Abstract
A series of novel sulphonamide derivatives was obtained from sulphanilamide which was N4-alkylated with ethyl bromoacetate followed by reaction with hydrazine hydrate. The hydrazide obtained was further reacted with various aromatic aldehydes. The novel sulphonamides were characterised by infrared, mass spectrometry, 1H- and 13C-NMR and purity was determined by high-performance liquid chromatography (HPLC). Human (h) carbonic anhydrase (CA, EC 4.2.1.1) isoforms hCA I and II and Mycobacterium tuberculosis β-CA encoded by the gene Rv3273 (mtCA 3) inhibition activity was investigated with the synthesised compounds which showed promising inhibition. The KIs were in the range of 54.6 nM–1.8 µM against hCA I, in the range of 32.1 nM–5.5 µM against hCA II and of 127 nM–2.12 µM against mtCA 3.
1. Introduction
Sulphonamides are interesting biologically active compounds. There are numerous sulphonamide drugs available on the markets for the treatment of various diseasesCitation1. Sulphonamide derivatives such as acetazolamide, methazolamide, ethoxzolamide, dichlorophenamide, dorzolamide and brinzolamide have been clinically used for decades as inhibitors of the zinc enzyme carbonic anhydrase (CA, EC 4.2.1.1). A diverse research trend in the past years has led to the obtaining of diuretic, anti-glaucoma, anti-cancer, anti-convulsant, anti-diabetic, and anti-obesity agents based on CA inhibitors (CAIs) of the sulphonamide typeCitation2–6. Sulphonamides act as strong CAIs by binding as anions to the zinc metal ion within the enzyme active siteCitation7.
CA has various roles in physiological events such as carbon dioxide and bicarbonate transport processes, respiration, pH balancing, CO2 homeostasis, electrolyte secretion, biosynthetic reactionsCitation1. Distinct, evolutionarily non-related gene families of CAs are present in various organisms, out of which the α-class is present in humans, as 15 different isoforms (hCA I–XIV). hCA I is present in red blood cells and in many tissues but its physiological function is still unknown; however, it is known that hCA I is associated with retinal and cerebral edema, and the inhibition of CA I may be helpful in curing such conditionsCitation1–5. hCA II, the physiologically dominant isoform, is another enzyme which is associated with several disease conditions such as epilepsy, edema, glaucoma and altitude sicknessCitation1–5. Furthermore, it has also emerged in the past few years that these enzymes can be used as potential target for designing anti-infective drugs with a novel mechanism of actionCitation6.
In this article, we report a synthetic strategy for the generation and characterisation of some sulphonamide derivatives. The novel sulphonamides were purified and characterised using IR, Mass spectrometry, 1H and 13C-NMR for confirmation of their structure, and purity of the compounds was determined by using HPLC techniques. The newly synthesised compounds were analysed as inhibitors of human hCA I and II, and the bacterial, β-class enzyme from Mycobacterium tuberculosis (mtCA 3) encoded by the gene Rv3273Citation8.
2. Material and method
2.1. Chemistry
All the reagents and solvents were obtained from commercial suppliers and were used as received unless otherwise indicated. Solvents were dried, wherever necessary, according to standard procedures. All reactions were performed under N2 atmosphere, unless otherwise indicated. Analytical silica gel 60 F254-coated TLC plates were purchased from Sigma-Aldrich (Milan, Italy), and were visualised with UV light. IR spectra (ATR) were recorded on a Quest ATR Diamond Accessory (Black) P31482 & Shimadzu 8100 infrared spectrophotometer. 1H-NMR was recorded at 300 MHz in DMSO-d6 as solvent using TMS as an internal reference standard at Sophisticated Analytical Instrument Facility (SAIF). Molecular ion peaks of some of the synthesised compounds were recorded using LCMS at Laxai- Avanti Life Sciences Pvt. Ltd Hyderabad, India. Melting points were recorded using a Veego® (VMP)-D capillary melting point apparatus (Veego Instruments Corp., Mumbai, India) and are uncorrected. Percent Purity of synthesised compounds was determined by performing RP-HPLC.
General procedure for synthesis of ethyl 2-((4-sulphamoylphenyl) amino) acetate (2)Citation9
In a solution of sulphanilamide (0.01 mol) in absolute ethanol (20 ml), ethyl bromoacetate (0.01 mol) and anhydrous potassium carbonate (0.6 g) were added and the reaction mixture was heated under reflux for 12 h. The potassium salt was filtered off and excess of ethanol was removed under reduced pressure. The residue solidifies on cooling to give compound (2). Yield: 70%, Rf: 0.72 (chloroform:methanol 9:1); M.P.: 141–144 °C; IR (ATR) cm−1: 3356 (N–H str of NH2), 3271 (N–H str), 2993 (Ar C–H str), 2904 (aliphatic C–H str), 1728 (C = O str), 1597 (C = C str), and 1138 (S = O str).
General procedure for synthesis of 4-((2-hydrazinyl-2-oxoethyl)amino) benzenesulphonamide (3)Citation10
In a solution of compound (2) (0.01 mol) in absolute ethanol (15 ml), hydrazine hydrate (0.02 mol) was added and the reaction mixture was heated under reflux for 4 h. The excess of ethanol was removed under vacuum and the reaction mixture was allowed to cool. The reaction mixture was then diluted with ice-cold water. The precipitate obtained was filtered, washed with cold water, dried and recrystallised from ethanol. Yield: 50%; Rf: 0.46 (chloroform:methanol 9:1); M.P.: 174–177 °C, 1H-NMR (300 MHz, DMSO-d6) δ ppm: 9.15 (s, 1H, −CONH), 7.51–7.47 (dd, 2H, Ar–H), 6.93 (s, 2H, −SO2−NH2), 6.62–6.61 (dd, 2H, Ar–H), 6.58 (t, 1H, Ar–NH), 4.25 (d, 2H, −CH2−), 3.69–3.67 (d, 2H, −NH2); IR (ATR, cm−1): 3344 (N − H str of NH2), 3294 (N − H str), 3034 (Ar C − H str), 2943 (aliphatic C–H str), 1654 (C = O str), 1589 (Ar C = C), and 1139 (S = O str).
General procedure for synthesis of (E)-1-(4-chlorophenyl)-3-phenylprop-2-en-1-one derivatives (4a–4k)Citation11–13
4-Chloro-acetophenone (0.01 mol) and various substituted aromatic aldehydes (0.01 mol) were mixed in ethanol (40 ml) in a conical flask placed in an ice bath. To this, 60% NaOH solution (10 ml) was added dropwise with continuous stirring for 30 min. The mixing was continued for another 2–3 h maintaining the ice bath. The mixture was kept in a refrigerator overnight. Reaction completion was confirmed by TLC (hexane:ethyl acetate = 2:1). Then, it was diluted with ice-cold water, filtered, washed well with cold water, dried in air and recrystallised from rectified methanol.
General procedure for synthesis of 4-aryl/heteroaryl but-3-en-2-one derivatives (4l–4u)Citation14
A mixture of aldehyde (1 equiv) and acetone (13.6 equiv) was added in aqueous 60% NaOH solution. The mixture was stirred at 40 °C. Reaction completion was confirmed by TLC (hexane:ethyl acetate = 2:1). On completion of reaction, 25 cm3 of water were added to reaction mixture to afford the crude as an oil. The product was extracted with AcOEt (3 × 25 cm3) and dried in air. Reaction with 4-((2-hydrazinyl-2-oxoethyl)amino) benzenesulphonamide (3) led to the final products.
General procedure for synthesis of 4-((2-(3-(4-chlorophenyl)-5-aryl/heteroaryl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino)benzenesulphonamide derivatives (5a–5k)Citation15
A mixture of chalcone (E)-1-(4-chlorophenyl)-3-aryl/heteroaryl-prop-2-en-1-one derivatives (4a–4k) (0.01 mol) and 4-((2-hydrazinyl-2-oxoethyl) amino) benzenesulphonamide (3) (0.02 mol) in 20 ml ethanol was refluxed for 2 h. To this, alcoholic KOH solution (10 ml, 60%) was added dropwise with continuous stirring for 30 min. The reaction mixture was refluxed further for 2 h and stirred overnight. Reaction was monitored by TLC using chloroform:methanol (0.8:0.2). The resulting solution was poured on ice-cold water. Precipitate obtained was filtered and recrystallised from ethanol.
