Abstract
A series of quinazoline derivatives with benzylidene hydrazine carboxamide were designed and synthesised as EGFR inhibitors. Most compounds exhibited exceptional anti-proliferative activity against A549, HepG2, MCF-7 and H1975 cells. Furthermore, six compounds demonstrated excellent inhibition activity against EGFRWT with the IC50 value both less than 2 nM. Among the six compounds, 44 exhibited the strongest activity (0.4 nM) and potently inhibited EGFRL858R/T790M (0.1 μM). Excitingly, the most potent compound 14 showed excellent enzyme inhibitory activity with 6.3 nM and 8.4 nM for both EGFRWT and EGFRT790M/L858R. The result of AO single staining and Annexin V/PI staining showed that the compound 14 and 44 could induce remarkable apoptosis of A549 cells. The compound 14 arrested the cell cycle at the S phase and compound 44 arrested the cell cycle at the G0 phase in A549 cells. These preliminary results demonstrate that compound 14 and 44 may be promising lead compound-targeting EGFR.
Introduction
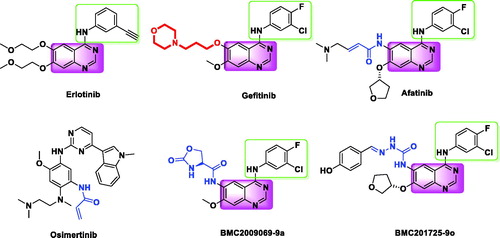
Epidermal growth factor receptor (EGFR) is overexpressed in several human tumours including non-small cell lung cancer (NSCLC)Citation1. As a target of NSCLC treatment, EGFR has become a hotspot in anti-tumour research in past few yearsCitation2–4. Some EGFR small molecule inhibitors have yielded good results in tumour targeted therapies. The first generation of EGFR inhibitors erlotinib and gefitinib () has achieved remarkable benefits in patients carrying “sensitizing mutations” such as L858R and exon-19 deletionsCitation5. Unfortunately, most patients develop resistance after 10–14 months of treatment with gefitinib and erlotinib, and the statistics show that gatekeeper residue (T790M) mutation was detected in 50%Citation6,Citation7.
For this mutation, covalent inhibitors were developed to overcome these resistances. Afatinib, a typical covalent inhibitor and an ATP-competitive anilinoquinazoline derivative, was approved by the US FDA in 2013 for advanced NSCLC patients with active mutant EGFRCitation8. However, afatinib contains a reactive “warhead” which can irreversibly bind to proteins other than the target, resulting in a toxic burden that limits its clinical utilityCitation6,Citation8,Citation9. AZD9291, a third-generation irreversible inhibitor and approved by the FDA in November 2015 for the treatment of EGFR T790M mutation in NSCLC patients, has a 200-fold selectivity for the T790M/L858R double mutant over wild-type EGFRCitation10. These inhibitors contain an electrophilic Michael addition receptor moiety that can covalently bind to the conserved cysteine residue (Cys797) of EGFR at the lip of the ATP binding cleft of EGFR to facilitate the occupation of the EGFR ATP binding site and overcome the resistance caused by the T790MCitation11.
A recent study exposed the occurrence of the tertiary point mutation C797S in 40% of the patients treated with AZD9291Citation12. This mutation causes the drug molecules to lose covalent interactions, thereby resulting in a decrease in inhibitory activity. These data indicated that there is a great need to solve the mutation problem without relying on covalent reaction with Cys797. Therefore, developing noncovalent inhibitors strategy to overcome the T790M mutation attracted the researchers’ attentionCitation13,Citation14. Chen et al.Citation15 reported that compound BMC2009069-9a is a novel noncovalent inhibitors candidate with the potency to overcome the problem of T790M.
