay mixture containing tested compounds was shaken at 37 °C over night and then centrifuged at 2000 rpm for 20 min, followed by the analysis with HPLC. A C18 column (150 nm × 4.6 mm, 5 μm) was used with the mobile phase of methanol-0.1% triethylamine (TEA) (85:15, v/v) at a flow rate of 1.0 ml.min−1 and the detection wavelength of 283 nm at 32 °C. Experiments were conducted in triplicate and log p values were calculated.
Results and discussion
Chemistry
The synthetic routes of benzamide derivatives (compound 4a–4c) and picolinamide derivatives (compound 7a–7i) are outlined in Schemes 1 and 2. First, benzamide or picolinamide reacted with a series of amines (p-aminophenol, m-aminophenol, o-aminophenol) in the presence of oxalyl chloride and TEA in dichloromethane to gain compounds 3a–3c, respectively. Then chloroethyldimethylamine in acetone was applied to generate compounds 4a–4c in the presence of K2CO3 and NaI. The final products were purified by silica gel chromatography and characterised by 1H NMR, IR and MS. The synthesis pathway of these two series compounds had a little difference. Benzamide can react easily with oxalyl chloride and gain benzoyl chloride with an excellent yield, but no perfect products gained to synthesise picolinamide amides from picolinamide using this method, so another pathway was used to generate picolinamide amides with DCC, HOBT as catalysts.
Structure–effect relationship of new synthesised compounds on the inhibition against AChE and BChE
The half maximal inhibitory concentration (IC50 values) of new synthesised compounds for AChE and BChE as well as the selectivity for AChE were summarised in . It indicated that the alteration of substituted position of dimethylamine significantly influenced the activity and the selectivity of compounds. Among them, the inhibitory potency and selectivity of benzamide and picolinamide amide derivatives against AChE was ranked by the following order: Para-substituted dimethylamine > meta-substituted dimethylamine > ortho-substituted dimethylamine. However, interestingly, the inhibitory potency of almost compounds against BChE was ranked by the order: ortho-substituted dimethylamine > meta-substituted dimethylamine > para-substituted dimethylamine. Among them, compound 7a with IC50 values of 2.49 ± 0.19 µM, showed more potent than Rivastigmine (IC50 = 10.54 ± 0.26 µM) In addition compound 7a revealed the highest selectivity for AChE (Ratio: 99.40).
Table 1. Cholinesterase inhibitory activity and log p values of benzamide and picolinamide derivatives.
Log p values of new synthesised compounds were ranged from 1.12 to 1.42, which indicated that all the compounds are possible sufficiently lipophilic to pass the blood brain barrier (BBB) in vivoCitation24.
Compound 7a was selected for kinetic studies. The linear Lineweaver–Burk equation of the Michaelis–Menten was applied to evaluate the inhibition profile. The analysis of the steady-state inhibition data of compound 7a was shown in Supplement data: Table 2. According to the analysis, Km but not Vmax increased with the increasing concentration of compound 7a, which presented a mixed-type inhibition. The competitive inhibition constant (Ki) and the non-competitive constant (Ki’) are 4.69 μM and 3.28 μM, respectively.
Molecular docking was conducted for compound to study the possible inhibition mode with AChE or BChE. 3 D structure of compound 7a was established by virtue of the builder interface of MOE program and docked into the active site of the protein after energy being minimised (For AChE, −22.7507 kcal.mol−1; For BChE, −16.6987 kcal.mol−1). As shown in Supplement data, compound 7a exhibited multiple points binding modes with AChE (Supplement data: Figure 2 (A,B)). The binding points of compound 7a with AChE were Trp84(3.92 Å), Trp279(4.12 Å) and Tyr334(3.89 Å) (Supplement data: Table 3), while the binding points with BChE was Tyr252(4.43 Å) (Supplement data: Figure 2(A,B); Table 3). For both of them, the conformation of the side chain conformed to the shape of the mid-gorge, but compound 7a could not combine with Trp82 in BChE, which was an important amino acid in catalysis domain. The results above were possible to partial explain its potent and selective inhibition for AChE.
Conclusions
In summary, a new series of benzamide and policnamide derivatives were designed, synthesised and evaluated for their effects on AChE and BChE. SAR investigation showed that all compounds with Para-substituted dimethylamine side chain had more potent inhibition activity and selectivity against AChE compared with Meta- or Ortho-substituted ones. In addition, the inhibition potency against AChE of picolinamide derivatives was more potent than that of benzamide derivatives. Based on these results earlier, it seems that an important rule is discovered and possibly applied in the future: we can alter the substituted position of nitrogen-containing side chain to gain selective and/or potent AChE inhibitors conveniently, which can be helpful for the development of potential AChE inhibitors for the treatment of AD.
IENZ_1399885_Supplementary_Material.pdf
Download PDF (397.2 KB)Disclosure statement
The authors confirm that this article content has no conflict of interest.
Additional information
Funding
References
- Kenichi O, Naoya O, Kengo I, et al. Effects of imaging modalities, brain atlases and feature selection on prediction of Alzheimer's disease. J Neurosci Methods 2015;256:168–83.