General procedure for synthesis of 4-((2-(3-methyl-5-ary/heteroaryl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino)benzenesulphonamidederivatives (5l–5u)Citation16
4-Aryl/heteroaryl-but-3-en-2-one derivatives (4l–4u) (0.01 mol) were dissolved in hot glacial acetic acid. To this solution 4-((2-hydrazinyl-2-oxoethyl)amino)benzenesulphonamide (3) (0.015 mol) was added and refluxed. Reaction was monitored by TLC using chloroform:methanol (9:1). The resulting solution was poured on ice-cold water. Precipitate obtained was filtered and recrystallised from 90% ethanol.
General synthesis of 4-((2-(arylmethylidene)hydrazinyl)-2-oxoethyl)amino)benzene sulphonamide derivatives (6a–6j)Citation17–19
In a 250-ml flask, equimolar quantity of hydrazide (3) (0.05 mol) and aromatic aldehyde (0.05 mol) were dissolved in EtOH (50 ml). Glacial acetic acid (2–3 ml) was added to adjust the pH to 5–6. The reaction mixture was refluxed for 3 h. The progress of reactions was monitored using TLC using hexane:ethyl acetate (8:2) as the mobile phase. After completion of reaction (as seen from TLC), the mixture was poured onto the crushed ice and the precipitated product was filtered, washed twice with ice-cold water, dried and recrystallised from water:ethanol.
Using the above general procedure, the following compounds were prepared and characterised.
4-((2-(3-(4-Chlorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino) benzenesulphonamide (5a)
Yield: 70%; Rf: 0.53 (chloroform:methanol 9:1); M.P.: 140–144 °C; purity (HPLC): 88.87%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.98–7.86 (dd, 2H, Ar–H), 7.57–7.50 (m, 4H, Ar–H), 7.47–7.20 (m, 5H, Ar–H), 6.93 (s, 2H, –SO2NH2), 6.67–6.64 (dd, 2H, Ar–H), 6.59–6.55 (t, 1H, Ar–NH), 5.61–5.56 (dd, 1H, Hx), 4.49–4.30 (dd, 2H, –CH2–), 3.93–3.83 (dd, 1H, Ha), 3.21–3.13 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 167.07, 154.42, 151.38, 142.22, 135.8, 131.15, 130.11, 129.17, 129.00, 128.85, 127.67, 125.91, 111.62, 60.61, 45.15, 42.2; IR (ATR, cm−1): 3390, 3306 (N–H str of NH2), 3234 (N–H str), 3074 (Ar C–H str), 2939 (aliphatic C–H str), 1683 (C = O str), 1155 (S = O str), 738 (C–Cl str); LC-MS (ESI; M)+: 468.5.
4-((2-(3-(4-Chlorophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino) benzenesulphonamide (5b)
Yield: 68%; Rf: 0.46 (chloroform:methanol 9:1); M.P.: 179–181 °C; purity (HPLC): 98.02%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.86–7.84 (dd, 2H, Ar–H), 7.54–7.49 (m, 4H, Ar–H), 7.28–7.24 (dd, 2H, Ar–H), 7.10–7.06 (t, 2H, Ar–H), 6.89 (s 2H, −SO2NH2−), 6.66–6.64 (dd, 2H, Ar–H), 6.48–6.45 (t, 1H, Ar–NH), 5.62–5.58 (dd, 1H, Hx), 4.46–4.30 (dd, 2H, −CH2−), 3.91–3.84 (dd, 1H, Ha), 3.20–3.15 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 167.19, 160.64, 154.47, 151.43, 138.36, 135.78, 131.17, 130.12, 129.22, 128.94, 128.14, 127.67, 115.85, 111.65, 59.98, 45.15, 42.12; IR (ATR, cm−1): 3390, 3308 (N–H of NH2), 3236 (N–H str), 3074 (Ar C–H str), 2924 (aliphatic C–H str), 1683 (C = O str), 1157 (S = O str), 738 (C–Cl str); LC-MS (ESI; M)+: 486.8.
4-((2-(3-(4-Chlorophenyl)-5-(4-bromophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino) benzenesulphonamide (5c)
Yield: 75%; Rf: 0.44 (chloroform:methanol 9:1); M.P.: 182–185 °C; purity (HPLC): 99.11%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.88–7.86 (dd, 2H, Ar–H), 7.57–7.47 (m, 4H, Ar–H), 7.29–7.20 (dd, 2H, Ar–H), 7.16–7.10 (t, 2H, Ar–H), 6.93 (s, 2H, –SO2NH2–), 6.66–6.63 (dd, 2H, Ar–H), 6.58–6.55 (t, 1H, Ar–NH), 5.62–5.56 (dd, 1H, Hx), 4.42–4.35 (dd, 2H, –CH2–), 3.92–3.82 (dd, 1H, Ha), 3.22–3.15 (dd, 1H, Ha); 13C-NMR (100 MHz, DMSO-d6): δ = 166.81, 151.5, 142.96, 137.27, 133.84, 131.56, 131.09, 129.27, 128.74, 128.19, 127.7, 123.78, 111.62, 44.00; IR (ATR, cm−1): 3362, 3300 (N–H of NH2), 3230 (N–H str), 3080 (Ar C–H str), 2846 (aliphatic C–H str), 1683 (C = O str), 1155 (S = O str), 736 (C–Cl str), 580 (C–Br str).
4-((2-(3-(4-Chlorophenyl)-5-(4-methylphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5d)
Yield: 70%; Rf: 0.57 (chloroform:methanol 9:1); M.P.: 151–154 °C; purity (HPLC): 100%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.85–7.80 (dd, 2H, Ar–H), 7.58–7.50 (m, 4H, Ar–H), 7.10 (s, 4H, Ar–H), 6.89 (s, 2H, SO2NH2), 6.70–6.64 (dd, 2H, Ar–H), 6.45–6.42 (t, 1H, Ar–NH), 5.57–5.53 (dd, 1H, Hx), 4.46–4.30 (dd, 2H, CH2), 3.98–3.81 (dd, 1H, Ha), 3.18–3.12 (dd, 1H, Hb), 2.27 (s, 3H, Ar–CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 167.02, 154.44, 151.4, 139.28, 137.23, 135.76, 131.16, 130.32, 129.19, 128.84, 128.14, 127.69, 125.89, 111.64, 60.41, 45.16, 42.2, 21.12; IR (ATR, cm−1): 3362, 3294 (N–H of NH2), 3232 (N–H str), 3082 (Ar C–H str), 2853 (aliphatic C–H str), 1680 (C = O str), 1151 (S = O str), 740 (C–Cl str).
4-((2-(3-(4-Chlorophenyl)-5-(pyridin-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino) benzenesulphonamide (5e)
Yield: 71%; Rf: 0.41 (chloroform:methanol 9:1); M.P.: 161–164 °C; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 8.5 (s, 2H, Pyr–H), 7.88–7.85 (dd, 2H, Ar–H), 7.57–7.51 (m, 4H, Ar–H), 7.26–7.24 (dd, 2H, Ar–H), 6.93 (s, 2H, SO2NH2), 6.68–6.58 (dd, 2H, Ar–H), 6.61–6.58 (t, 1H, Ar–NH), 5.63–5.57 (dd, 1H, Hx), 4.54–4.32 (dd, 2H, CH2), 3.95–3.85 (dd, 1H, Ha), 3.25–3.17 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 168.78, 154.19, 151.09, 149.92, 149.12, 135.22, 130.73, 129.75, 128.86, 127.18, 120.81, 111.24, 59.23, 44.56, 41.12; IR (ATR, cm−1): 3390, 3309 (N–H of NH2), 3238 (N–H str), 3074 (Ar C–H str), 2926 (aliphatic C–H str), 1685 (C = O str), 1521 (C = N str), 1153 (S = O str), 736 (C–Cl str); LC-MS (ESI; M)+: 469.10.
