Abstract
Carbonic anhydrase (CA, EC 4.2.1.1) IX is regarded as a tumour hypoxia marker and CA inhibitors have been proposed as a new class of antitumor agents, with one such agent in Phase II clinical trials. The expression of some CAs, in particular the isoforms CA IX and CA XII, has been correlated with tumour aggressiveness and progression in several cancers. The aim of this study was to evaluate the possibility that CA IX could represent a marker related to clear cell Renal Cell Carcinoma (ccRCC). Bcl-2 and Bax, and the activity of caspase-3, evaluated in tissue biopsies from patients, were congruent with resistance to apoptosis in ccRCCs with respect to healthy controls, respectively. In the same samples, the CA IX and pro-angiogenic factor VEGF expressions revealed that both these hypoxia responsive proteins were strongly increased in ccRCC with respect to controls. CA IX plasma concentration and CA activity were assessed in healthy volunteers and patients with benign kidney tumours and ccRCCs. CA IX expression levels were found strongly increased only in plasma from ccRCC subjects, whereas, CA activity was found similarly increased both in plasma from ccRCC and benign tumour patients, compared to healthy volunteers. These results show that the plasmatic level of CA IX, but not the CA total activity, can be considered a diagnostic marker of ccRCCs. Furthermore, as many reports exist relating CA IX inhibition to a better outcome to anticancer therapy in ccRCC, plasma levels of CA IX could be also predictive for response to therapy.
Introduction
Renal cell carcinoma (RCC) accounts for about 3% of all solid malignanciesCitation1 shows the highest aggressiveness among all urologic neoplasmsCitation2. It is the seventh most common cancer in men and the ninth most common in womenCitation3. Incidence worldwide is about 209,000 new cases per year and 102,000 deaths per year. The incidence of all stages of this cancer has increased over several years, contributing to a steadily increasing mortality rate per unit populationCitation3. One-third of patients with RCC have evidence of metastatic disease at the time of diagnosis and about half of those treated by radical surgery eventually relapse. Metastatic RCC is usually resistant to conventional chemotherapy whereas only immunotherapy with interferon alpha (IF-α) or interleukin-2 (IL-2) has been demonstrated to provide significant results in terms of disease-free survivalCitation4–6.
Over 80% of sporadic RCCs are clear cell carcinomas (ccRCC) which have been extensively investigated as regards their molecular signatures. A high percentage of ccRCCs has been shown to be associated with the inactivation of the von Hippel–Lindau (VHL) tumour suppressor geneCitation7. The VHL gene encodes a protein (pVHL) which is the substrate recognition component of a ubiquitin ligase complex targeting a protein transcription factor, hypoxia-inducible factor-1α (HIF-1α), for proteolysisCitation8,Citation9. Under hypoxic conditions, as well as in case of VHL inactivation, HIF-1α is stabilised and accumulates in the nucleus leading to subsequent over-expression of genes which are critical for tumour angiogenesis, glucose transport, epithelial proliferation, cell migration, and pH controlCitation10. Hypoxia in the tumour microenvironment is associated with poor prognosis and poor response to therapy, underlying the importance of studying the effect of potential anticancer drugs on the hypoxic pathway.
The carbonic anhydrase (CA, EC 4.2.1.1) family includes 16 catalytically active zinc metalloenzymes involved in the reversible hydration of carbon dioxide to bicarbonate and a protonCitation11,Citation12. These isoforms mainly differ in their catalytic activity, tissue distribution, and subcellular localisation. Indeed, there are cytosolic, mitochondrial, secreted, and membrane-bound isoenzymesCitation13. Among the latter, CA IX and CA XII were found to be overexpressed in a wide variety of human tumours, being involved in cancer aggressiveness and progressionCitation14,Citation15. In particular, CA IX is a downstream gene activated following either hypoxia (via HIF-1α) or VHL inactivationCitation16. CA IX has extensively been investigated in ccRCCs and a high percentage of these tumours (up to 94%) express CA IXCitation17,Citation18.