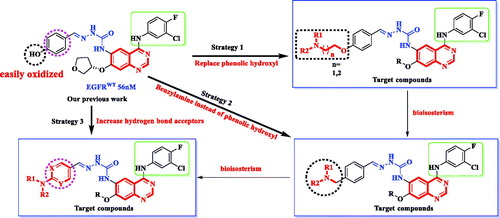
In our previous study, a series of quinazoline derivatives were designed and synthesised as noncovalent EGFR inhibitorsCitation16. The most promising compound BMC201725-9o exhibited inhibitory activity against EGFR (IC50 = 56 nM). Further research shows that BMC201725-9o can induce apoptosis in A549 cells, but shows weak inhibitory activity against EGFRL858R/T790M (IC50 > 1000 nM). In this study, in order to enhance the inhibitory activity of EGFR against L858R/T790M mutation and improve the target compounds properties, further structural modifications were mainly focused on the position C-7 of quinazoline. The goal of structural modification was to increase inhibitory activity against T790M by increasing the interaction of the compound with the ATP binding site and reduce oxidation of phenolic hydroxyl groups. Following this method, we synthesised one novel quinazoline derivatives by three modification strategies: (a) The introduction of a polar flexible chain on the oxygen atom allows the solubilised tail to better reach the solvent zone. (b) According to the principle of bio-electronic isosteres, oxygen atom is replaced by a more stable carbon atom. (c) Adding heteroatoms on benzene rings to increase hydrogen bonding receptors. The design strategy is shown in .
Herein we assessed antitumour activity of all target compounds against A549, HepG2, MCF-7, H1975 cancer cell lines. In addition, the EGFRWT and EGFRL858R/T790M kinase inhibitory activities of some potential compounds were evaluated. Among these designed compounds, compounds 14 and 44 showed the potential against cell and mutant EGFR kinases, which was demonstrated the potential for overcoming the T790M mutant. In addition, this article further disclosed studies data on docking studies, AO single staining, cell cycle and apoptosis of 14 and 44.
Materials and methods
Reagents and general methods
All reagents and solvents used were purchased from commercial sources without further purification. Flash chromatography was performed using silica gel (200–300 mesh). All reactions were monitored by TLC, using silica gel plates with fluorescence F254 and UV light visualisation. 1H NMR and11C NMR spectra were recorded on a Brucker AV-400 spectrometer at 400 MHz and Brucker AV-500 spectrometer at 125 MHz using deuterated solvents as an internal standard. Coupling constants (J) are expressed in hertz (Hz). Chemical shifts (d) are given in parts per million (ppm). High-resolution ESI- MS were recorded on an Applied Biosystems Q-STAR Elite ESI- LC- MS/MS mass spectrometer. The purity of compounds was determined with reverse-phase HPLC analysis to be over 95% (see Supporting information). HPLC instrument: Dionex Summit HPLC (Column: Diamonsil C18, 5.0 mm, 4.6 250 mm (Agilent Technologies); detector: PDA-100 photodiode array; injector: ASI-100 autoinjector; pump: p-680A). A flow rate of 1.0 ml/min was used with mobile phase of MeOH in H2O with 0.1% modifier (ammonia v/v).
Preparation of compounds 1–6
Compounds 1–6 were synthesised according to the procedures in our previous group researchCitation16.
Preparation of (S)-phenyl (4-((3-chloro-4-fluorophenyl) amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl) carbamate (7a). To the mixture of phenyl chloroformate (13.8 g, 88.5 mmol) and DIPEA (11.5 g, 88.5 mmol) in anhydrous 1,4-dioxane (80 ml), a solution of compound 6a (13.5 g, 35.4 mmol) in anhydrous 1,4-dioxane (60 ml) was slowly added at 10 °C. After the addition was completed, the mixture was warmed to room temperature for another 1.5 h, and the solvent was evaporated under reduced pressure. The residue was dissolved in dichloromethane (60 ml), and washed with water (3 × 20 ml), dried over anhydrous Na2SO4, concentrated under reduced pressure to afford (S)-phenyl(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy) quinazolin-6-yl) carbamate 7a as yellow oil (15.8 g, 90.3%), which were immediately used in the next step without further purification. ESI-MS m/z: [M + H]+ 496.1.