4-((2-(3-(4-Chlorophenyl)-5-(2-chlorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5f)
Yield: 67%; Rf: 0.47 (chloroform:methanol 9:1); M.P.: 159–162 °C; purity (HPLC): 100%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.88–7.85 (dd, 2H, Ar–H), 7.57–7.51 (m, 5H, Ar–H), 7.29–7.25 (dd, 2H, Ar–H), 7.16 (s, 1H, Ar–H), 6.93 (s, 2H, SO2NH2), 6.69–6.66 (dd, 2H, Ar–H), 6.63–6.59 (t, 1H, Ar–NH), 5.82–5.77 (dd, 1H, Hx), 4.57–4.38 (dd, 2H, CH2), 4.02–3.92 (dd, 1H, Ha), 3.29–3.23 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 167.29, 154.58, 151.42, 138.82, 135.87, 131.41, 131.24, 130.06, 129.98, 129.4, 129.19, 128.9, 127.89, 127.69, 126.9, 111.69, 58.37, 45.15, 41.1; IR (ATR, cm−1): 3267, 3207 (N–H of NH2), 3146 (N–H str), 3097 (Ar C–H str), 2947 (aliphatic C–H str), 1670 (C = O str), 1195 (S = O str), 759 (C–Cl str); LC-MS (ESI; M)+: 503.40.
4-((2-(3-(4-Chlorophenyl)-5-(2-hydroxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5g)
Yield: 72%; Rf: 0.41 (chloroform:methanol 9:1); M.P.: 161–163 °C; purity (HPLC): 98.58%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 10.85 (s, 1H, Ar–OH), 7.85–7.78 (m, 3H, Ar–H), 7.55–7.50 (m, 3H, Ar–H), 7.48–7.41 (dd, 3H, Ar–H), 6.92 (s, 2H, SO2NH2), 6.69–6.66 (dd, 3H, Ar–H), 6.48–6.45 (t, 1H, Ar–NH), 4.35–4.32 (dd, 2H, CH2), 4.01–3.99 (dd, 1H, Ha), 3.35 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 171.95, 151.54, 147.39, 137.25, 134.4, 131.04, 129.16, 128.73, 128.4, 128.17, 127.72, 111.63, 44.44; IR (ATR, cm−1): 3566 (OH str), 3356, 3300 (N–H of NH2), 3259 (N–H str), 3072 (Ar C–H str), 2924 (aliphatic C–H str), 1670 (C = O str), 1147 (S = O str), 729 (C–Cl str).
4-((2-(3-(4-Chlorophenyl)-5-(3,4-dimethoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino)benzenesulphonamide (5h)
Yield: 70%; Rf: 0.61 (chloroform:methanol 9:1); M.P.: 170–173 °C; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.88–7.82 (dd, 2H, Ar–H), 7.57–7.47 (m, 4H, Ar–H), 6.92 (s, 2H, SO2NH2), 6.87–6.80 (dd, 2H, Ar–H), 6.71–6.65 (dd, 2H, Ar–H), 6.63–6.59 (t, 1H, Ar–NH), 5.82–5.77 (dd, 1H, Hx), 4.57–4.38 (dd, 2H, CH2), 4.02–3.92 (dd, 1H), 3.29–3.23 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 167.18, 154.48, 149.29, 148.54, 135.67, 134.81, 131.16, 130.29, 129.22, 128.92, 127.66, 117.88, 112.31, 111.62, 110.01, 60.43, 56.02, 55.94; IR (ATR, cm−1): 3329 (N–H of NH2), 3209 (N–H str), 3087 (Ar C–H str), 2899 (aliphatic C–H str), 1689 (C = O str), 1153 (S = O str), 732 (C–Cl str).
4-((2-(3-(4-Chlorophenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5i)
Yield: 68%; Rf: 0.45 (chloroform:methanol 9:1); M.P.: 148–150 °C; purity (HPLC): 96.06%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 7.89–7.87 (dd, 2H, Ar–H), 7.54–7.50 (m, 4H, Ar–H), 7.37–7.35 (dd, 1H, Ar–H), 7.05–7.04 (dd, 1H, Ar–H), 6.94–6.92 (dd, 1H, Ar–H), 6.91 (s, 2H, SO2NH2), 6.66–6.64 (dd, 2H, Ar–H), 6.57–6.54 (t, 1H, Ar–NH), 5.93–5.89 (dd, 1H, Hx), 4.41–4.28 (dd, 2H, CH2), 3.90–3.83 (dd, 1H, Ha), 3.41–3.40 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 167.3, 154.67, 151.49, 135.8, 131.16, 130.1, 129.31, 129.03,127.66,127.14, 125.48, 125.2, 111.65, 56.14, 45.13, 41.89; IR (ATR, cm−1): 3390, 3308 (N–H of NH2), 3236 (N–H str), 3074 (Ar C–H str), 2924 (aliphatic C–H str), 1683 (C = O str), 1157 (S = O str), 738 (C–Cl str); LC-MS (ESI; M)+: 475.0.
4-((2-(5-(Anthracen-9-yl)-3-(4-chlorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5j)
Yield: 71%; Rf: 0.62 (chloroform:methanol 9:1); M.P.: 190–192 °C; purity (HPLC): 100%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 8.75–7.61 (m, 4H, Ar–H), 8.22–8.02 (m, 4H, Ar–H), 7.59–7.49 (m, 12H, Ar–H), 6.95–6.92 (dd, 3H, Hx, SO2NH2), 6.75–6.68 (dd, 2H, Ar–H), 6.58–6.55 (t, 1H, Ar–NH), 4.38–4.35 (dd, 2H, CH2), 4.01–4.00 (dd, 1H, Ha), 3.35 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 166.86, 151.57, 146.71, 137.28, 134.37, 131.66, 131.38, 131.15, 131.05, 130.07, 129.93, 129.81, 129.4, 128.74, 128.42, 128.21, 127.82, 127.74, 127.52, 125.89, 125.84, 125.55, 125.45, 111.63, 44.43, 44.17; IR (ATR, cm−1): 3342 (N–H of NH2), 3277 (N–H str), 3051 (Ar C–H str), 2862 (aliphatic C–H str), 1672 (C = O str), 1161 (S = O str), 761 (C–Cl str).
4-((2-(3-(4-Chlorophenyl)-5-(1H-indol-3-yl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5k)
Yield: 73%; Rf: 0.55 (chloroform:methanol 9:1); M.P.: 174–177 °C; purity (HPLC): 100%; 1H-NMR (300 MHz, DMSO-d6) δ ppm: 11.23 (s, 1H, Indole–NH), 8.23–8.15 (dd, 2H, Ar–H), 7.73–7.71 (dd, 1H, Ar–H), 7.57–7.55 (dd, 2H, Ar–H), 7.44 (dd, 1H, Ar–H), 7.22–7.18 (t, 2H, Ar–H), 6.90 (s, 2H, SO2NH2), 6.71–6.65 (t, 3H, Ar–H, Hx), 6.52–6.49 (t, 1H, Ar–NH), 4.37–4.31 (dd, 2H, CH2), 3.88–3.86 (dd, 1H, Ha), 3.50–3.43 (dd, 1H, Hb); 13C-NMR (100 MHz, DMSO-d6): δ = 165.68, 151.6, 141.54, 137.53, 131.47, 131.02, 130.3, 127.77, 124.76, 124.58, 122.93, 121.94, 120.98, 120.71, 112.26, 111.89, 111.75, 111.56, 56.67, 44.06; IR (ATR, cm−1): 3174 (N–H of NH2), 3113 (N–H str), 3041 (Ar C–H str), 2820 (aliphatic C–H str), 1672 (C = O str), 1122 (S = O str), 740 (C–Cl str).