Even if the main function of CA IX is to maintain intracellular pH homeostasis under hypoxic conditions that are common in solid tumours, it has been shown that CA IX also lowers E-cadherin-mediated cell adhesionCitation19. Contrary to other tumours, CA IX over-expression in RCC is associated with good prognosis and improved response to immunotherapyCitation20. This difference could be a reflection of molecular mechanisms responsible for CA IX expression: high CA IX expression in other tumours than ccRCC is hypoxia-related, whereas, is linked to VHL inactivation in ccRCC, where the latter is itself a marker of good prognosisCitation16. The fact that CA IX is not detectable in normal kidney tissue suggests that detection of both CA IX protein and activity in plasma may be a useful diagnostic marker and a predictive tool to evaluate the effectiveness of therapy.
Materials and methods
Patients and tissue collection
Tissue samples were obtained from eight patients with ccRCC (seven males and one female) median age 68.5 (range 58–77 years) who underwent surgical resections at the Department of General Surgery, of Alba hospital and of University of Florence. Cancer tissue (from the edge of the tumour) and adjacent normal tissue (at least 10 cm from the tumour) were excised from each surgical specimen. All patients were thoroughly informed about the aims of the study and gave written consent for the investigation in accordance with the ethical guidelines of our University and of Alba General Hospital. None of the patients had taken nonsteroidal anti-inflammatory drugs for at least 3 months before surgery. All tumours were adenocarcinomas classified as: three patients stage I, five patients stage II according to the American Joint Committee on Cancer Staging SystemCitation21; none had any colloid component. Samples were frozen at −80 °C for western blot analysis and at −20 °C for VEGF determination and caspase-3 activity evaluation until processing.
Plasma samples were obtained from 24 individuals: eight patients with benign tumours (six males and two females) median age 56.5 (range 44–77 years), eight healthy volunteers (five males and three females) median age 66.3 (range 54–68 years), eight with ccRCC (same patients as above) to evaluate CA IX plasma concentration and CA catalytic activity.
Western blot analysis
Representative Western Blots were carried out in order to show CA IX, VEGF, pro-caspase 3, BAX, and BCL2 expression in normal and tumour tissue of patient with ccRCC. Human renal tissues were homogenised in RIPA buffer and incubated on ice for 1 h, and then centrifuged at 10,000g at 4 °C for 15 min. Following quantification by BCA assay kit (Thermo Fisher Scientific, Milan, Italy), 25 µg of total proteins from each supernatant were separated by SDS-PAGE under reducing conditions and then transferred to PVDF membrane. The membrane was blocked in 5% non-fat milk in 1% TBST, and then incubated overnight at 4 °C, respectively, with: 1:200 anti-CA IX monoclonal murine antibody M75Citation22, recognising N-terminal extracellular PG domain of human CA IX. 1:1000 anti-VEGF monoclonal antibody; 1:1000 anti-procaspase-3 monoclonal antibody; 1:1000 anti-BAX monoclonal antibody; 1:1000 anti-BCL2 monoclonal antibody, were all from Santa Cruz Biotechnology (Los Angeles, CA).
Membrane-bound secondary antibodies (1:12,000) (Santa Cruz Biotechnology, Los Angeles, CA) were detected by ECL following the instructions of the manufacturer (Amersham, Freiburg, Germany). To ensure equal loading amounts, blots were stripped and reprobed using a 1:2000 monoclonal antibody raised against human tubulin (Los Angeles, Santa Cruz, CA).