Preparation of phenyl (4-((3-chloro-4-fluorophenyl) amino)-7-methoxy quinazolin-6-yl)carbamate (7b). To the mixture of phenyl chloroformate (13.8 g, 88.5 mmol) and DIPEA (11.5 g, 88.5 mmol) in anhydrous 1,4-dioxane (60 ml), a solution of compound 6 b (11.3 g, 35.4 mmol) in anhydrous 1,4-dioxane (30 ml) was slowly added at 10 °C. After the addition was completed, the mixture was warmed to room temperature for another 1.5 h, and the solution was poured into water with stirring for 15 min. The precipitate was filtered and washed with water, dried to furnish phenyl (4-((3-chloro-4-fluorophenyl) amino)-7-methoxy quinazolin-6-yl) carbamate 7 b as light yellow solid (14.2 g, 91.6%)Citation14, which were immediately used in the next step without further purification. ESI-MS m/z: [M + H]+ 439.1. 1H NMR (400 MHz, DMSO-d6) δ 9.36 (d, J = 14.2 Hz, 2H), 8.37 (s, 1H), 8.18 (d, J = 5.0 Hz, 1H), 7.86–7.74 (m, 1H), 7.38 (d, J = 4.2 Hz, 2H), 7.15 (s, 2H), 7.10 (s, 1H), 6.75 (d, J = 7.7 Hz, 3H), 3.96 (s, 3H).
Preparation of N-(4-((3-chloro-4-fluorophenyl)amino)-7-(cyclopentyloxy)quinazolin-6-yl) hydrazinecarboxamide (8a). A mixture of 7a (4.2 g, 8.40 mmol) and 80% hydrazine monohydrate (3 ml) in 1,4-dioxane (20 ml) was refluxed for 2 h and monitored by TLC. After cooling to room temperature, the yellow solid was filtered off and washed with 1,4-dioxane and water, and dried to afford the (S)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3 -yl)oxy)quinazolin-6-yl)hydrazine carboxamide 8a as a white solid (2.4 g, 65.2%)Citation16. Mp 262.3–263.7 °C. ESI-MS m/z: [M + H]+ 434.1. 1H NMR (400 MHz, DMSO-d6) δ 9.78 (s, 1H), 9.28 (s, 1H), 8.92 (s, 1H), 8.43 (d, J = 12.7 Hz, 1H), 8.07 (d, J = 6.6 Hz, 1H), 7.92 (s, 1H), 7.76 (d, J = 8.6 Hz, 1H), 7.40 (t, J = 9.1 Hz, 1H), 7.24 (s, 1H), 5.36 (s, 1H), 4.68 (s, 2H), 4.01 (dd, J = 10.2, 4.0 Hz, 1H), 3.98–3.86 (m, 2H), 3.82 (dd, J = 12.4, 7.9 Hz, 1H), 2.37 (td, J = 12.2, 7.5 Hz, 1H), 2.15–1.84 (m, 1H).
N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxy quinazolin-6-yl)hydrazine carboxamide (8b). The synthesis of compound 8 b was similar to the compound 8a. A mixture of 7 b (4.0 g, 9.12 mmol) and 80% hydrazine monohydrate (5 ml) in 1,4-dioxane (20 ml) was refluxed for 2 h and monitored by TLC. After cooling to room temperature, the white solid was filtered off and washed with water, and dried to afford the N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxy quinazolin-6-yl) hydrazine carboxamide 8 b as a white solid (2.4 g, 68.5%) [16]. Mp 263.8–264.6 °C. ESI-MS m/z: [M + H]+ 376.1. 1H NMR (400 MHz, DMSO-d6) δ 9.76 (s, 1H), 9.20 (s, 1H), 8.91 (s, 1H), 8.47 (s, 1H), 8.07 (d, J = 4.9 Hz, 1H), 7.90 (s, 1H), 7.81–7.69 (m, 1H), 7.40 (t, J = 9.1 Hz, 1H), 7.27 (s, 1H), 4.67 (s, 2H), 4.04 (s, 3H).
General procedure for the preparation of compounds 9–54
To a solution of 8a–b (0.46 mmol) in Ethanol/DMF (4 ml), 1.1 equiv of aldehydes and 98% sulfuric acid (1 drop) were added, and the mixture was refluxed for 9–10 h until TLC showed the completion of the reaction. After cooling to room temperature, the precipitate was filtered and dried to yield 9–54 which were purified by isopropanolCitation16.
