Abstract
Ring A halogenated 13α-, 13β-, and 17-deoxy-13α-estrone derivatives were synthesised with N-halosuccinimides as electrophile triggers. Substitutions occurred at positions C-2 and/or C-4. The potential inhibitory action of the halogenated estrones on human aromatase, steroid sulfatase, or 17β-hydroxysteroid dehydrogenase 1 activity was investigated via in vitro radiosubstrate incubation. Potent submicromolar or low micromolar inhibitors were identified with occasional dual or multiple inhibitory properties. Valuable structure–activity relationships were established from the comparison of the inhibitory data obtained. Kinetic experiments performed with selected compounds revealed competitive reversible inhibition mechanisms against 17β-hydroxysteroid dehydrogenase 1 and competitive irreversible manner in the inhibition of the steroid sulfatase enzyme.
Graphical Abstract
Keywords:
Introduction
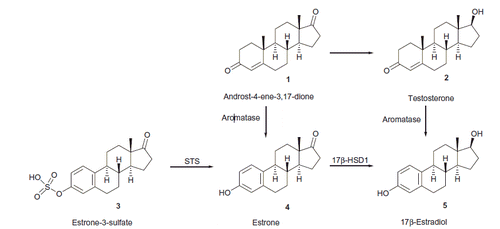
Estrogens play an important role in cell proliferation and their overproduction stimulates the growth of hormone-sensitive cells, leading to hormone-dependent diseases, such as breast and endometrial cancerCitation1. Inhibition of enzymes involved in the final steps of estrogen biosynthesis is a powerful route to prevent the proliferative action of estrogens. Cytochrome P450 aromatase is responsible for the conversion of nonaromatic androgens 1 and 2 to estrone (E1, 4, Scheme 1) or 17β-estradiol (E2, 5), respectively. Estrogens are originated not only from nonaromatic steroids, but also from estrone-3-sulfate (E1S) 3, which exists as a large circulatory reservoir. E1S is transported into cells by organic anion transporters (OATPs) and several other members of the SoLute Carrier (SLC) protein familyCitation2. After entering the cells, E1 is released from the sulfate ester by steroid sulfatase (STS). The next, hormone-activating process is the formation of active hormone E2 from E1, which is mainly catalyzed by 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1). Activities of STS and 17β-HSD1 are higher in breast cancer tissue compared to other tissues, that is this may be the main route of local estrogen production in the tumorsCitation3,Citation4.
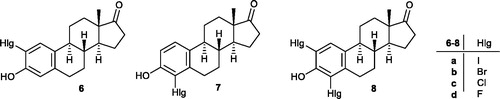
Inhibition of the above-mentioned enzymes may be achieved by inhibitors designed on the estrane core. A-ring halogenation of E1 results in 2- and 4-regioisomeric (6, 7) and/or 2,4-bis-substituted compounds (8, )Citation5–7. The substitution pattern of the aromatic ring and the nature of the introduced halogen greatly influence the inhibitory properties of estrone derivatives halogenated at the A-ring. Compounds obtained by introduction of the same substituent to different positions of the aromatic ring may possess high binding affinities to different enzymes. 2-Bromo- (6b) or 2-chloroestrone (6c) are potent aromatase inhibitors with low micromolar IC50 valuesCitation5. Their 4-substituted counterparts (7b,c) display only moderate aromatase inhibitory potential. Möller et al. described that 2-haloestrones (6b,c) as 17β-HSD1 inhibitors can suppress the E1–E2 conversion with IC50 values in the submicromolar rangeCitation6. 4-Halogenated counterparts (7) have not been tested against 17β-HSD1 by this research group. However, the 4-haloestrones (7b,d) are known to be efficient STS inhibitorsCitation7. Thus, introduction of Br or F onto C-4 of E1 led to a significant increase in STS inhibitory potential. None of the mentioned references discusses the affinity of 2-iodo- and 4-iodoestrones (6a, 7a) or 2,4-bis-compounds (8) for these three enzymes.
Concerning the results obtained so far for certain A-ring halogenated estrones it seems that substitution of E1 at C-2 may enhance aromatase and 17β-HSD1 inhibitory potential, but 4-halogenation may lead to efficient STS inhibitors.
The use of the estrone-based inhibitors of the mentioned steroidogenic enzymes in the therapy is limited because of their retained estrogenic activity. The availability of inhibitors acting selectively without hormonal behavior would be of particular interest. Literature data reveal that estrogenic effect of estrone is a 5–35% extent of that of 17β-estradiolCitation8–10. Chloro substitution at position C-4 of estrone or 17β-estradiol retains the estrogenicity, however, 4-bromo and 4-iodo, as well as 2-chloro, -bromo, and -iodo compounds exert suppressed effect compared to that of the parent compounds. Data vary according to the methods applied, but it can be stated that estrogenic effect decreases with the increasing size of the introduced halogen. C-2 substituted estrone or 17β-estradiol compounds are usually less estrogenic than their C-4 counterparts. The 2,4-disubstituted analogs, nevertheless, exert negligible estrogenic potentialCitation8,Citation10,Citation11. Certain other chemical modifications of the estrane skeleton, such as the inversion of the configuration at C-13 or the opening of ring D, may result in the complete loss of hormonal activityCitation12–15. We have promising preliminary results concerning the design, synthesis and biochemical evaluation of 17β-HSD1 inhibitors based on hormonally inactive 13α-estrane coreCitation16. 13α-Estrone (9, Scheme 2) itself was proved to be a potent inhibitor with an IC50 comparable to that of the natural substrate E1. Additionally, the previously synthesised 13α-estrone derivatives (10a,b–12a,b) brominated or iodinated in the A-ring exerted low micromolar or submicromolar inhibitory potentialCitation17. Chlorinations in the 13α-estrone series leading to 10c–12c have not been performed up to now. Concerning recent promising results, that 13α-estrone derivatives might possess valuable 17β-HSD1 inhibitory potential, it seemed rational to evaluate these compounds as potential aromatase or STS inhibitors, too.