Semiquantitative reverse transcription-PCR (RT-PCR)
Total RNA was isolated according to the manufacturer’s protocol (NucleoSpin® RNA II, Macherey-Nagel, Philadelphia, PA) and reverse transcribed (Omniscript, Qiagen, Milan, Italy) by using random primers. The RNA purity was validated by absorbance ratio. A typical PCR reaction (HotStarTaq, Qiagen, Milan, Italy) was prepared for amplification of CA IX mRNA, and calibration was performed by amplification of the same cDNA sample with primers for the housekeeping GAPDH mRNA. Primer sequences were as follows: CA IX 5-TAAGCAGCTCCACACCCTCT-3 (sense) and 5-TCTCATCTGCACAAGGAACG-3 (antisense), and the product size was 250 pb; for GAPDH, 5-GAGTCAACGGATTTGGTCGT-3 (sense) and 5-TTGATTTTGGAGGGATCTCG-3 (antisense) were used, giving a product size of 238 pb (Public Software Primer3; Whitehead Institute, Cambridge, MA). Amplification was performed as follow: 30 s of denaturation at 94 °C, 30 s of annealing at 56 °C, and 1 min of extension at 72 °C for 30 cycles. Amplification products were highlighted with ethidium bromide on 1.5% agarose gel. The intensities of the bands corresponding to the amplified products were quantified by densitometric analysis.
Caspase-3 activity determination
The activity of caspase-3 was determined by use of a fluorescent substrate according to the method describedCitation23. The Ac-Asp-Glu-Val-Asp-AMC (Ac-DEVD-AMC; Bachem AG, Bubendorf, Switzerland) was used as a fluorescent substrate for caspase-3Citation24. Tissues were homogenised with 10 mmol/L HEPES (pH7.4) containing 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate, 42 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L DTT, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL leupeptin, and 1 μg/mL pepstatin A. The homogenate was then centrifuged at 10,000g for 10 min. Supernatants containing 250 μg of total protein were incubated with 40 μM of the caspase-3 substrate Ac-DEVD-AMC for 60 min at 37 °C. At the end of incubation, substrate cleavage was determined fluorometrically (Spectrofluo JY3 D, Jobin Yvon, Paris, France) with a λ excitation at 380 nm and a λ emission at 460 nm. The data were expressed as arbitrary units per milligram of protein. One unit enzyme activity was defined as the amount of the enzyme required to cleave 40 µmoles Ac-DEVD-AMC for 60 min at 37 °C. The determinations were done in quintuplicate.
Determination of VEGF production
The concentration of VEGF in the tissues was measured using a commercially available ELISA kit according to the manufacturer’s instructions (Immunoassay Kit Human VEGF; Biosource International., Camarillo, CA). Samples were incubated in microtiter plates precoated with a monoclonal antibody specific for VEGF. After incubation at room temperature for 2 h and washing, a substrate solution was added. Colour development was stopped after 30 min at room temperature, and the colour intensity was read at 450 nm within 30 min. Protein concentration in the tissues was determined by the Pierce-BCA method (Thermo Fisher Scientific, Los Angeles, CA), using BSA as standard. Determinations were performed in triplicate and VEGF values were expressed as pg/μg protein.
Determination of plasmatic concentration of CA IX
Plasmatic CA IX was measured using a quantitative sandwich enzyme immunoassay technique (Kit Quantikine® Human Carbonic Anhydrase IX/CA9 Immunoassay, R&D Systems, Inc., McKinley Place NE, Minneapolis, MN).
Plasma samples were washed three times with 0.9% NaCl and then haemolysed in cold water. The ghosts and any intact cell were removed by centrifugation at 15,000g for 10 min at 4 °C, and the pH of the haemolysate was adjusted to 7.4 with solid Tris-base. Samples absorbance was then measured at 280 nm.
Determination of plasmatic activity of CA
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5), and 20 mM Na2SO4, following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10 s. The CO2 concentrations ranged from 1.7–17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock inhibitor solution (0.1 mM) was prepared in water and dilutions up to 0.01 nM were done. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature (prior to assay, in order to allow for the formation of the EI complex). The inhibition constants were obtained by non-linear least-squares methods using PRISM3, as reported earlierCitation25 and represent the mean from at least three different determinations.
Statistical analysis
Results are expressed as means ± SD. Multiple comparisons were performed by the Student test. Statistical significances were accepted at p < .05.