4-((2-(3-Methyl-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino) benzene sulphonamide (5l)
Yield: 79%; Rf: 0.63 (chloroform:methanol 9:1); M.P.: 211– 213 °C; purity (HPLC): 99.16%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.68–7.61 (dd, 2H, Ar–H), 7.53–7.51 (m, 3H, Ar–H), 7.47–7.45 (dd, 2H, Ar–H), 7.34–7.32 (dd, 1H, Hx), 7.19–7.08 (dd, 1H, Hb), 6.95 (S, 2H, SO2–NH2), 6.67–6.64 (dd, 2H, Ar–H), 6.51–6.48 (t, 1H, Ar–NH), 4.27–4.22 (dd, 2H, CH2), 3.99–3.95 (dd, 1H, CHHa), 2.08 (s, 3H, CH3); IR (ATR) cm−1: 3404 (N–H str of NH2), 3302 (N–H str), 3078 (aromatic C–H str), 2885 (aliphatic C–H str), 1693 (C = O str), 1610 (C = N), 1143 (S = O str); LC-MS (ESI; M + 1)+: 373.1.
4-((2-(5-(4-Methoxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5m)
Yield: 85%; Rf: 0.65 (chloroform:methanol 9:1); M.P.: 235 –237 °C; purity (HPLC): 98.89%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.58–7.56 (dd, 2H, Ar–H), 7.50–7.48 (dd, 2H, Ar–H), 7.46–7.44 (dd, 1H, Hx), 6.95–6.93 (dd, 1H, Hb), 6.92–6.90 (dd, 2H, Ar–H), 6.8 (S, 2H, SO2–NH2), 6.69–6.67 (dd, 2H, Ar–H), 6.32–6.30 (t, 1H, Ar–NH), 4.26–4.25 (dd, 2H, CH2), 4.0–3.99 (dd, 1H, CHHa), 3.79 (s, 3H, OCH3), 2.08 (s, 3H, CH3); IR (ATR) cm−1: 3404 (N–H str of NH2), 3333 (N–H str), 3248 (O–C str) 3030 (Ar C–H str), 2839 (aliphatic C–H str), 1660 (C = O str), 1604 (C = N), 1153 (S = O str); LC-MS (ESI; M + 1)+: 403.1.
4-((2-(5-(4-Chlorophenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5n)
Yield: 85%; Rf: 0.61 (chloroform:methanol 9:1); M.P.: 221– 225 °C; purity (HPLC): 98.89%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.61–7.54 (dd, 2H, Ar–H), 7.54–7.51 (dd, 2H, Ar–H), 7.43–7.33 (dd, 2H, Ar–H), 7.01–6.99 (dd, 1H, Hx), 6.97–6.93 (dd, 1H, Hb), 6.87 (S, 2H, SO2–NH2), 6.69–6.67 (dd, 2H, Ar–H), 6.32–6.30 (t, 1H, Ar–NH), 4.26–4.25 (dd, 2H, CH2), 3.99–3.40 (dd, 1H, CHHa), 2.1 (s, 3H, CH3); IR (ATR) cm−1: 3408 (N–H str of NH2), 3288 (N–H str), 2885 (aliphatic C–H str), 1697 (C = O str), 1608 (C = N), 1145 (S = O str), 810 (C–Cl str); LC-MS (ESI; M + 1)+: 407.1.
4-((2-(3-Methyl-5-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzene sulphonamide (5o)
Yield: 78%; Rf: 0.7 (chloroform:methanol 9:1); M.P.: 233–235 °C; purity (HPLC): 99.46%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.76–7.75 (dd, 2H, Ar–H), 7.57–7.55 (dd, 2H, Ar–H), 7.35–7.34 (dd, 1H, Hx), 7.11–7.09 (dd, 2H, Ar–H), 6.87 (S, 2H, SO2–NH2), 6.84–6.81 (dd, 1H, Hb), 6.64–6.60 (t, 1H, Ar–NH), 6.62–6.60 (dd, 2H, Ar–H), 4.24–4.23 (dd, 2H, CH2), 3.97–3.96 (dd, 1H, CHHa), 2.27 (s, 3H, Ar–CH3), 2.04 (s, 3H, CH3); IR (ATR) cm−1: 3319 (NH str of NH2), 3232 (NH str), 3113 (Ar C–H str), 2916 (aliphatic C–H str), 1668 (C = O str), 1600 (C = N), 1151 (S = O str); LC-MS (ESI; M + 1)+: 387.1.
4-((2-(5-(4-Fluorophenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5p)
Yield: 76%; Rf: 0.81 (chloroform:methanol 9:1); M.P.: 217–219 °C; purity (HPLC): 99.67%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.68–7.64 (dd, 2H, Ar–H), 7.53–7.51 (dd, 2H, Ar–H), 7.28–7.22 (dd, 2H, Ar–H), 7.12–7.08 (dd, 1H, Hx), 6.94 (S, 2H, SO2–NH2), 6.68–6.66 (dd, 1H, Hb), 6.68–6.66 (dd, 2H, Ar–H), 6.52–6.49 (t, 1H, Ar–NH), 4.27–4.25 (dd, 2H, CH2), 3.99–3.97 (dd, 1H, CHHa), 2.1 (s, 3H, CH3); IR (ATR) cm−1: 3406 (NH str of NH2), 3294 (NH str), 3051 (Ar C–H str), 2901 (aliphatic C–H str), 1693 (C = O str), 1600 (C = N), 1307 (C–F str), 1145 (S = O str); LC-MS (ESI; M + 1)+: 391.23.
4-((2-(5-(2-Chlorophenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5q)
Yield: 76%; Rf: 0.64 (chloroform:methanol 9:1); M.P.: 202–205 °C; purity (HPLC): 99.40%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.54–7.52 (dd, 2H, Ar–H), 7.47–7.41 (dd, 1H, Hx), 7.37–7.28 (m, 4H, Ar–H), 7.00–6.94 (dd, 1H, Hb), 6.90 (S, 2H, SO2–NH2), 6.68–6.66 (dd, 2H, Ar–H), 6.46–6.43 (t, 1H, Ar–NH), 4.27–4.25 (dd, 2H, CH2), 4.0–3.9 (dd, 1H, CHHa), 2.1 (s, 3H, CH3); IR (ATR) cm−1: 3406 (NH str of NH2), 3279 (NH str), 3064 (Ar C–H str), 2885 (aliphatic C–H str), 1693 (C = O str), 1610 (C = N), 1141 (S = O str), 746 (C–Cl str); LC-MS (ESI; M + 1)+: 407.1.
4-((2-(5-(2-Hydroxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5r)
Yield: 86%; Rf: 0.59 (chloroform:methanol 9:1); M.P.: 211–213 °C; purity (HPLC): 99.35%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.69 (S, 1H, Ar–OH), 7.55–7.53 (dd, 2H, Ar–H), 7.50–7.48 (dd, 1H, Ar–H), 7.25–7.2 (dd, 1H, Ar–H), 7.09–7.05 (dd, 1H, Ar–H), 6.97–6.92 (dd, 1H, Hx), 6.87–6.856 (dd, 1H, Hb), 6.85 (S, 2H, SO2–NH2), 6.80–6.76 (dd, 1H, Ar–H), 6.67–6.65 (dd, 2H, Ar–H), 6.34–6.31 (t, 1H, Ar–NH), 4.24–4.23 (dd, 2H, CH2), 3.97–3.96 (dd, 1H, CHHa), 2.1 (s, 3H, CH3); IR (ATR) cm−1: 3336 (NH str), 3252 (OH str), 2893 (aliphatic C–H str), 1658 (C = O str), 1600 (C = N), 1153 (S = O str); LC-MS (ESI; M + 1)+: 389.1.