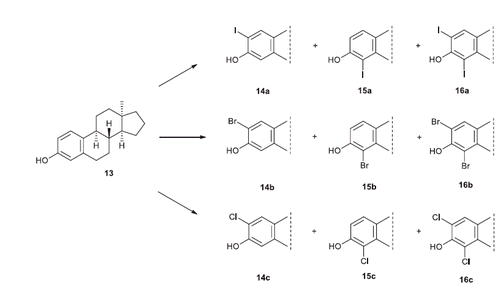
Based on the above-mentioned literature results here we aimed to perform aromatic halogenations on 13α-estrone (9) and its 17-deoxy counterpart (13, Scheme 2, 3). Chlorination of 13α-estrone (9) and introduction of chlorine, bromine or iodine onto positions 2 and/or 4 of 17-deoxy-13α-estrone (13) were planned. Synthesis of the halogenated 13β-estrone derivatives (6a,b,c–8a,b,c) was also designed with the aim of preparing the 13β-counterparts for comparative investigations (). We additionally intended to investigate the potential in vitro inhibitory effects of the basic and the halogenated compounds (6a,b,c–8a,b,c; 9–16) towards aromatase, STS and 17β-HSD1 enzymes. Comparison of the inhibitory results and evaluation of structure–activity relationships were also planned. Representatives from the most effective test compounds were selected for mechanistic and kinetic investigations.
Materials and methods
Chemical syntheses and characterisation data of the reported compounds, as well as experimental conditions of enzymatic assays performed are described in the Supporting Information.
Results and discussion
Chemistry
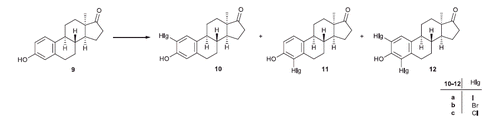
We recently described the halogenations of the aromatic ring of 9 with different groups at position 3 (OH, OMe, OBn)Citation17. Bromination or iodination was carried out with N-halosuccinimides as electrophile triggers in different solvents. In the 3-OH series both 2- and 4-substituted regioisomers were formed and bis-substitutions also occurred. The mixtures of the desired products (10a,b–12a,b) could efficiently be separated by flash chromatography.
As a completion of our previous results, here we performed the chlorination of the aromatic ring of 9 in order to get the appropriate 2-, 4-, or 2,4-bis-chloro derivatives (10c–12c) for further structure–activity examinations. Reactions with N-chlorosuccinimide led to a mixture of products (10c–12c, Scheme 2, , Entries 1, 2), which were separated by flash chromatography.
Table 1. Synthesis of halogenated compounds (6–8, 10c–12c, 14–16)
Expecting to acquire valuable structure–activity relationship data, it seemed reasonable to synthesise the appropriate halogenated 13α compounds in the 17-deoxy series, too. The halogenations of 17-deoxy-13α-estrone 13 were carried out using N-halosuccinimides in different solvents (Scheme 3, ). Iodination of 13 with 1.5 equivalent of NIS in trifluoroacetic acid yielded the mixture of the two regioisomers (14a and 15a) and the bis-compound (16a) in a ratio of 2:1:1 (, Entry 3). Bromination of 13 with 1.1 equivalent of reagent in dichloromethane led to a 4:3:1 mixture of 14b, 15b and 16b (, Entry 4). Chlorination of 13 with 1.5 equivalent of NCS yielded the 2:1 mixture of the two regioisomers 14c and 15c (, Entry 5). The bis-chloro compound (16c) could be synthesised by using 3.0 equivalent of NCS (, Entry 6).
In order to have the appropriate 13β-estrone derivatives (6a,b,c–8a,b,c) for the comparative enzyme inhibition studies, halogenations were carried out in this series, too. Our primary goal was to get the two regioisomers and the bis-compounds for the in vitro tests; therefore the regioselectivity was inessential. Different conditions were needed for the convenient synthesis of mono- and disubstituted 13β compounds. Monosubstitutions occurred using nearly 1 equiv. of N-halosuccinimide (, Entries 7, 9, 11), but the excess (nearly 2 equiv.) of the halogenating agent led to disubstitution (, Entries 8, 10, 12), too. The aimed halogenations at C-2 and/or C-4 were achieved and the flash chromatographic separations of the reaction mixtures furnished the desired compounds (6–8).
Enzyme inhibition studies
Aromatase, STS and 17β-HSD1 belong to different enzyme families with distinct catalytic mechanismsCitation18–20. Specific inhibitors of these enzymes may be developed because of the differences in their active sitesCitation4. Dual or multiple inhibition might also be of value since inhibition of only one of these three enzymes is not adequate in treatment of hormone-dependent breast cancer. Since aromatase is needed for the synthesis of E1, hormone-dependent breast cancer may be more effectively treated by dual inhibition of aromatase and STS. Among the inhibitors of the mentioned three enzymes, aromatase inhibitors are clinically the most effective for hormone-dependent breast cancer.
Beside their efficiency, the estrogen deprivation is accompanied with resistance and side effects. It would be crucial to design new drug candidates without such side effects. Innovative treatment strategies combining inhibitors of STS or 17β-HSD1 with aromatase inhibitor could lower the dose of the latter and extend the disease progression. The tumor-selective lowering of E2 levels could be achieved by the use of inhibitors of these two enzymes together with the aromatase inhibitor.