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(dimethylamino)benzylidene)hydrazine-1-carboxamide (9). This compound was obtained as white solid in 88% yield. Mp 236.4–239.4 °C. ESI-MS m/z: [M + H]+ 507.1. 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H), 9.83 (s, 1H), 9.07 (s, 1H), 8.92 (d, J = 8.4 Hz, 1H), 8.51 (s, 1H), 8.10 (d, J = 6.6 Hz, 1H), 7.88 (d, J = 11.7 Hz, 1H), 7.79 (s, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.46–7.37 (m, 1H), 7.32 (s, 1H), 6.79 (d, J = 8.5 Hz, 2H), 4.13 (s, 3H), 2.98 (s, 6H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(dimethylamino)benzylidene)hydrazine-1-carboxamide (10). This compound was obtained as yellow solid in 76% yield. Mp 238.4–241.2 °C. ESI-MS m/z: [M + H]+ 563.1. 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H), 9.86 (s, 1H), 9.13 (s, 1H), 9.00 (s, 1H), 8.50 (s, 1H), 8.08 (d, J = 6.1 Hz, 1H), 7.89 (s, 1H), 7.78 (s, 1H), 7.54 (d, J = 8.1 Hz, 2H), 7.41 (t, J = 9.0 Hz, 1H), 7.32 (s, 1H), 6.73 (d, J = 8.3 Hz, 2H), 5.45 (s, 1H), 4.04 (s, 2H), 4.01–3.95 (m, 1H), 3.89 (d, J = 4.5 Hz, 1H), 2.98 (s, 6H), 2.43 (dd, J = 13.8, 6.7 Hz, 1H), 2.18 (s, 1H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(diethylamino)benzylidene)hydrazine-1-carboxamide (11). This compound was obtained as white solid in 86% yield. Mp 226.1–229.4 °C. ESI-MS m/z: [M + H]+ 535.1. 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H), 9.83 (s, 1H), 9.07 (s, 1H), 8.92 (d, J = 8.4 Hz, 1H), 8.51 (s, 1H), 8.10 (d, J = 6.6 Hz, 1H), 7.88 (d, J = 11.7 Hz, 1H), 7.79 (s, 1H), 7.53 (d, J = 8.7 Hz, 2H), 7.46–7.37 (m, 1H), 7.32 (s, 1H), 6.79 (d, J = 8.5 Hz, 2H), 4.13 (s, 3H), 2.98 (s, 6H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(diethylamino)benzylidene)hydrazine-1-carboxamide (12). This compound was obtained as white solid in 84% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 591.2. 1H NMR (400 MHz, DMSO) δ 10.85 (dd, J = 48.5, 28.5 Hz, 1H), 10.13–9.58 (m, 1H), 9.09 (s, 1H), 8.98 (s, 1H), 8.40 (s, 1H), 8.02 (s, 1H), 7.87 (s, 1H), 7.68 (s, 1H), 7.51 (d, J = 8.7 Hz, 2H), 7.36 (s, 1H), 7.24 (s, 1H), 6.68 (d, J = 8.8 Hz, 2H), 5.42 (s, 1H), 4.05 (dt, J = 15.6, 6.6 Hz, 2H), 4.00–3.94 (m, 1H), 3.88 (dt, J = 12.7, 6.4 Hz, 1H), 3.40 (s, 4H), 2.42 (dd, J = 13.9, 6.2 Hz, 1H), 2.22–2.12 (m, 1H), 1.12 (t, J = 6.9 Hz, 6H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-((5-morpholinothiazol-2-yl)methylene)hydrazine-1-carboxamide (13). This compound was obtained as white solid in 86% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 612.1. 