4-((2(5-(4-Hydroxyphenyl)-3-methyl-4,5-dihydro-1H-pyrazol-1- yl)-2-oxoethyl) amino)benzenesulphonamide (5s)
Yield: 77%; Rf: 0.50 (chloroform:methanol 9:1);M.P.: 243–246 °C; purity (HPLC): 99.57%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 9.52 (S, 1H, Ar–OH), 7.55–7.53 (dd, 2H, Ar–H), 7.36–7.3 (dd, 2H, Ar–H), 6.92–6.86 (dd, 1H, Hb), 6.84 (S, 2H, SO2–NH2), 6.76–6.74 (dd, 2H, Ar–H), 6.67–6.64 (dd, 2H, Ar–H), 6.54–6.53 (dd, 1H, Hx), 6.52–6.49 (t, 1H, Ar–NH), 4.23–4.21 (dd, 2H, CH2), 3.96–3.95 (dd, 1H, CHHa), 2.06 (s, 3H, CH3); IR (ATR) cm−1: 3389 (NH str of NH2), 3315 (NH str), 3263 (OH str), 3020 (Ar C–H str), 2902 (aliphatic C–H str), 1680 (C = O str), 1602 (C = N), 1143 (S = O str); LC-MS (ESI; M + 1)+: 389.1.
4-((2-(5-(4-(Dimethylamino)phenyl)-3-methyl-4,5-dihydro-1Hpyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide) (5t)
Yield: 86%; Rf: 0.7 (chloroform:methanol 9:1); M.P.: 239–240 °C; purity (HPLC): 99.26%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.53–7.51 (dd, 2H, Ar–H), 7.43–7.41 (dd, 2H, Ar–H), 6.99–6.95 (dd, 1H, Hb), 6.93 (S, 2H, SO2–NH2), 6.72–6.70 (dd, 2H, Ar–H), 6.72–6.70 (dd, 1H, Hx), 6.68–6.66 (dd, 2H, Ar–H), 6.50–6.47 (t, 1H, Ar–NH) , 4.25–4.24 (dd, 2H, CH2), 3.97–3.95 (dd, 1H, CHHa), 2.07 (s, 3H, CH3); IR (ATR) cm−1: 3325 (N–H str of NH2), 3236 (N–H str), 3107 (Ar C–H str), 2899 (aliphatic C–H str), 1660 (C = O str), 1602 (C = N str), 1311 (C–N ter. amine str), 1238 (C–N aromatic str) 1153 (S = O str); LC-MS (ESI; M + 1)+: 416.1.
4-((2-(3-Methyl-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl) amino)benzenesulphonamide (5u)
Yield: 82%; Rf: 0.63 (chloroform:methanol 9:1); M.P.: 197–199 °C; purity (HPLC): 99.099%; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 7.55–7.53 (dd, 2H, Ar–H), 7.38–7.37 (dd, 1H, Ar–H), 7.13–7.08 (dd, 1H, Ar–H), 7.08–7.06 (dd, 1H, Hb), 7.01–7.00 (dd, 1H, Ar–H), 6.83 (S, 2H, SO2–NH2), 6.66–6.64 (dd, 2H, Ar–H), 6.46–6.45 (dd, 1H, Hx), 6.26 (t, 1H, Ar–NH), 4.23–4.22 (dd, 2H, CH2), 3.97–3.96 (dd, 1H, CHHa), 2.05 (s, 3H, CH3); IR (ATR) cm−1: 3402 (NH str of NH2), 3296 (NH str), 3074 (Ar C–H str), 2881 (aliphatic C–H str), 1691 (C = O str), 1600 (C = N), 1143 (S = O str); LC-MS (ESI; M + 1)+: 379.1.
4-((2-(2-Benzylidenehydrazinyl)-2-oxoetehyl) amino) benzenesulphonamide (6a)
Yield: 80%; Rf: 0.69; M.P.: 223–226 °C; 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.51 (s, 1H, NH), 8.02 (s, 1H, CH), 7.72–7.67 (m, 4H), 7.45–7.38 (m, 5H), 6.87 (s, 2H, NH2), 6.32 (t, 1H, NH), 4.33 (d, 2H, CH2); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.49 (CH2), 170.60 (C = O), 143.67 (N = CH); IR (ATR) cm−1: 3313 (N–H str of NH2), 3255 (N–H str), 3173 (Ar C–H str), 3070 (aliphatic C–H str), 1681 (C = N str), 1145 (S = O str); LC-MS (ESI; M + 1)+: 333.2.
4-((2-(2-(4-Methylbenzylidene) hydrazinyl)-2-oxoethyl)amino)benzene sulphonamide (6b)
Yield: 85%; Rf: 0.75; M.P.: 220–225 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.44 (s, 1H, NH), 7.98 (s, 1H, CH), 7.59–7.57 (m, 4H), 7.23–7.19 (dd, 2H), 6.86 (s, 2H, NH2), 6.70–6.65 (dd, 2H), 6.30 (t,1H, NH), 4.29 (d, 2H, CH2), 2.39 (s,3H, CH3); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.47 (CH2), 170.44 (C = O), 143.78 (N = CH), 21.03 (CH3); IR (ATR) cm−1: 3319 (N–H str of NH2), 3257 (N–H str), 3194 (Ar C–H str), 3078 (aliphatic C–H str), 1683 (C = N str), 1145 (S = O str); LC-MS (ESI; M)+: 346.9.
4-((2-(2-(2-Hydroxybenzylidene)hydrazinyl)-2-oxoethyl) amino) benzenesulphonamide (6c)
Yield: 85%; Rf: 0.82; M.P.: 213–216 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.50 (s, 1H, NH), 10.01 (s, 1H, OH), 8.31 (s, 1H, CH), 7.58–7.54 (dd, 2H), 7.45–7.43 (d,1H), 7.27–7.21 (d,1H), 6.86 (s, 2H, NH2), 6.69–6.65 (m, 4H), 6.41 (t, 1H, NH), 4.29 (d, 2H, CH2); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.48 (CH2), 170.10 (C = O), 144.10 (N = CH); IR (ATR) cm−1: 3443 (O-H str), 3369 (N–H str of NH2), 3265 (N–H str), 3113 (Ar C–H str), 3093 (aliphatic C–H str), 1681 (C = N str), 1120 (S = O str); LC-MS (ESI; M)+: 348.3.
4-((2-Oxo-2-(2-(pyridin-2-ylmethylene) hydrazinyl) ethyl) amino) benzenesulphonamide (6d)
Yield 80%; Rf: 0.78; M.P.: 245–250 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.74 (s, 1H, NH), 8.02 (s, 1H, CH), 7.95–7.93 (m, 4H), 7.86–7.79 (d, 1H), 7.39–7.36 (d, 1H), 6.91 (s, 2H, NH2), 6.46 (t, 1H, NH), 4.36 (d, 2H, CH2); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.50 (CH2), 170.82 (C = O), 144.19 (N = CH); IR (ATR) cm−1: 3354 (N–H str of NH2), 3236 (N–H str), 3097 (Ar C–H str), 3072 (aliphatic C–H str), 1685 (C = N str), 1145 (S = O str); LC-MS (ESI; M + 1)+: 334.3.
4-((2-(2-(3,4-Dimethoxybenzylidene)hydrazinyl)-2-oxoethyl)amino) benzenesulphonamide (6e)
Yield: 86%; Rf: 0.84; M.P.: 230–233 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.40 (s, 1H, NH), 7.94 (s, 1H, CH), 7.57–7.54 (m, 4H), 7.20–6.94 (m, 3H), 6.88 (s, 2H, NH2), 6.69 (t, 1H, NH), 4.31 (d, 2H, CH2), 3.85 (s, 6H, 2-OCH3); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.48 (CH2), 170.28 (C = O), 143.46 (N = CH), 55.11 (2O–CH3); IR (ATR) cm−1: 3306 (N–H str of NH2), 3248 (N–H str), 3171 (Ar C–H str), 3086 (aliphatic C–H str), 1683 (C = N str), 1143 (S = O str), 1255 (C–O str); LC-MS (ESI; M + 1)+: 393.29.