Literature data reveal that three-dimensional structures of these enzymes have not met expectations in drug design, but they are useful in understanding the catalytic mechanisms and inhibitor bindingCitation4. Aside from the differences in the active sites and catalytic mechanisms, these three enzymes may be inhibited by similar, estrone-based inhibitors. Slight differences in the structures of the potential inhibitors, involving regioisomerism, may influence not only the extent but also the nature of inhibition. This seems to be true for the ring A halogenated derivatives in the 13β-estrone seriesCitation5–7. Literature data suggest that substitutions at C-2 are advantageous concerning 17β-HSD1 and aromatase, but 4-regioisomers are better STS inhibitors. Nevertheless, there is no thorough comparative investigation in the literature concerning the aromatase, STS and 17β-HSD1 inhibitory activities of 2-, 4-, and 2,4-bis-chloro, -bromo, and -iodo estrones. The literature data are incomplete in this sense. The involvement of 17-keto- and 17-deoxy-13α-estrone compounds in the structure–activity determinations seems also reasonable. To the best of our knowledge, there has not been found any exact correlation between the structural characteristics of the investigated estrone derivative (regarding the conformation and the presence of the 17-keto function) and good aromatase, STS, or 17β-HSD1 inhibitory potential.
As a part of our efforts to get valuable pieces of information, which could help the development of more potent single or multiple inhibitors of estrogen biosynthesis, we describe here the evaluation of halogenated 17-keto-13β-, 17-keto-13α-, and 17-deoxy-13α-estrone derivatives (6a,b,c–8a,b,c, 9–16) as 17β-HSD1, aromatase, and/or STS inhibitors.
Enzyme inhibition studies were performed by in vitro radiosubstrate incubations using human term placenta cytosol and microsomas as enzyme sources. Aromatase inhibition was measured on testosterone (2) to E2 (5) conversion, STS inhibition was investigated via hydrolytic release of E1 (4) from E1S (3), whereas the influence on 17β-HSD1 was tested by the transformation of (4) to E2 (5). Relative conversions compared to non-inhibited controls (100%) were measured in the presence of 10 µM concentration of the test compound. For more efficient compounds, IC50 values were determined and inhibitory potentials were assessed also in comparison to IC50 data of the corresponding substrate. Reference IC50 parameters measured for the substrates and the basic compound E1 (4) are listed in . Mechanistic and kinetic investigations were performed and inhibitory constants (Ki) were determined for selected compounds.
Table 2. Reference IC50 parameters of the substrate compounds. Relative conversions (Rel. conv., control incubation with no inhibition is 100%) measured in the presence of 10 μM concentration of the compound tested. Mean ± SD, n = 3.
Aromatase
According to literature information, introduction of F, Cl, Br, Me, and formyl groups to C-2 of E1 affords compounds with high binding affinity to aromatase enzymeCitation5. 2-Bromoestrone (6b) proved to be an efficient inhibitor with an IC50 value of 2.4 µM, whereas 2-chloroestrone (6c, Ki = 0.13 µM) seemed to be more potent displaying about a 20-fold enhancement in its affinity compared to E1 (Ki = 2.50 µM). Concerning 6c as a powerful competitive inhibitor, no evidence of enzymatic generation of a reactive substance was observed. Exact correlations between inhibitory activity and size and/or electronegativity of substituents at C-2 could not be established. Inhibitory activities of estrone analogs were found to be higher than those of the corresponding estradiol derivatives. Consequently, a 17-carbonyl function plays a crucial role in the binding of estrogens to the active site of aromatase enzyme, as observed in the cases of the androgen derivativesCitation21–24. Halogenation at the C-4 position, except for fluorination, markedly decreased affinities. Osawa et al. suggested that substrates bind to the active site of aromatase through two conformations [β-side up (normal) and α-side up (upside down)], or have the opportunity and space to rotate around the binding siteCitation25. In the β-side up binding mode, C-2 is located close to the heme. This binding allows the catalysis of 2-hydroxylation. The estradiol molecule may rotate by 180° through the O(3)–O(17) axis, resulting in the α-side up binding mode, which allows 4-hydroxylation but to a lesser extent. The higher inhibitory potential of C-2-substituted E1 analogs compared with those of their C-4 substituted counterparts may be related to the high aromatase C-2 hydroxylation activities. These literature results indicate that concerning estrone-based aromatase inhibitors, the nature of the C-17 substituent, the substitution pattern of the aromatic ring, and the conformation of the compound greatly influence their inhibitory behavior.
In this contribution we also report in vitro aromatase inhibition tests of the synthesised 13β- and 13α-estrone derivatives. Certain 2-halogenated 13β-estrone derivatives (6b and 6c) displayed low micromolar inhibition (). 2-Chloroestrone (6c) was found to be the most effective with its IC50 value of 6.0 µM. 2-Bromoestrone (6b) was slightly less potent (IC50 = 8.7 µM). These results are in a good agreement with those of Numazawa et al.Citation5. 2-Iodoestrone (6a) displayed weaker inhibition with a relative conversion of 66% at a 10 µM test concentration (IC50 > 10 µM). Nevertheless, both derivatives have enhanced efficiency compared to their parent compound E1. The results obtained for the 2-halogenated 13β-estrone derivatives reveal that the inhibitory potential decreases with the increasing size of the halogen substituent.
Table 3. In vitro inhibition of enzyme activities by the test compounds.
Other test compounds including 13α-estrone (9), its 17-deoxy counterpart (13), and their halogenated derivatives (10–12, 14–16) exerted very weak inhibitory effect: their relative conversion data are higher than 80% at a 10 µM test concentration. The empirical rules previously established in the 13β-series have not been observable in the 13α-estrone series, while the affinity for aromatase enzyme of the two basic 13α-estrone derivatives (9 and 13) could not be improved by attaching halogens onto ring A. This might be explained by the lack of ability of 13α-estrones for binding to the active site, because of their core-modified structure.