Results
CA IX expression in RCC
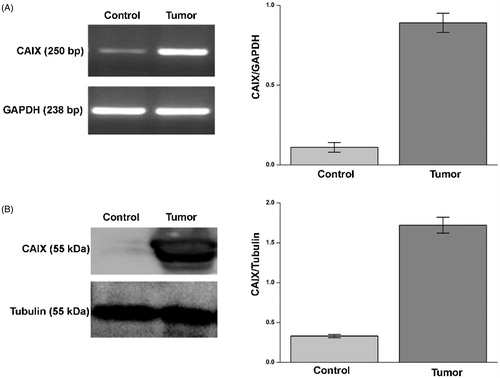
For each patient we determined the expression of CA IX both in tumour and corresponding normal tissue by semiquantitative RT-PCR and Western blot analysis. As expected, CA IX mRNA and protein were more expressed in tumour tissue compared to normal tissue (, panels A and B).
Figure 1. CA IX expression in ccRCC samples. mRNA levels (panel A, left side) evaluated by RT-PCR and protein levels (panel B, left side) evaluated by WB of CA IX in normal tissue (Control) and tumour sample (tumour) of a representative patient. GAPDH housekeeping gene amplification products and tubulin housekeeping protein were used to normalise values from RT-PCR and western blot, respectively; in right side of panel A (RT-PCR) and B (WB) are reported the densitometric values of samples from eight patients. Differences were significant for p ≤ .005 with t-test.
VEGF concentration
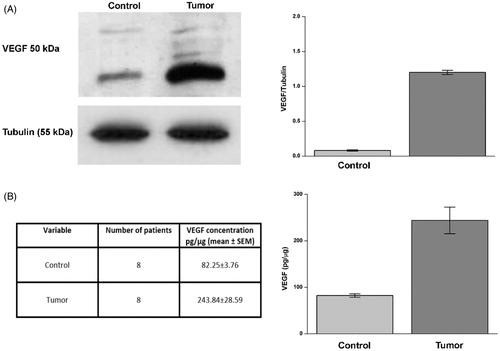
VEGF concentration has been evaluated in normal and matching tumour tissue from eight patients with ccRCC. Similarly to CA IX, VEGF is a downstream gene activated following hypoxia and/or VHL inactivationCitation10,Citation26. In tumour tissue, VEGF concentration was very high as assessed by Western blot and ELISA assays, with an average value of 243.84 pg/µg of protein, as compared to normal tissue where the VEGF average concentration was 82.25 pg/µg of protein (, panels A and B).
Figure 2. Evaluation of VEGF in ccRCC samples. Protein levels evaluated by WB (panel A) or ELISA (panel B) of VEGF in normal tissue (Control) and tumour sample (tumour). In panel A, right side, are reported the densitometric values of samples of WB from eight patients (differences were significant for p ≤ .005 with t-test). In panel A, left side, WB of VEGF from a representative patient is reported. Tubulin housekeeping protein was used to normalise values in WB. VEGF concentration by ELISA from same patients (panel B, left and right side) is expressed as pg/µg. Differences were significant for p ≤ .005 with t-test.
Apoptosis markers in ccRCC
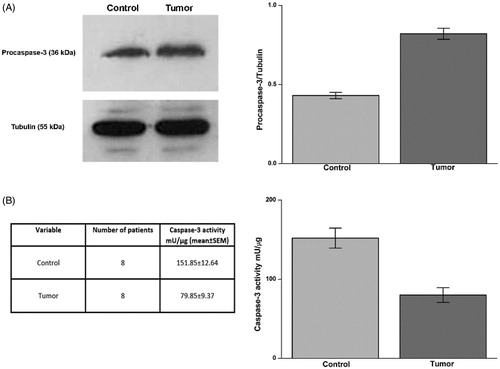
We studied the expression of procaspase-3 by Western blot and caspase-3 activity by a fluorescent substrate in normal and tumour tissue from each patient. The expression of procaspase-3, the inactive precursor of the apoptosis effector caspase-3, was higher in tumour tissue in comparison to healthy tissue; accordingly, caspase-3 activity was significantly higher in the normal tissue (average activity 154.94 mU/µg) than in tumour tissue (average activity 79.85 mU/µg) (, panels A and B).