1H NMR (400 MHz, DMSO-d6) δ 11.04 (s, 1H), 9.85 (s, 1H), 9.02 (s, 1H), 8.86 (s, 1H), 8.50 (s, 1H), 8.08 (d, J = 6.8 Hz, 2H), 7.77 (dt, J = 7.4, 3.5 Hz, 1H), 7.58 (s, 1H), 7.42 (t, J = 9.1 Hz, 1H), 7.30 (s, 1H), 5.43 (s, 1H), 4.15–4.05 (m, 2H), 3.96 (q, J = 8.0 Hz, 1H), 3.88 (td, J = 8.2, 4.2 Hz, 1H), 3.74 (t, J = 4.9 Hz, 4H), 3.51 (t, J = 4.9 Hz, 4H), 2.40 (dt, J = 14.4, 7.1 Hz, 1H), 2.25–2.14 (m, 1H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-((2-(pyrrolidin-1-yl)pyrimidin-5-yl)methylene)hydrazine-1-carboxamide (14). This compound was obtained as yellow solid in 85% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 591.1. 1H NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H), 10.05 (s, 1H), 9.01 (s, 2H), 8.66 (s, 2H), 8.55 (s, 1H), 8.08 (dd, J = 6.8, 2.6 Hz, 1H), 7.89 (s, 1H), 7.77 (dd, J = 8.8, 4.0 Hz, 1H), 7.44 (t, J = 9.1 Hz, 1H), 7.32 (s, 1H), 5.43 (d, J = 5.3 Hz, 1H), 4.02 (dd, J = 10.1, 3.8 Hz, 2H), 3.98 (d, J = 9.2 Hz, 1H), 3.82 (dt, J = 8.4, 4.1 Hz, 1H), 3.56–3.52 (m, 4H), 2.47–2.39 (m, 1H), 2.16 (dd, J = 13.1, 6.4 Hz, 1H), 1.99–1.93 (m, 4H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-((5-(pyrrolidin-1-yl)furan-2-yl)methylene)hydrazine-1-carboxamide (15). This compound was obtained as yellow solid in 78% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 579.1. 1H NMR (400 MHz, DMSO) δ 11.07 (s, 1H), 10.06 (s, 1H), 9.09 (s, 1H), 8.65 (s, 1H), 8.55 (s, 1H), 8.08 (dd, J = 6.8, 2.6 Hz, 1H), 7.89 (s, 1H), 7.77 (dd, J = 8.8, 4.0 Hz, 1H), 7.44 (t, J = 9.1 Hz, 1H), 7.32 (s, 1H), 7.01 (s, 1H) 6.41 (s, 1H),5.43 (d, J = 5.3 Hz, 1H), 4.02 (dd, J = 10.1, 3.8 Hz, 2H), 3.98 (d, J = 9.2 Hz, 1H), 3.82 (dt, J = 8.4, 4.1 Hz, 1H), 3.42–3.62 (m, 4H), 2.47–2.39 (m, 1H), 2.16 (dd, J = 13.1, 6.4 Hz, 1H), 1.93–1.89 (m, 4H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-((5-(dimethylamino)thiophen-2-yl)methylene)hydrazine-1-carboxamide (16). This compound was obtained as white solid in 89% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 569.1. 1H NMR (400 MHz, DMSO-d6) δ 10.78 (s, 1H), 9.84 (s, 1H), 9.03 (s, 1H), 8.89 (s, 1H), 8.49 (s, 1H), 8.09 (s, 1H), 7.98 (s, 1H), 7.78 (s, 1H), 7.40 (d, J = 9.1 Hz, 1H), 7.29 (s, 1H), 7.10 (s, 1H), 5.88 (s, 1H), 5.41 (s, 1H), 4.10 (s, 2H), 3.99 (s, 1H), 3.86 (s, 1H), 2.99 (d, J = 9.6 Hz, 6H), 2.44–2.36 (m, 1H), 2.28–2.18 (m, 1H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-((6-(pyrrolidin-1-yl)pyridin-3-yl)methylene) hydrazine-1-carboxamide (17). This compound was obtained as yellow solid in 77% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 534.1. 