STS
Numerous STS inhibitors have already been described in the literatureCitation7. Estrone aryl sulfamates are known as irreversible, suicide inhibitors. EMATE is a highly potent STS inhibitor, but because of its estrogenic activity it is not an adequate antitumor drug candidate. As literature data show the 17-deoxy analog of EMATE (NOMATE) displays similar STS inhibitory potential as its 17-keto counterpartCitation26,Citation27. This suggests that the presence of the 17-keto function is not essential for the effective inhibition of 3-sulfamates. E1 displays weak binding to STS, but its certain counterparts substituted in ring A exert substantial inhibition. This proves that appropriately substituted 3-OH E1 derivatives may also be good inhibitor candidates. It was established that substitution at C-4 of E1 with relatively small electron withdrawing-groups, such as F, Br, CN, formyl, or NO2, lead to improvement in inhibitory potency, which may be attributed to H-bonding and/or steric or other interactions. It is known that 4-formylestrone is a time- and concentration-dependent irreversible inhibitor of STSCitation7, and it inactivates the enzyme by reacting with active site residues. Phan et al. proposed that the 4-formyl function is involved in Schiff base formation with amino groups in appropriate side-chains including Lys-368, Lys-134, and Arg-79Citation7. As reported in the literature, the 3-OH function of the inhibitor may be involved in H-bonding with certain amino acid residues, with His346 and/or formylGly75 hydrate among others. Concerning hydrogen-bonding abilities, these side-chains are bifunctional. His346 may accept proton through its π-cloud and nitrogen, but may donate its NH proton. FormylGly75 hydrate may establish hydrogen bonds with its carbonyl group as a proton acceptor, whereas its OH group may behave as a proton donor. On the part of the steroid, both the OH function and its phenolate may form H-bonds or specific interactions. The electron-withdrawing properties of the introduced ring A substituents may greatly influence the polarisation and the acidity of the 3-OH group. This substituent effect depends on the position, number and nature of the introduced groups. Certain substituents at the ortho positions may additionally be involved in intramolecular H-bonding with the 3-OH group, which may reduce the affinity of the inhibitor to the enzyme. Phan et al. have not found direct correspondence between the estimated pKa values (taken from the corresponding o-substituted phenols) and the inhibitor potentials of their examined compoundsCitation7.
Taking into account the above-mentioned literature results, it can be stated that not only the presence of a 3-O-sulfamoyl group but also the introduction of a relatively small electron-withdrawing group to carbon 4 of E1 may be a general possibility to enhance the potency of estrone-based STS inhibitors.
Here we start with the evaluation of the 2- and/or 4-chlorinated, brominated or iodinated 13β-estrone derivatives (6a,b,c–8a,b,c). The 4-iodo compound (7a) exerted outstanding submicromolar inhibition (). Its 0.23 µM IC50 value indicates a 22-fold higher affinity compared to the E1S substrate and an affinity increased by 100-fold compared to E1. 6a its 2-sbstituted counterpart exerted 100-fold weaker inhibition according to its IC50 value. 4-Bromo derivative 7b displayed submicromolar inhibitory potential with an IC50 value nearly twice as high as that of its 2-counterpart (6b). Phan et al. recently reported that the inhibition potential of 6c is modest, whereas its 4-counterpart 7b displays considerable inhibitionCitation7. Even so we found that both isomers are potent inhibitors. This difference may be ascribed to different substrates used in the two methods. Phan et al. used an artificial substrate (4-methylumbelliferyl sulfate), whereas we applied the natural substrate estrone-3-sulfate (3). The different binding specificity of these substrates may result in different inhibition results. Nevertheless, our results are in good agreement with those obtained recently by Phan et al. concerning the effect of the regioisomerism on the inhibitory potentialCitation7. The two chlorinated regioisomers (6c and 7c) displayed commensurate inhibitory properties in the low micromolar range. Concerning all data obtained for the monosubstituted compounds (6, 7), it can be stated that introduction of a large halogen substituent onto C-4 is particularly advantageous. The 2,4-bis-iodo compound (8a) had no marked influence on the conversion of E1S–E1. The bis-bromo compound (8b) displayed low micromolar inhibition, commensurable with that of its bis-chloro counterpart (8c).
With all compounds and their inhibitory data in hand we were additionally interested in the investigation of the potential correlation between their predicted pKa values and the measured inhibitory potentials. pKa values were calculated using computer software available onlineCitation28. Data are not listed separately. Predicted pKa values suggest that our monosubstituted derivatives bear protonated 3-OH function (pKa > 7.6), whereas bis compounds (pKa = 6.8–7.4) are partially or completely deprotonated under the physiological conditions (pH =7.3) applied in our experiments. The obtained inhibition potentials do not have apparent direct relationship with the number and electronegativity of the introduced halogens, neither reflect the predicted pKa data. Differences observed between inhibitory potentials of the two regioisomers indicate that electronic properties of the introduced halogens are not the determining factors in binding interactions of the 3-OH groupCitation7.
In the 13α-estrone series, the inhibitory potential of the compounds tested greatly depended on both the nature and the position of the halogen introduced. Introduction of iodine onto ring A was advantageous over substitution with smaller halogens (Br or Cl). 4-Bromo and 4-iodo compounds (11a and 11b) were effective inhibitors with IC50 values of 8.5 and 6.0 µM. These regioisomers displayed better inhibitory potentials than their two counterparts (10a and 10b). In the iodinated series, the best inhibitor was bis-iodo derivative 12a with its low micromolar IC50 value. The chlorinated compounds (10c–12c) displayed weak inhibition with relative conversions over 50%.
The data obtained for the halogenated 17-deoxy-13α-estrone derivatives (14–16) reveal that all monohalogenated compounds (14, 15), independently from the regioisomerism, are potent STS inhibitors in the low micromolar range. In general, these compounds displayed higher inhibitory potential than those of their 17-keto counterparts (10, 11). We found that the presence of the 17-keto function on the halogenated 13α-estrane core is not essential for STS inhibitory activity. Additionally, empirical rules established previously do not predominate in this series. Furthermore, the IC50 values of the two regioisomers are comparable and the nature of introduced halogen is not crucial. Concerning the bis-substituted compounds (16a–16c), their inhibitory potential greatly depended on the nature of the halogen introduced. Surprisingly, the most potent 16c compound in the 17-deoxy series, displaying an IC50 value of 1.3 µM, bears the smallest halogens. Compound 16c exerts inhibition comparable to that of 4-bromoestrone 7b (IC50 = 1.3 and 0.89 µM, respectively), but lower to that of 4-iodoestrone 7a (IC50 = 0.23 µM).