4-((2-(2-(4-Methoxybenzylidene) hydrazinyl)-2-oxoethyl) amino) benzenesulphonamide (6f)
Yield: 85%; Rf: 0.79; M.P.: 240–245 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.40 (s, 1H, NH), 7.96 (s, 1H, CH), 7.66–7.61 (m, 4H), 6.99–6.95 (dd, 2H), 6.90 (s, 2H, NH2), 6.70–6.65 (dd, 2H), 6.43–6.41 (t,1H, NH), 4.29 (d, 2H, CH2), 3.81 (s, 3H, OCH3); 13C-NMR (400 MHz, DMSO-d6): δ ppm 43.52 (CH2), 170.37 (C = O), 143.71 (N = CH), 55.49 (O–CH3); IR (ATR) cm−1: 3294 (N–H str of NH2), 3275 (N–H str), 3198 (Ar C–H str), 3099 (aliphatic C–H str), 1680 (C = Nstr), 1143 (S = O str); LC-MS (ESI; M)+: 362.9.
4-((2-(2-(4-Hydroxybenzylidene)hydrazinyl)-2-oxoethyl) amino) benzenesulphonamide (6g)
Yield 80%; Rf: 0.81; M.P.: 214–218 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.25 (s, 1H, NH), 9.73 (s, 1H, OH), 7.85 (s, 1H, CH), 7.50–7.43 (m, 4H), 6.77–6.72 (dd, 2H), 6.63–6.60 (dd, 2H), 6.82 (s, 2H, NH2), 6.58 (t, 1H, NH), 4.20 (d, 2H, NH); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.48 (CH2), 170.10 (C = O), 144.10 (N = CH); IR (ATR) cm−1: 3392 (O-H str), 3313 (N–H str of NH2), 3292 (N–H str), 3211 (Ar C–H str), 3022 (aliphatic C–H str), 1685 (C = N str), 1136 (S = O str); LC-MS (ESI; M + 1)+: 349.3.
4-((2-(2-(4-Hydroxy-3,5-dimethoxybenzylidene)hydrazinyl)-2-oxoethyl)amino) benzenesulphonamide (6h)
Yield: 85%; Rf: 0.85; M.P.: 255–260 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.33 (s, 1H, NH), 9.83 (s, 1H, OH), 7.82 (s, 1H, CH), 7.51–7.47 (dd, 2H), 6.83–6.81 (dd, 2H), 6.88 (s, 2H, NH2), 6.63–6.56 (s,2H), 6.35 (t,1H, NH), 4.26 (d, 2H, CH2), 3.78 (s, 6H, 2OCH3); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.48 (CH2), 170.29 (C = O), 147.98 (N = CH), 55.95 (O–CH3); IR (ATR) cm−1: 3408 (O-H str), 3304 (N–H str of NH2), 3279 (N–H str), 3196 (Ar C–H str), 3106 (aliphatic C–H str), 1685 (C = N str), 1143 (S = O str), 1219 (C–O str), LC-MS (ESI; M + 1)+: 409.3.
4-((2-Oxo-2-(2-(3,4,5-trimethoxybenzylidene) hydrazinyl)ethyl) amino) benzenesulphonamide (6i)
Yield: 80%; Rf: 0.80; M.P.: 250–253 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.33 (s, 1H, NH), 7.82 (s, 1H, CH), 7.51–7.47 (dd, 2H), 6.83–6.81 (dd, 2H), 6.88 (s, 2H, NH2), 6.63–6.56 (dd, 2H), 6.35 (t, 1H, NH), 4.26 (d, 2H, CH2), 3.78 (s, 6H, 2OCH3), 3.82 (s, 3H, OCH3); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 40.21 (CH2), 170.47 (C = O), 143.62 (N = CH), 55.11 (O–CH3), 60.03 (2O–CH3); IR (ATR) cm−1: 3304 (N–H str of NH2), 3271 (N–H str), 3201 (Ar C–H str), 3107 (aliphatic C–H str), 1683 (C = Nstr), 1144 (S = O str), 1236 (C–O str); LC-MS (ESI; M + 1)+: 423.3.
4-((2-(2-(2,3-Dimethoxybenzylidene)hydrazinyl)-2-oxoethyl)amino)benzenesulphonamide (6j)
Yield: 80%; Rf: 0.77; M.P.: 240–244 °C. 1H-NMR (400 MHz, DMSO-d6) δ ppm: 11.40 (s, 1H, NH), 7.94 (s, 1H, CH), 7.57–7.54 (dd, 2H), 7.32–7.31 (dd, 2H), 7.20–6.94 (m, 3H), 6.88 (s, 2H, NH2), 6.69–6.65 (t, 1H, NH), 4.31 (d, 2H, CH2), 3.85 (s, 6H, OCH3); 13C-NMR (400 MHz, DMSO-d6) δ ppm: 43.48 (CH2), 170.28 (C = O), 143.46 (N = CH), 55.11 (2O–CH3); IR (ATR) cm−1: 3387 (N–H str of NH2), 3255 (N–H str), 3186 (Ar C–H str), 3070 (aliphatic C–H str), 1687 (C = Nstr), 1172 (S = O str), 1230 (C–O str); LC-MS (ESI; M)+: 392.9.
2.2. CA activity/inhibition studies
An Sx.18Mv-R Applied Photophysics (Oxford, UK) stopped-flow instrument has been used to assay the catalytic activity of various CA isozymes for CO2 hydration reactionCitation20. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.5) or 10 mM Tris (pH 8.5) as buffers, and 0.1 M Na2SO4 (for maintaining constant ionic strength, which is not inhibitory against these enzymes), following the CA-catalysed CO2 hydration reaction for a period of 10 s at 25 °C. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and activation constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial rate. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitors (10 mM) were prepared in distilled-deionised diluted to 1 nM using the assay buffer. Inhibitor and enzyme solutions were pre-incubated together for 15 min (standard assay at room temperature) prior to assay, in order to allow for the formation of the enzyme–inhibitor complex. The inhibition constant (KI), was obtained by considering the classical Michaelis–Menten equation and the Cheng-Prusoff algorithm by using non-linear least squares fitting as reported earlierCitation21–24.
3. Results and discussion
3.1. Chemistry
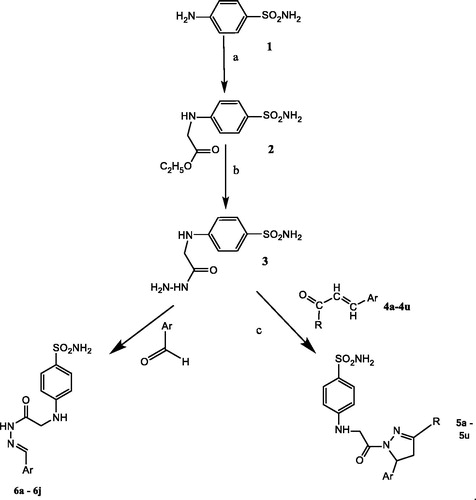
The key intermediate 1 (ethyl 2-((4-sulphamoylphenyl) amino) acetate) was obtained in good yields by the reaction of sulphanilamide with ethyl bromoacetate in the presence of potassium carbonate (no sulphonamide N-alkylation occurred), Scheme 1. During optimisation, the same reaction was performed with sodium carbonate instead of potassium carbonate. The results were not satisfactory, and whence the equimolar ratio of both the reactants in the presence of K2CO3 and ethanol as a solvent was used to obtain compound 1. 4-((2-Hydrazinyl-2-oxoethyl)amino) benzene sulphonamide (2) was obtained by the reaction of hydrazine hydrate with 1 in equimolar ratios. The reaction was refluxed for 3 h at 70 °C producing the compound with yields of 70–72%. Reaction of 2 with various substituted aromatic aldehydes afforded derivatives 6. When using unsaturated aldehydes, a cyclisation reaction occurred after the Schiff base formation, leading to compounds 5 (Scheme 1).
Scheme 1. Synthesis of compounds 5a–5u and 6a–6j. Reagents and conditions were: (a) Br–CH2COOC2H5, K2CO3, EtOH, reflux, 12 h; (b) NH2NH2·H2O, EtOH, reflux, 4–6 h. (c) For compounds 5a–5k EtOH, KOH, reflux, 4 h, overnight stirring and for 5l–5u glacial acetic acid, reflux.