1H NMR (400 MHz, DMSO-d6) δ 10.87 (s, 1H), 9.81 (s, 1H), 9.00 (s, 1H), 8.90 (s, 1H), 8.50 (s, 1H), 8.24 (s, 1H), 8.09 (s, 1H), 7.89 (s, 2H), 7.78 (s, 1H), 7.45–7.36 (m, 1H), 7.30 (s, 1H), 6.59 (d, J = 9.1 Hz, 1H), 4.10 (d, J = 4.9 Hz, 3H), 3.43 (s, 4H), 1.95 (s, 4H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-((6-(pyrrolidin-1-yl)pyridin-3-yl)methylene)hydrazine-1-carboxamide (18). This compound was obtained as white solid in 79% yield. Mp 236.4–238.9 °C. ESI-MS m/z: [M + H]+ 590.2. 1H NMR (400 MHz, DMSO-d6) δ 10.95 (s, 1H), 9.84 (s, 1H), 9.08 (s, 1H), 8.99 (s, 1H), 8.50 (s, 1H), 8.23 (d, J = 2.1 Hz, 1H), 8.09 (dd, J = 6.9, 2.6 Hz, 1H), 7.94 (d, J = 9.8 Hz, 1H), 7.90 (s, 1H), 7.80–7.75 (m, 1H), 7.41 (t, J = 9.0 Hz, 1H), 7.31 (s, 1H), 6.50 (d, J = 8.8 Hz, 1H), 5.44 (s, 1H), 4.02 (d, J = 3.2 Hz, 2H), 4.00–3.93 (m, 1H), 3.86 (d, J = 5.0 Hz, 1H), 3.44 (s, 4H), 2.46–2.36 (m, 1H), 2.20–2.11 (m, 1H), 1.96 (s, 4H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-((dimethylamino)methyl)benzylidene)hydrazine-1-carboxamide (19). This compound was obtained as white solid in 84% yield. Mp 226.5–229.4 °C. ESI-MS m/z: [M + H]+ 521.1. 1H NMR (400 MHz, DMSO-d6) δ 11.12 (s, 1H), 9.84 (s, 1H), 9.08 (s, 1H), 8.92 (s, 1H), 8.52 (s, 1H), 8.11 (d, J = 6.7 Hz, 1H), 8.02 (s, 1H), 7.78 (s, 1H), 7.68 (d, J = 7.8 Hz, 2H), 7.40 (d, J = 7.2 Hz, 3H), 7.33 (s, 1H), 4.12 (s, 3H), 3.42 (s, 2H), 2.16 (s, 6H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-((diethylamino)methyl)benzylidene)hydrazine-1-carboxamide (20). This compound was obtained as white solid in 86% yield. Mp 226.1–229.6 °C. ESI-MS m/z: [M + H]+ 549.2. 1H NMR (400 MHz, DMSO-d6) δ 11.12 (s, 1H), 9.84 (s, 1H), 9.08 (s, 1H), 8.92 (s, 1H), 8.52 (s, 1H), 8.11 (d, J = 6.7 Hz, 1H), 8.02 (s, 1H), 7.78 (s, 1H), 7.68 (d, J = 7.8 Hz, 2H), 7.40 (d, J = 7.2 Hz, 3H), 7.33 (s, 1H), 4.12 (s, 3H), 3.42 (s, 2H), 2.46–2.54(m, 4H) 1.46 (s, 6H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(pyrrolidin-1-ylmethyl)benzylidene)hydrazine-1-carboxamide (21). This compound was obtained as yellow solid in 89% yield. Mp 231.4–234.5 °C. ESI-MS m/z: [M + H]+ 547.1. 1H NMR (400 MHz, DMSO-d6) δ 11.13 (s, 1H), 9.85 (s, 1H), 9.08 (s, 1H), 8.93 (s, 1H), 8.52 (s, 1H), 8.10 (s, 1H), 8.01 (s, 1H), 7.79 (s, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.42 (s, 3H), 7.33 (s, 1H), 4.12 (s, 3H), 3.60 (s, 2H), 2.44 (s, 4H), 1.70 (s, 4H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(piperidin-1-ylmethyl)benzylidene)hydrazine-1-carboxamide (22). This compound was obtained as yellow solid in 92% yield. Mp 235.4–237.5 °C. ESI-MS m/z: [M + H]+ 561.2. 