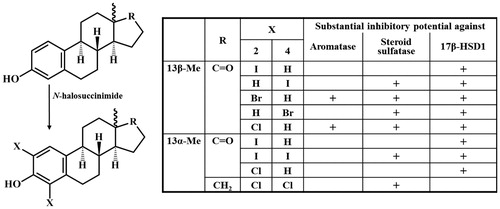
The most potent representative test compounds 7a and 16c were selected for mechanistic and kinetic investigations. In the reversibility tests, placental microsomas were preincubated with high concentration of the inhibitor and enzyme activities were measured after dilution of the samples. Relative conversions in these preincubated samples (, III and V) show suppressed enzyme activities, similar to those obtained for incubations with high inhibitor concentrations (, II). Results indicate that 7a and 16c molecules do not dissociate upon dilution and they are bound to the enzyme in an irreversible manner. The time dependence of the reversibility test reveals that irreversible binding is nearly completed within a short 2.5 min time for both compounds (, III).
Figure 2. Investigation of STS inhibition reversibility of selected 13β-estrone compounds 7a, 16c. Inhibitor compounds were preincubated with human placental microsomes for 10 and 20 min. Following a 50-fold dilution step and 20 min secondary incubation to allow dissociation, the usual enzyme activity measurement was applied. Mean ± SD of three separate experiments. Experimental conditions: I No preincubation, 7a 0.03 μM; 16c 0.2 μM, II No preincubation, 7a 1.5 μM; 16c 10 μM, III Preincubation, 2.5 min, 7a 1.5 μM; 16c 10 μM, IV Preincubation, 10 min, 7a 1.5 μM; 16c 10 μM, V Preincubation, 20 min, 7a 1.5 μM; 16c 10 μM.
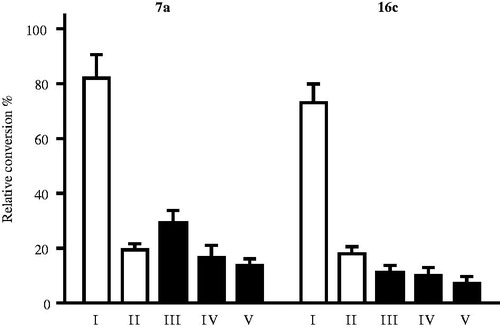
Kinetic analyses of selected compounds 7a and 16c were performed by measurement of enzyme activities using different fixed substrate concentrations and varied inhibitor concentrations. Lines of the Dixon’s plots intersect in the second quadrant revealing competitive inhibition mechanisms (, B). To confirm the competitive nature of the inhibition of 7a, replot of slopes vs. 1/substrate concentration was constructed (inset B/7a in . The resulting straight line passing through the origin supports the kinetics obtained from the Dixon’s plotCitation29,Citation30. Phan and coworkers investigated 4-nitroestrone as a representative estrone derivatives substituted at position C-4 with various electron-withdrawing functions. They have found the binding to be noncompetitive indicating that 4-nitroestrone is bound to the enzyme outside of its active siteCitation7. Our test compounds, 4-iodoestrone 7a and 2,4-bis-chloro-17-deoxy-13α-estrone 16c selected for the mechanistic investigations displayed a competitive mechanism, which alludes to binding within the active site of the enzyme.
Figure 3. Concentration-dependent STS inhibition (A) and Dixon’s kinetic analysis (B) of selected 13β-estrone compounds 7a, 16c. Inset in B/7a shows the secondary plot of slopes of the Dixon’s lines vs. 1/substrate concentration.
Ki parameters were determined from the intersections of the Dixon’s plots, and these values were found to be 0.36 µM for 7a and 1.9 µM for the 16c. These measured Ki values reflected inhibitory potentials ranked according to the IC50 data.
17β-hsd1
The crystal structure of 17β-HSD1 in its complex with E2 has been recently reportedCitation31. On the basis of this structure, molecular dynamic simulations and ligand–protein docking studies showed that there is an unoccupied lipophilic tunnel to the exterior of the protein located near the C-2 atom of E2Citation6. These results may serve as the basis for the development of potent inhibitors, while the binding affinity of the potential inhibitor might be enhanced by introducing a lipophilic moiety to position 2. Möller et al.Citation6 have found that 2-bromo and 2-chloro derivatives of E1 exerted potent inhibition and displayed similar binding affinity to that of the parent compound. We previously extended their research to the 13α-estrone series by performing brominations or iodinations on its aromatic ringCitation17. All synthesised 3-OH derivatives (10a,b–12a,b) displayed outstanding low or submicromolar 17β-HSD1 inhibitory activities, but their IC50 values depended on the number, the nature and the position of the A-ring substituents (). The iodo derivatives (10a–12a) proved to be more potent than their bromo counterparts (10b–12b). The finding, that the 2,4-bis-iodo compound (12a) displays a submicromolar IC50 of 0.38 µM, was a novel resultCitation17.
Concerning that the iodo derivatives in the 13α-estrone series displayed high inhibitory potential, the iodo counterparts in the 13β-estrone series (6a–8a) have also been tested. Both iodo regioisomers (6a, 7a) exerted outstanding inhibition, but the 2-iodo compound (6a) proved to be more potent with an IC50 value of 0.064 µM (). 2-Bromo- and 2-chloro-13β-estrone (6b and 6c) exerted somewhat weaker potentials as their iodo counterpart (6a), nevertheless, we observed their improved binding affinity compared to the parent compound E1.