The structures of 4-((2-(3-alky/aryl-5-ary/heteroaryl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino)benzenesulphonamides 5a–5u were confirmed by mass spectrometry (MS), Fourier-transform infrared spectroscopy (FTIR) and 1H and 13C-nuclear magnetic resonance (NMR). The structures of compounds were analysed by FTIR spectra which revealed that N–H stretches of amines in the region 3500–3000 cm−1. Spectra revealed presence of C = O stretching vibrations of amide in the region 1695–1630 cm−1, aliphatic C–H stretching vibration was observed in 2920–2800 cm−1 and C = N stretching vibration was observed at 1615–1564 cm−1. Further evidence for formation of target compound was obtained from 1H-NMR spectra which provided diagnostic tool for the positional elucidation of the protons. The Ar–NH proton was appeared at δ = 6.58 ppm as triplet. The formed pyrazoline was confirmed with doublet of doublet for CH2 giving signal δ = 4.0–3.8 ppm (Ha), Hb at δ 7.1–6.6 ppm (compounds 5l–5u) and at δ = 3.26–3.13 ppm (compounds 5a–5k), CH giving signal at δ = 7.4–7.1 ppm (Hx of series 5l–5u) and δ = 5.61–5.56 ppm (Hx of series 5a–5k). The characteristic doublet signal of aromatic protons was observed between δ = 7.9 and 6.6 ppm. The NH protons of SO2–NH2 as singlet between δ = 6.94 and 6.80 ppm was observed. Singlet in the range of δ = 6.59–6.2 ppm for Ar–NH protons was observed. The characteristic singlet signal of CH3 protons in series 5l–5u was observed between δ = 2.1 and 2.04 ppm. Mass spectroscopy was done for newly synthesised compounds. The base peak m/z for the compounds were found as (M + 1)+ with respective to their molecular weight except for 5i it is was found to be (M)+.
High-performance liquid chromatography (HPLC) was done for newly synthesised compounds. Using area normalisation method, the percent purity for the compounds was found to be above 88%. The structures of novel Schiff bases 6a–6j were confirmed by MS, FTIR and 1H and 13C-NMR. The IR spectra displayed an intense absorption band in the range of 1615–1630 cm−1, characteristic of the carbonyl groups. Additionally, intense bands, originating from the stretching vibration of the C = N group of the azomethine were observed at 1685 and 3313 cm−1 for N–H str of NH2 following the aliphatic C–H str displayed vibration at 3022 cm−1. Further, we observed 1H and 13C-NMR interpretation for singlet peak at chemical shift δ range in 7.95–8.02 ppm and 143.71–144.10 ppm, confirming the presence of azomethine group in the compound, respectively ( and ).
Table 1. Physicochemical properties of 4-((2-(3-alky/aryl-5-ary/heteroaryl-4,5-dihydro-1H-pyrazol-1-yl)-2-oxoethyl)amino)benzenesulphonamide derivatives (5a–5u).
Table 2. Physicochemical properties of 4-((2-(arylmethylidene)hydrazinyl)-2-oxoethyl)amino) benzene sulphonamide derivatives (6a–6j).
3.2. CA inhibition
The compounds 5 and 6 reported here were investigated as inhibitors of three CAs involved in crucial physiologic processes and known to act as drug targets, i.e. the human (h) isoforms hCA I and II (belonging to the α-CA class) and the bacterial enzyme mtCA3 from Mycobacterium tuberculosis (a β-class CA) (). Acetazolamide (AAZ), a clinically used sulphonamide has been employed as standard inhibitor in the assay.
Table 3. hCA I, II and mtCA 3 inhibition data of compounds 5 and 6 reported in the article, by a stopped-flow CO2 hydrase assayCitation20.
As seen from data of , all investigated compounds inhibited the three enzymes, but generally with a medium potency. Thus, the inhibition constants (KIs) were in the range of 54.6 nM–1.8 µM against hCA I, in the range of 32.1 nM–5.5 µM against hCA II and of 127 nM–2.12 µM against mtCA 3, showing a quite flat structure–activity relationship, except for some particular cases which will be discussed in detail.
Thus, for hCA I, the best inhibitors were 5d, 5j and 6c, with KIs ranging between 54.6 and 93.4 nM, being thus much better inhibitors compared to the standard acetazolamide (KI of 250 nM, ). These compounds incorporate p-chlorophenyl and p-tolyl moieties (5d), p-chlorophenyl and 9-anthranyl (5j) moieties, and the 2-hydroxyphenyl fragment in the case of 6c, which are in fact not very different from those found in compounds showing a much worse inhibitory pattern (e.g. 5c, 5k, 6e, etc.). Thus, the explanation that we propose is that the quite long and flexible linker between the benzenesulphonamide fragment and the imine or heterocylic parts of the molecule, affords for a multitude of diverse orientation of the tail present in these compounds, which is probably detrimental to a tight binding, except for the few cases mentioned above, i.e. 5d, 5j and 6c, for which probably some of these conformations assure good interactions with the enzyme active site. However, for the majority of these derivatives, these various conformations/orientations may be not favourable, which explain why most of them have inhibition constants in the high nanomolar–micromolar range ().
More or less the same situation was observed for the inhibition of hCA II, but for this isoform the most effective inhibitors were 5e, 5r, 5s and 6c, with KIs ranging between 32.1 and 83.1 nM (). Compound 5e is the only derivative incorporating a 4-pyridyl moiety, which seems to be effective in inducing strong hCA II inhibitory effects, whereas the remaining ones incorporate 2- or 4-hydroxyphenyl groups. However, the change of these groups to halogens or to methoxy leads to a strong loss of inhibitory effects. The explanation we propose is the same as above for the discussion of hCA I inhibition data.mtCA3 was also effectively inhibited by several of the new compounds, such as 5e, 5g, 5j and 5k, which showed KIs ranging between 127 and 157 nM (acetazolamide has an inhibition constant of 104 nM, being only slightly more effective compared to these sulphonamides). However, the largest majority of these derivatives showed KIs in the range of 250 nM–2.12 µM, being thus much less effective inhibitors.
4. Conclusion
We report here a new series of sulphonamide derivatives, which was obtained by reaction of a hydrazide derivative with aromatic/heterocyclic aldehydes, followed by an eventual cyclisation to a five-membered heterocylic system. The compounds were designed to incorporate moieties known to induce effective inhibitory for CA isoforms involved in crucial physiologic or pathologic processes such as the cytosolic hCA I and hCA II and the bacterial enzyme mtCA3 from Mycobacterium tuberculosis. The compounds acted as effective-medium potency inhibitors, with KIs in the range of 54.6 nM–1.8 µM against hCA I, in the range of 32.1 nM–5.5 µM against hCA II and of 127 nM–2.12 µM against mtCA 3.
Acknowledgements
The authors are thankful to Dr V. J. Kadam, Principal, Bharati Vidyapeeth’s College of Pharmacy, Navi Mumbai for providing necessary research facilities in the department.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- (a) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. (b) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. (c) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (d) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77. (e) Supuran CT, Vullo D, Manole G, et al. Designing of novel carbonic anhydrase inhibitors and activators. Curr Med Chem Cardiovasc Hematol Agents 2004;2:49–68. (f) Taslimi P, Gulcin I, Ozgeris B, et al. The human carbonic anhydrase isoenzymes I and II (hCA I and II) inhibition effects of trimethoxyindane derivatives. J Enzyme Inhib Med Chem 2015;31:152–7.
- (a) Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J Enzyme Inhib Med Chem 2013;28:229–30. (b) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. (c) Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. (d) Abbate F, Winum JY, Potter BV, et al. Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isozyme II with EMATE, a dual inhibitor of carbonic anhydrases and steroid sulfatase. Bioorg Med Chem Lett 2004;14:231–4.
- (a) Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;2:681–91. (b) Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Exp Opin Ther Pat 2013;23:705–16. (c) Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Exp Opin Ther Pat 2013;23:725–35. (d) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:E25.