- Anand R, Gill KD, Mahdi AA, et al. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology 2014;76:27–50.
- Klafki H, Staufenbiel M, Kornhuber J, et al. Therapeutic approaches to Alzheimer’s disease. Brain 2006; 129:2840–55.
- Gulçin İ, Abbasova M, Taslimi P, et al. Synthesis and biological evaluation of aminomethyl and alkoxymethyl derivatives as carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase inhibitors. J Enzyme Inhib Med Chem 2017;32:1174–82.
- Akıncıoğlu A, Kocaman E, Akıncıoğlu H, et al. The synthesis of novel sulfamides derived from β-benzylphenethylamines as acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase enzymes inhibitors. Bioorg Chem 2017;74:238–50.
- Jiang YY, Gao HW, Turdu G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer's disease: a review. Bioorg Chem 2017;75:50–61.
- Bayrak C, Taslimi P, Gülçin I, et al. The first synthesis of 4-phenylbutenone derivative bromophenols including natural products and their inhibition profiles for carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Bioorg Chem 2017;72:359–66.
- Yang X, Qiang XM, Li Y, et al. Pyridoxine-resveratrol hybrids Mannich base derivatives as novel dual inhibitors of AChE and MAO-B with antioxidant and metal-chelating properties for the treatment of Alzheimer’s disease. Bioorg Chem 2017;71:305–14.
- Tommonaro G, García-Font N, Vitale RM, et al. Avarol derivatives as competitive AChE inhibitors, non hepatotoxic and neuroprotective agents for Alzheimer’s disease. Eur J Med Chem 2016;122:326–38.
- Liu HR, Huang XQ, Lou DH, et al. Synthesis and acetylcholinesterase inhibitory activity of Mannich base derivatives flavokawain B. Bioorg Med Chem Lett 2014;24:4749–53.
- Liu HR, Liu XJ, Fan HQ, et al. Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2014;22:6124–33.
- Liu HR, Zhou C, Fan HQ, et al. Novel potent and selective acetylcholinesterase inhibitors as potential drugs for the treatment of Alzheimer’s disease: synthesis, pharmacological evaluation, and molecular modeling of amino-alkyl-substituted fluoro-chalcones derivatives. Chem Biol Drug Des 2015;86:517–22.
- Liu HR, Fan HQ, Gao XH, et al. Design, synthesis and preliminary structure–activity relationship investigation of nitrogen-containing chalcone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors: a further study based on Flavokawain B Mannich base derivatives. J Enzyme Inhib Med Chem 2015;17:1–10.
- Mahdavi M, Mohseni Lavi M, Yekta R, et al. Evaluation of the cytotoxic, apoptosis inducing activity and molecular docking of spiroquinazolinone benzamide derivatives in MCF-7 breast cancer cells. Chem Biol Interact 2016;260:232–42.
- Khan GS, Pilkington LI, Barker D. Synthesis and biological activity of benzamide DNA minor groove binders. Bioorg Med Chem Lett 2016;26:804–8.
- Ryu JH, Kim S, Han HY, et al. Synthesis and biological evaluation of picolinamides as potent inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). Bioorg Med Chem Lett 2015;25:695–700.
- Vu HN, Kim JY, Hassan AHE, et al. Synthesis and biological evaluation of picolinamides and thiazole-2-carboxamides as mGluR5 (metabotropic glutamate receptor 5) antagonists. Bioorg Med Chem Lett 2016;26:140–4.
- Peng DY, Sun Q, Zhu XL, et al. Design, synthesis, and bioevaluation of benzamides: novel acetylcholinesterase inhibitors with multi-functions on butylcholinesterase, Aβ aggregation, and β-secretase. Bioorg Med Chem 2012;20:6739–50.
- Alpan AS, Parlar S, Carlino L, et al. Synthesis, biological activity and molecular modeling studies on 1H-benzimidazole derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2013;21:4928–37.
- Skrzypek A, Matysiak J, Niewiadomy A, et al. Synthesis and biological evaluation of 1,3,4-thiadiazole analogues as novel AChE and BuChE inhibitors. Eur J Med Chem 2013;62:311–9.
- Bozkurt B, Coban G, Kaya GI, et al. Alkaloid profiling, anticholinesterase activity and molecular modeling study of Galanthus elwesii. S Afr J Bot 2017;113:119–27.
- Aouani I, Sellami B, Lahbib K, et al. Efficient synthesis of novel dialkyl-3-cyanopropylphosphate derivatives and evaluation of their anticholinesterase activity. Bioorg Chem 2017;72:301–7.
- Yu H, Li WM, Kan KKW, et al. The physicochemical properties and the in vivo AChE inhibition of two potential anti-Alzheimer agents, bis(12)-hupyridone and bis(7)-tacrine. J Pharm Biomed Anal 2008;46:75–81.
- Glave WR, Hansch CJ. Relationship between lipophilic character and anesthetic activity. J Pharm Sci 1972;61:589–91.