Efficiency of the 4-substituted counterparts (7a, 7b) was found to be slightly weaker. These results are in a good agreement with empirical rules established previously concerning the two substitution to be up against the four substitution. New inhibition results of the iodo compounds (6a–7a) are particularly remarkable, and the inhibition potential of the 2-iodo regioisomer is outstanding. Additionally, all chlorinated derivatives (6c–8c) displayed outstanding commensurate submicromolar inhibition.
The three newly synthesised chloro 13α-estrone compounds (10c–12c) proved to be potent inhibitors. The 2-regioisomer (10c) seemed to be the most prominent with its submicromolar IC50 value of 0.33 µM. This value is commensurate with that of compound 6c. This result indicates a twofold better binding than that of E1, and an affinity increased by fourfold in comparison to parent compound 13α-estrone 9. The 4-chloro and the 2,4-bis-chloro derivatives (11c and 12c) exerted somewhat weaker inhibitions (IC50 values were found to be 2.6 and 2.2 µM, respectively).
Comparing recent results obtained for bromo and iodo compounds in both the 13α- and 13β-series with those of the chloro-13α derivatives, it can be stated that there is less difference in the inhibitory potential of the two regioisomers in the 13α-series than in the natural estrone series except for the chloro-13α compounds. The inhibitory potential of 13α-estrone (9) increased four-fold by adding chlorine, a relatively small electron-withdrawing group, to C-2.
We were interested in the comparison of the inhibitory results concerning the 17-keto and the 17-deoxy-13α derivatives. The basic deoxy compound 13 displayed a surprising but outstanding inhibitory potential comparable with that of reference E1, the natural substrate of the enzyme. We expected that this promising result might be improved by the introduction of halogens to the aromatic ring of 13. Nevertheless, the 1.1 µM IC50 value of 13 could not be lowered significantly. All halogenated derivatives (14–16) displayed comparable or higher values than that obtained for compound 13. In this series the 2-halogenated compounds (14a–c) were found to be the best inhibitors (IC50 values 2.6, 1.3, and 2.9 µM, respectively). The 4-chloro (15c) and the 2,4-bis-bromo derivative (16b) exerted modest inhibition (IC50=4–5 µM), whereas other 17-deoxy-13α derivatives displayed weaker inhibition: their relative conversions exceeded 50% indicating IC50 > 10 µM.
It was established that the advantage of the 2-substitution over the 4-halogenation shows up in the 17-deoxy series, too. However, none of the new 17-deoxy inhibitor candidates displayed submicromolar IC50 values.
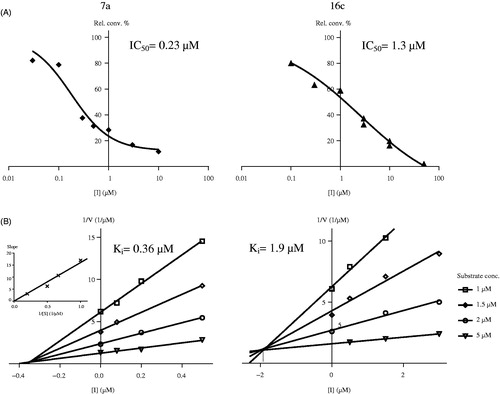
Potent 13α-estrone derivatives, basic compound 9, its 4-iodo (11a) and 2,4-bis-iodo derivative 12a, were selected for mechanistic and kinetic studies. To provide some information about the mechanism of action, we performed reversibility tests by preincubation of inhibitors with human placental cytosol. shows relative conversions obtained for the selected inhibitors according to preincubation conditions I–IV. The results indicate that the relative conversions in preincubated and diluted samples (, III and IV), were similar to that obtained for incubation with the lower concentration of the inhibitor (, I). This means that inhibitors 9, 11a and 12a can be released from binding by dilution that is they bind to the enzyme in a reversible manner.
Figure 4. Investigation of 17β-HSD1 inhibition reversibility of selected 13α-estrone compounds 9, 11a, 12a. Inhibitor compounds were preincubated with human placental cytosol. Following a 50-fold dilution step, the usual enzyme activity measurement was applied. Mean ± SD of three separate experiments. Experimental conditions: I No preincubation, 0.2 μM, II No preincubation, 10 μM, III Preincubation, 10 μM, 2.5 min, IV Preincubation, 10 μM, 20 min.
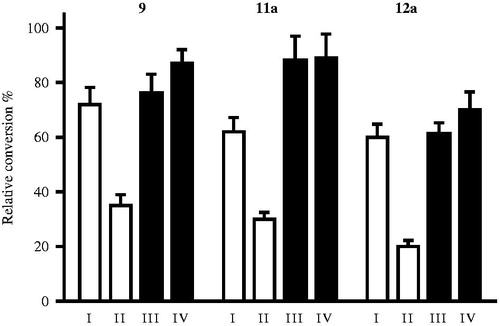
In order to characterise the inhibition type and determine the Ki, inhibition experiments were performed for the selected inhibitors 9, 11a and 12a at different fixed substrate concentrations in the presence of cofactor excess. On the Dixon’s plot, data for each substrate concentration fall on straight lines which intersect in the second quadrant, alluding to competitive inhibition mechanism (, B)Citation29,Citation30. Inhibitors that bind to the steroid site of 17β-HSD1 can bind to both the free enzyme and the binary enzyme–cofactor complex by random kinetic mechanism. This mixed-type inhibition, nevertheless, is simplified if the enzyme is saturated with cofactor first and displays competitive patternsCitation32. Reversible and apparently competitive mechanism of the inhibition observed in our experiments shows that inhibitors 9, 11a, and 12a are bound in the substrate-binding cavity of 17β-HSD1 with non-covalent interactions.
Figure 5. Concentration-dependent 17β-HSD1 inhibition (A) and Dixon’s kinetic analysis (B) of selected 13α-estrone compounds 9, 11a, 12a.