- (a) Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Exp Opin Ther Pat. 2013;2:737–49. (b) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites. 2017;7:E48. (c) Ward C, Langdon SP, Mullen P, et al. 171–9. (d) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;1:3102–8. (e) Casey JR, Morgan PE, Vullo D, et al. Carbonic anhydrase inhibitors. Design of selective, membrane-impermeant inhibitors targeting the human tumor-associated isozyme IX. J Med Chem 2004;47:2337–47.
- (a) Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Exp Rev Neurother 2016;1:961–8. (b) Di Cesare Mannelli L, Micheli L, Carta F, et al. 894–9. (c) Margheri F, Ceruso M, Carta F, et al. 1(Suppl. 4):60–3. (d) Bua S, Di Cesare Mannelli L, Vullo D, et al. Design and synthesis of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 2017;60:1159–70.
- (a) Capasso C, Supuran CT. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;3:325–32. (b) Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Exp Opin Ther Targets 2015;1:1689–704. (c) Vermelho AB, da Silva Cardoso V, Ricci Junior E, et al. 139–46. (d) de Menezes Dda R, Calvet CM, Rodrigues GC, et al. 964–73. (e) Nocentini A, Cadoni R, Dumy P, et al. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J Enzyme Inhib Med Chem 2018;33:286–9.
- (a) Lomelino CL, Supuran CT, McKenna R. Non-classical inhibition of carbonic anhydrase. Int J Mol Sci. 2016;1:E1150. (b) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;2:759–72. (c) Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs–antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87. (d) Supuran CT, Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J Enzyme Inhib Med Chem 2016;31:1254–60. (e) Diaz JR, Fernández Baldo M, Echeverría G, et al. A substituted sulfonamide and its Co (II), Cu (II), and Zn (II) complexes as potential antifungal agents. J Enzyme Inhib Med Chem 2016;31(Suppl. 2):51–62.
- (a) Nishimori I, Minakuchi T, Vullo D, et al. Carbonic anhydrase inhibitors. Cloning, characterization, and inhibition studies of a new beta-carbonic anhydrase from Mycobacterium tuberculosis. J Med Chem 2009;5:3116–20. (b) Güzel O, Maresca A, Scozzafava A, et al. Discovery of low nanomolar and subnanomolar inhibitors of the mycobacterial beta-carbonic anhydrases Rv1284 and Rv3273. J Med Chem 2009;5:4063–7. (c) Maresca A, Scozzafava A, Vullo D, Supuran CT. Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three β-class carbonic anhydrases from Mycobacterium tuberculosis. J Enzyme Inhib Med Chem 2013;28:384–7.
- Daoud KM, Al-Obaydi AW. Synthesis and antibacterial activity of some hydrazides, substituted thiosemicarbazide, 1,3,4-oxadiazoles, thiadiazoles and 1,2,4-triazoles. Nat J Chem 2008;31:531–42.
- Qurrat-ul-Ain, Uzma A, Jamal RA, et al. Alpha-glucosidase and carbonic anhydrase inhibition studies of Pd(II)-hydrazide complexes. Arab J Chem. 2015;1–12.
- Suvitha S, Siddig A, Mohammed M, Syam M. Synthesis of chalcones with anticancer activities. Molecules 2012;17:6179–95.
- (a) Detsi A, Majdalani M, Kontogiorgis CA, et al. Natural and synthetic 20-hydroxy-chalcones and aurones: synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg Med Chem 2009;1:8073–85. (b) Peperidou A, Bua S, Bozdag M, et al. Novel 6- and 7-substituted coumarins with inhibitory action against lipoxygenase and tumor-associated carbonic anhydrase IX. Molecules 2018;23:E153.
- Panchal A, Kunjadia P, Patel P. Synthesis and biological evaluation of chalcone derivatives linked triazoles. Int J Pharm Sci Drug Res 2011;3:331–7.
- Rayar A, Veitía M, Ferroud C. An efficient and selective microwave-assisted Claisen-Schmidt reaction for the synthesis of functionalized benzalacetones. SpringerPlus 2015;4:221.
- Khalil N, Ahmed E, El-Nassan H. Synthesis, characterization, and biological evaluation of certain 1,3-thiazolone derivatives bearing pyrazoline moiety as potential anti-breast cancer agents. Med Chem Res 2012;22:1021–7.
- Raiford L, Peterson W. Identification of phenylhydrazones and isomeric pyrazolines obtained from chalcones. J Org Chem 1937;1:544–51.
- Pandeya S, Sriram D, Nath G, DeClercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur J Pharm Sci 1999;9:25–31.
- Singasane N. Synthesis and biological screening of some organic compounds. Mumbai, India: University of Mumbai; 2011.
- Yogeeswari P, Sriram D, Sunil J, et al. Anticonvulsant and neurotoxicity evaluation of some 6-chlorobenzothiazolyl-2-thiosemicarbazones. Eur J Med Chem 2002;37:231–6.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Menchise V, De Simone G, Alterio V, et al. Carbonic anhydrase inhibitors: stacking with Phe131 determines active site binding region of inhibitors as exemplified by the X-ray crystal structure of a membrane-impermeant antitumor sulfonamide complexed with isozyme II. J Med Chem 2005;4:5721–7. (b) Supuran CT, Mincione F, Scozzafava A, et al. Carbonic anhydrase inhibitors—part 52. Metal complexes of heterocyclic sulfonamides: a new class of strong topical intraocular pressure-lowering agents in rabbits. Eur J Med Chem 1998;33:247–54. (c) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg Med Chem Lett 2005;1:3102–8. (d) Şentürk M, Gülçin İ, Beydemir Ş, et al. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9. (e) Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2012;2:138–47. (f) Dogne JM, Hanson J, Supuran C, Pratico D. Coxibs and cardiovascular side-effects: from light to shadow. Curr Pharm Des 2006;12:971–5.
- (a) Krall N, Pretto F, Decurtins W, et al. A small‐molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl 2014;5:4231–5. (b) Rehman SU, Chohan ZH, Gulnaz F, et al. 333–40. (c) Clare BW, Supuran CT. Carbonic anhydrase activators. 3: Structure‐activity correlations for a series of isozyme II activators. J Pharm Sci 1994;8:768–73. (d) Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31. (e) Chohan ZH, Munawar A, Supuran CT. Transition metal ion complexes of Schiff-bases. Synthesis, characterization and antibacterial properties. Met Based Drugs, 2001;8:137–43. (f) Zimmerman SA, Ferry JG, Supuran CT. Inhibition of the archaeal β-class (Cab) and γ-class (Cam) carbonic anhydrases. Curr Top Med Chem. 2007;7:901–8. (g) De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- (a) Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;3:431–8. (b) Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Commun (Camb) 2010;4:8371–3. (c) Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;3:689–94. (d) De Simone G, Langella E, Esposito D, et al. Insights into the binding mode of sulphamates and sulphamides to hCA II: crystallographic studies and binding free energy calculations. J Enzyme Inhib Med Chem 2017;3:1002–11. (e) Di Fiore A, De Simone G, Alterio V, et al. The anticonvulsant sulfamide JNJ-26990990 and its S,S-dioxide analog strongly inhibit carbonic anhydrases: solution and X-ray crystallographic studies. Org Biomol Chem 2016;14:4853–8.
- (a) Carta F, Birkmann A, Pfaff T, et al. Lead development of thiazolylsulfonamides with carbonic anhydrase inhibitory action. J Med Chem 2017;6:3154–64. (b) Supuran CT, Kalinin S, Tanç M, et al. Isoform-selective inhibitory profile of 2-imidazoline-substituted benzene sulfonamides against a panel of human carbonic anhydrases. J Enzyme Inhib Med Chem 2016;31:97–202. (c) Pettersen EO, Ebbesen P, Gieling RG, et al. Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem 2015;3:689–721. (d) De Vita D, Angeli A, Pandolfi F, et al. Inhibition of the α-carbonic anhydrase from Vibrio cholerae with amides and sulfonamides incorporating imidazole moieties. J Enzyme Inhib Med Chem 2017;3:798–804. (e) Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;4:7697–9. (f) Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long lasting, topical intraocular pressure lowering properties. J Med Chem 2002;45:1466–76.