Figure 3. Procaspase-3 expression and caspase-3 activity in ccRCC samples. Protein levels evaluated by WB (panel A, left side) of Procaspase-3 in normal tissue (Control) and tumour sample (tumour) of a representative patient. Tubulin housekeeping protein was used to normalise values in WB. In panel A, right side, are reported the densitometric values of samples from eight patients. Caspase-3 activity in samples was evaluated as reported in material and methods and data are reported in panel B, left and right side. Differences were significant for p ≤ .005 with t-test.
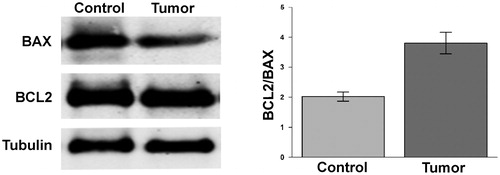
We analysed by Western blot the expression of the pro-apoptotic protein BAX and of the anti-apoptotic protein BCL2. The BCL2/BAX ratio in each renal biopsy analysed was higher in tumour tissue with respect to control tissue due to reduced BAX level ().
Figure 4. Ratio BAX/BCL2 in ccRCC samples. Protein levels of BCL2 and BAX evaluated by WB in normal tissue (Control) and tumour sample (tumour) of a representative patient. Tubulin was used to normalise values in WB. In left panel are reported the ratio between the values of BCL2 versus BAX. Differences were significant for p ≤ .005.
Plasma CA IX concentration and CA activity
CA IX plasma concentration and CA activity were evaluated on plasma samples obtained by 24 patients (eight with ccRCC, eight with benign renal tumour, and eight healthy volunteers as described in Material and methods). The average concentration of CA IX in the plasma of patients with ccRCC was 93.48 pg/ml, a value significantly higher compared to healthy controls (6.23 pg/ml) and to patients with benign tumours (11.86 pg/ml) (). There was a significant correlation between ccRCC and plasma CA IX concentration.
Figure 5. Evaluation of CA IX plasmatic concentration and CA enzymatic activity. Plasmatic concentration of CA IX evaluated by ELISA (upper panel). Plasmatic activity of CA (bottom panel) evaluated as reported in material and methods. Differences were significant for p ≤ .005 with t-test.
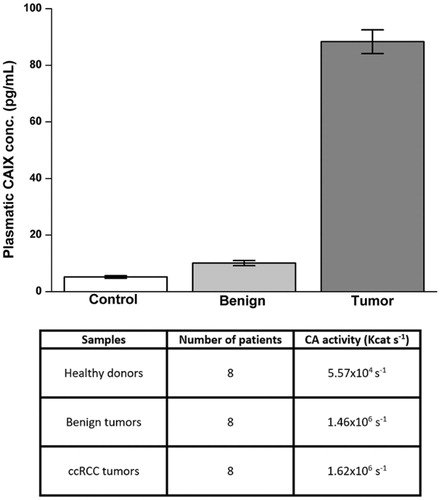
In the same samples of plasma the CA activity was evaluated. No particular differences were observed between the mean activity of CA in plasma samples of patients with ccRCC (kcat 1.62 × 106 s−1) and benign tumour (kcat 1.46 × 106 s−1). On the contrary, the average value observed in plasma samples of healthy subjects was much lower (kcat 5.57 × 104 s−1) (). These results show that during tumuorigenesis, some mechanisms lead to an increase in plasma CA IX concentration, and an increase in CA catalytic activity even in benign tumours.
These data suggest that other CA isoenzymes in addition to CA IX could be up-regulated in non-malignant cancer, contributing to plasmatic total enzymatic activity.