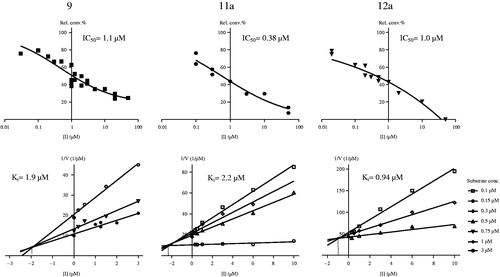
Ki parameters were determined from the intersections, and they were found to be 1.9, 2.2, and 0.94 µM for compounds 9, 11a, and 12a, respectively. Inhibitory potentials on the basis of these Ki data were comparable to those obtained by the IC50 values.
The 3-OH group of E1 and related inhibitors plays an important role in the binding to 17β-HSD1 forming a hydrogen-bonding system with the His221 and Glu282 residues at the recognition end of the active siteCitation33,Citation34. However, literature data show high affinity of certain 3-OMe derivatives indicating that ligands may establish effective binding even in the absence of proton on the C-3 substituentCitation16,Citation17,Citation35–37. Halogen substituents at C-2 and/or C-4 position modify binding abilities of the 3-OH substituent. The electron-withdrawing effect increases polarisation of the O–H bond and may induce deprotonation of this group under physiological pH conditions. ortho Substituents might also be able to form intramolecular hydrogen bond with the 3-OH group, which is disadvantageousCitation6.
Our inhibition results demonstrate that introduction of halogen atoms to C-2 and/or to C-4 position increases affinity to 17β-HSD1. However, there does not appear to be a direct relationship between the number and electronegativity of the halogens and the inhibitor potency.
Multiple or specific inhibition
Certain dual 17β-HSD1 and STS inhibitors were identified in both the 13β- and 13α-estrone series. Two 4-halo-17-keto-13β compounds (7a and 7b) elicited submicromolar inhibitory effect towards both enzymes. Certain additional 17-keto compounds (6b, 6c, 7c, 8c, 12a) possess dual inhibitory properties with IC50 values in submicromolar or low micromolar range. In the 17-deoxy-13α-estrone series, all two-halogenated compounds (14), the 2,4-bis-bromo- (16b) and 4-chloro derivative (15c) exerted potent low micromolar dual action. Two compounds, namely 2-bromo- and 2-chloro-13β-estrones 6b and 6c exerted considerable inhibitions towards the three investigated enzymes.
It is interesting to note that inhibitory potentials of iodo derivatives in the 13β-estrone series display outstanding variations. 2-Iodo compound 6a is a highly specific 17β-HSD1 inhibitor, but 7a its 4-counterpart has dual STS and 17β-HSD1 inhibitory potential. The disubstituted derivative (8a) exerts weak effects towards the enzymes investigated.
Our results confirm that structurally different enzymes with distinct catalytic mechanisms might be inhibited by the same inhibitor compounds. Newly detected multiple 17β-HSD1, STS and/or aromatase inhibitors might be superior to compounds affecting the action of only a single enzyme. These multiple inhibitors may serve as good candidates for efficient suppression of local estrogen production in breast cancer tissues.
Conclusions
Extensive research has been carried out in recent decades concerning enzyme inhibitors able to block estrogen biosynthesis. An armament of aromatase inhibitors is available by now in the medical practice; nevertheless, research work is continued to eliminate side effects and resistance developed by these medicationsCitation38. Numerous compounds have also been evaluated as potential STS or 17β-HSD1 inhibitors, but these efforts have not been crowned with success, as none of the drug candidates has been clinically introduced for the treatment of estrogen-dependent diseasesCitation3,Citation32,Citation39. In order to develop potent new inhibitors of estrogen biosynthesis, a profound understanding of the enzymatic mechanisms and the structure–function relationships is essential. Our results obtained for aromatase, STS and 17β-HSD1 inhibition of 13α- and 13β-estrone compounds bearing halo substituents on their ring A make valuable contribution to this aim.
Supplemental Material
Download PDF (437.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81–151.
- Izumi S, Nozaki Y, Komori T, et al. Substrate-dependent inhibition of organic anion transporting polypeptide 1B1: comparative analysis with prototypical probe substrates estradiol-17β-glucuronide, estrone-3-sulfate, and sulfobromophthalein. Drug Metab Dispos 2013;41:1859–66.
- Thomas MP, Potter BVL. Estrogen O-sulfamates and their analogues: clinical steroid sulfatase inhibitors with broad potential. J Steroid Biochem Mol Biol 2015;153:160–9.
- Hong Y, Chen S. Aromatase, estrone sulfatase, and 17β-hydroxysteroid dehydrogenase: structure-function studies and inhibitor development. Mol Cell Endocrinol 2011;340:120–6.
- Numazawa M, Ando M, Watari Y, et al. Structure-activity relationships of 2-, 4-, or 6-substituted estrogens as aromatase inhibitors. J Steroid Biochem Mol Biol 2005;96:51–8.
- Möller G, Deluca D, Gege C, et al. Structure-based design, synthesis and in vitro characterization of potent 17β-hydroxysteroid dehydrogenase type 1 inhibitors based on 2-substitutions of estrone and D-homo-estrone. Bioorg Med Chem Lett 2009;19:6740–4.
- Phan CM, Liu Y, Kim BM, et al. Inhibition of steroid sulfatase with 4-substituted estrone and estradiol derivatives. Bioorg Med Chem 2011;19:5999–6005.
- Kuruto-Niwa R, Ito T, Goto H, et al. Estrogenic activity of the chlorinated derivatives of estrogens and flavonoids using a GFP expression system. Environ Toxicol Pharmacol 2007;23:121–8.
- Zhu BT, Han GZ, Shim JY, et al. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: insights into the structural determinants favoring a differential subtype binding. Endocrinology 2006;147:4132–50.
- Nakamura H, Shiozawa T, Terao Y, et al. By-products produced by the reaction of estrogens with hypochlorous acid and their estrogen activities. J Health Sci 2006;52:124–31.
- Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 1997;62:268–303.
- Schönecker B, Lange C, Kötteritzsch M, et al. Conformational design for 13α-steroids. J Org Chem 2000;65:5487–97.
- Ayan D, Roy J, Maltais R, Poirier D. Impact of estradiol structural modifications (18-methyl and/or 17-hydroxy inversion of configuration) on the in vitro and in vivo estrogenic activity. J. Steroid Biochem Mol Biol 2011;127:324–30.
- Penov Gasi KM, Miljkovic DA, Medic Mijacevic LD, et al. Synthesis, X-ray crystal structure and biological activity of 16-amino-17-substituted-D-homo steroid derivatives. Steroids 2003;68:667–76.
- Jovanovic-Santa S, Petrovic J, Andric S, et al. Synthesis, structure, and screening of estrogenic and antiestrogenic activity of new 3,17-substituted- 16,17-seco-estratriene derivatives. Bioorg Chem 2003;31:475–84.
- Herman BE, Szabó J, Bacsa I, et al. Comparative investigation of the in vitro inhibitory potencies of 13-epimeric estrones and D-secoestrones towards 17β-hydroxysteroid dehydrogenase type 1. J. Enzyme Inhib Med Chem 2016;31:61–9.
- Bacsa I, Jójárt R, Schneider G, et al. Synthesis of A-ring halogenated 13α-estrone derivatives as potential 17β-HSD1 inhibitors. Steroids 2015;104:230–6.
- Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 2009;457:219–23.
- Ghosh D. Human sulfatases: a structural perspective to catalysis. Cell Mol. Life Sci 2007;64:2013–22.
- Marchais-Oberwinkler S, Henn C, Möller G, et al. 17β-Hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. J Steroid Biochem Mol Biol 2011;125:66–82.
- Kellis JT, Jr, Vickery LE. Purification and characterization of human placental aromatase cytochrome P-450. J Biol Chem 1987;262:4413–20.
- Yoshida N, Osawa Y. Purification of human placental aromatase cytochrome P-450 with monoclonal antibody and its characterization. Biochemistry 1991;30:3003–10.
- Sherwin PF, McMullan PC, Covey DF. Effects of steroid D-ring modification on suicide inactivation and competitive inhibition of aromatase by analogues of androsta-1,4-diene-3,17-dione. J Med Chem 1989;32:651–8.
- Numazawa M, Kamiyama T, Tachibana M, Oshibe M. Synthesis and structure−activity relationships of 6-substituted androst-4-ene analogs as aromatase inhibitors. J Med Chem 1996;39:2245–52.
- Osawa Y, Higashiyama T, Toma Y, Yarborough C. Diverse function of aromatase and the N-terminal sequence deleted form. J Steroid Biochem Mol Biol 1997;61:117–26.
- Nussbaumer P, Billich A. Steroid sulfatase inhibitors. Med Res Rev 2004;24:529–76.
- Ahmed V, Liu Y, Taylor SD. Multiple pathways for the irreversible inhibition of steroid sulfatase with quinone methide-generating suicide inhibitors. ChemBioChem 2009;10:1457–61.
- (a) SciFinder (Advanced Chemistry Development (ACD/Labs) Software V11.02), https://scifinder.cas.org, (accessed September 2017); (b) ACE and JChem pKa calculator, https://ace.chem.illinois.edu/ace/public/pKa.jsp, (accessed September 2017).
- Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems, New edn., New York: Wiley-Interscience, 1993.
- Cortés A, Cascante M, Cárdenas ML, Cornish-Bowden A. Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: new ways of analysing data. Biochem J 2001;357:263–8.
- Breton R, Housset D, Mazza C, Fontecilla-Camps JC. The structure of a complex of human 17beta-hydroxysteroid dehydrogenase with estradiol and NADP + identifies two principal targets for the design of inhibitors. Structure 1996;4:905–15.
- Penning TM. Human hydroxysteroid dehydrogenases and pre-receptor regulation: insights into inhibitor design and evaluation. J Steroid Biochem Mol Biol 2011;125:46–56.
- Huang YW, Pineau I, Chang HJ, et al. Critical residues for the specificity of cofactors and substrates in human estrogenic 17beta-hydroxysteroid dehydrogenase 1: variants designed from the three-dimensional structure of the enzyme. Mol. Endocrinol 2001;15:2010–20.
- Mazumdar M, Fournier D, Zhu DW, et al. Binary and ternary crystal structure analyses of a novel inhibitor with 17beta-HSD type 1: a lead compound for breast cancer therapy. Biochem J 2009;424:357–66. doi: 10.1042/BJ20091020.
- Langer L, Alexander JA, Engels LL. Human placental estradiol-17beta dehydrogenase. II. Kinetics and substrate specificities. J Biol Chem 1959;234:2609–14.
- Blomquist CH, Kotts CE, Hakanson EY. Inhibiton of human placental 17beta-hydroxysteroid dehydrogenase by steroids and nonsteroidal alcohols: aspects of inhibitor structure and binding specificity. Arch Biochem Biophys 1978;186:35–41.
- Szabó J, Bacsa I, Wölfling J, et al. Mernyák, Synthesis and in vitro pharmacological evaluation of N-[(1-benzyl-1,2,3-triazol-4-yl)methyl]-carboxamides on d-secoestrone scaffolds. J Enzyme Inhib Med Chem 2016;31:574–9.
- Yadav MR, Barmade MA, Tamboli RS, Murumkar PR. Developing steroidal aromatase inhibitors-an effective armament to win the battle against breast cancer. Eur J Med Chem 2015;105:1–38.
- Rizner TL. The important roles of steroid sulfatase and sulfotransferases in gynecological diseases. Front Pharmacol 2016;7:30.