Discussion and conclusions
CA IX has been shown to be up-regulated in a number of human cancer cells as a consequence of either hypoxia-induced or constitutive HIF-1 activation, whereas, it is not expressed in their normal counterparts, with the exception of gastric mucosaCitation14,Citation15. Although these characteristics make CA IX an interesting target for novel approaches in the anticancer therapy, the exact role of CA IX in tumour growth and progression started only recently to be delineated. It has been shown that CA IX activity contributes to the acidification of hypoxic tumours through the decrease of the extracellular pHCitation27,Citation28. A low pH has been associated with tumorigenic transformation, chromosomal rearrangements, extracellular matrix breakdown, tumour cell migration and invasionCitation29. Some new CA inhibitors with high affinity for CA IX isoforms have been shown to have potent antitumor activity in several cancer cell lines and one of them progressed to Phase II clinical trials recentlyCitation12,Citation13,Citation15,Citation30,Citation31. However, the antiproliferative effect of CA inhibitors might be due to their effect not only on CA IX but on other CA isoenzymes, namely the other tumour-associated isoforms CA XII, CA II, and/or the mitochondrial isoform CA VACitation32.
In this study, we evaluated the possibility that CA IX could be a marker for the ccRCC diagnosis. In fact, we observed that CA IX is widely expressed in tumour tissue of patients with ccRCC as compared to healthy kidney tissue. CA IX probably plays an important role in regulating growth factors, such as VEGF and metalloproteinases which are involved in metastases. VEGF is highly expressed in the majority of RCCsCitation33. Similarly to CA IX, VEGF is one of the HIF-1 target genes induced by either intratumoral hypoxia or VHL inactivationCitation34,Citation10. Accordingly, with VHL status, we found elevated VEGF levels in the ccRCC tissue samples as compared to normal tissue, and on this basis, we hypothesise that CA IX plays an important role in amplifying the VEGF signalling process by HIF-1α in ccRCC. VEGF may represent a marker of metastatic potential, and the signalling pathway of this growth factor represents a promising therapeutic approach for the treatment of metastatic RCC. As McIntyre et al.Citation35 demonstrated, in colon cancer and glioblastoma, that inhibition of tumour hypoxic response by CA IX inhibition, potentiates the antiangiogenic therapy, we hypothesise that the same could be true also for ccRCC, highlighting CA IX as a therapeutic target alone or in combination with anti-VEGF therapy. Furthermore, in the same tumour samples, we observed both a reduced activity of caspase-3, and a decrease of BAX, shifting BCL2/BAX levels towards an antiapoptotic ratio. Hypoxia is a characteristic feature of locally advanced solid tumours resulting from an imbalance between oxygen supply and consumption. Tumour cell responses to hypoxic stress can be enhanced by proteomic and genomic changes, resulting in loss of apoptotic potentialCitation36. These new cell variants have advantages over less adapted cells in a hypoxic microenvironment and expand through clonal selection, often becoming the dominant cell type. Our findings clearly show that VEGF is abundantly expressed in tumour tissue, and VEGF over-expression together with CA activity could be correlated with the observed anti-apoptotic phenotype in ccRCC.
Evaluating the plasma concentrations in healthy subjects, patients with ccRCC and benign tumours, we observed that patients with ccRCC had a significant higher plasma concentration, whereas, no particular difference have been observed in total CA activity, between patients with ccRCC and benign tumours; while in healthy subjects, both CA IX concentration and CA activity were lower. We assume that the CA IX plasma concentrations, but not total CA activity, could be a marker of ccRCC. In spite of the relatively low number of samples, these results are homogeneous for each population and may open new prospectives for future, larger scale studies.
Disclosure statement
No potential conflict of interest is reported by the authors.
Additional information
Funding
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin 2010;60:277–300.
- Lam JS, Belldegrun AS, Figlin RA, et al. Tissue array-based predictions of pathobiology, prognosis, and response to treatment for renal cell carcinoma therapy. Clin Cancer Res 2004;10:6304s–9s.
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373:1119–32.
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289–96.
- Coppin C, Le L, Porzsolt F, et al. Targeted therapy for advanced renal cell carcinoma. Cochrane Database Syst Rev 2008;CD006017.
- Yang Y, Xia F, Hermance N, et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell Biol 2011;31:2774–86.
- Clark PE. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int 2009;76:939–45.
- Blankenship C, Naglich JG, Whaley JM, et al. Alternate choice of initiation codon produces a biologically active product of the von Hippel Lindau gene with tumor suppressor activity. Oncogene 1999;18:1529–35.
- Cockman ME, Masson N, Mole DR, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem 2000;275:25733–41.
- Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol 2004;22:4991–5004.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- a) Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74. b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. c) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- a) Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229. b) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. c) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. d) Capasso C, Supuran CT. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32.
- a) Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res 2013;23:6160–5. b) Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 2013;23:737–49.
- a) Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31. b) Guler OO, De Simone G, Supuran CT. Drug design studies of the novel antitumor targets carbonic anhydrase IX and XII. Curr Med Chem 2010;17:1516–26. c) Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902.
- a) Wykoff CC, Beasley NJP, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 2000;60:7075–83. b) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nature Rev Drug Discov 2011;10:767–77.
- Bui MHT, Seligson D, Han K, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 2003;9:802–11.
- Patard JJ, Fergelot P, Karakiewicz PI, et al. Low CA IX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer 2008;123:395–400.
- Švastová E, Žilka N, Zat’ovičová M, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with β-catenin. Exp Cell Res 2003;290:332–45.
- Sandlund J, Oosterwijk E, Grankvist K, et al. Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int 2007;100:556–60.
- Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual, 8th ed. Berlin: Springer Verlag; 2017.
- Zatovičová M, Tarábková K, Švastová E, et al. Monoclonal antibodies generated in carbonic anhydrase IX-deficient mice recognize different domains of tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol Methods 2003;282:117–34.
- Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem 1997;272:25719–23.
- Masini E, Pierpaoli S, Marzocca C, et al. Protective effects of a plant histaminase in myocardial ischaemia and reperfusion injury in vivo. Biochem Biophys Res Commun 2003;309:432–9.
- Steele RM, Benedini F, Biondi S, et al. Nitric oxide-donating carbonic anhydrase inhibitors for the treatment of open-angle glaucoma. Bioorg Med Chem Lett 2009;19:6565–70.
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 2007;26:281–90.
- Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 2007;26:299–310.
- a) Swietach P, Patiar S, Supuran CT, et al. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J Biol Chem 2009;284: 20299–310. b) Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8.
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2009;4:891–9.
- a) Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76. b) Morsy SMI, Badawi AM, Cecchi A, et al. Carbonic anhydrase inhibitors. Biphenylsulfonamides with inhibitory action towards the transmembrane, tumor-associated isozymes IX possess cytotoxic activity against human colon, lung and breast cancer cell lines. J Enzyme Inhib Med Chem 2009;24:499–505. c) Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long lasting, topical intraocular pressure lowering properties. J Med Chem 2002;45:1466–76. d) Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: perfluoroalkyl/aryl-substituted derivatives of aromatic/heterocyclic sulfonamides as topical intraocular pressure-lowering agents with prolonged duration of action. J Med Chem 2000;43:4542–51.
- a) Cianchi F, Vinci MC, Supuran CT, et al. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther 2010;334:710–19. b) Andreucci E, Peppicelli S, Carta F, et al. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J Mol Med (Berl) 2010;95:1341–53. c) Stanica L, Gheorghiu M, Stan M, et al. Quantitative assessment of specific carbonic anhydrase inhibitors effect on hypoxic cells using electrical impedance assays. J Enzyme Inhib Med Chem 2017;32:1079–90.
- a) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2006;27:E48. b) Thiry A, Dognè JM, Masereel B. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006;27:566–73.
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24.
- Levy AP, Levy NS, Iliopoulos O, et al. Regulation of vascular endothelial growth factor by hypoxia and its modulation by the von Hippel-Lindau tumor suppressor gene. Kidney Int 1997;51:575–8.
- McIntyre A, Patiar S, Wigfield S, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res 2012;18:3100–11.
- Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory, mechanisms, and cellular response. Oncologist 2004;9:4–9.