1H NMR (400 MHz, DMSO-d6) δ 11.14 (s, 1H), 9.86 (s, 1H), 9.08 (s, 1H), 8.93 (s, 1H), 8.53 (s, 1H), 8.10 (s, 1H), 8.02 (s, 1H), 7.79 (s, 1H), 7.68 (d, J = 7.8 Hz, 2H), 7.43 (s, 1H), 7.40 (s, 1H), 7.34 (s, 2H), 4.13 (d, J = 2.3 Hz, 3H), 3.46 (s, 2H), 2.34 (s, 4H), 1.51 (s, 4H), 1.40 (s, 2H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(morpholinomethyl)benzylidene)hydrazine-1-carboxamide (23). This compound was obtained as white solid in 87% yield. Mp 236.4–239.5 °C. ESI-MS m/z: [M + H]+ 563.1. 1H NMR (400 MHz, DMSO-d6) δ 11.15 (s, 1H), 9.87 (s, 1H), 9.10 (s, 1H), 8.93 (s, 1H), 8.56 (s, 1H), 8.12 (s, 1H), 8.03 (s, 1H), 7.80 (s, 1H), 7.78 (d, J = 7.8 Hz, 2H), 7.42 (s, 3H), 7.35 (s, 1H), 4.13 (d, J = 2.3 Hz, 3H), 3.66 (s, 2H), 3.45 (s, 4H), 2.44 (s, 4H).
(E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-((4-methylpiperazin-1-yl)methyl)benzylidene)hydrazine-1-carboxamide (24). This compound was obtained as yellow solid in 81% yield. Mp 231.4–235.8 °C. ESI-MS m/z: [M + H]+ 576.2. 1H NMR (400 MHz, DMSO-d6) δ 11.12 (s, 1H), 9.84 (s, 1H), 9.07 (s, 1H), 8.93 (s, 1H), 8.52 (s, 1H), 8.11 (s, 1H), 8.01 (s, 1H), 7.80 (s, 1H), 7.68 (d, J = 8.2 Hz, 2H), 7.44 (s, 1H), 7.41 (d, J = 7.5 Hz, 2H), 7.34 (s, 1H), 4.09 (d, J = 26.9 Hz, 3H), 3.49 (s, 2H), 2.53 (s, 4H), 2.39–2.30 (m, 4H), 2.15 (s, 3H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-((dimethylami no)methyl)benzylidene)hydrazine-1-carboxamide (25). This compound was obtained as white solid in 86% yield. Mp 236.4–239.5 °C. ESI-MS m/z: [M + H]+ 577.2. 1H NMR (400 MHz, DMSO-d6) δ 11.19 (s, 1H), 9.86 (s, 1H), 9.15 (s, 1H), 9.01 (s, 1H), 8.51 (s, 1H), 8.09 (dd, J = 6.9, 2.6 Hz, 1H), 8.02 (s, 1H), 7.80–7.75 (m, 1H), 7.69 (d, J = 7.9 Hz, 2H), 7.40 (d, J = 9.1 Hz, 1H), 7.37 (s, 1H), 7.34 (d, J = 6.3 Hz, 2H), 5.45 (s, 1H), 4.05 (d, J = 3.3 Hz, 2H), 3.97 (q, J = 7.7 Hz, 1H), 3.91–3.83 (m, 1H), 3.41 (s, 2H), 2.47–2.39 (m, 1H), 2.18 (d, J = 6.7 Hz, 1H), 2.1 (s, 6H)
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-((diethylamino) methyl)benzylidene)hydrazine-1-carboxamide (26).
This compound was obtained as yellow solid in 81% yield. Mp 226.4–231.5 °C. ESI-MS m/z: [M + H]+ 605.2. 1H NMR (400 MHz, DMSO-d6) δ 11.19 (s, 1H), 9.86 (s, 1H), 9.15 (s, 1H), 9.01 (s, 1H), 8.51 (s, 1H), 8.09 (dd, J = 6.9, 2.6 Hz, 1H), 8.02 (s, 1H), 7.80–7.75 (m, 1H), 7.69 (d, J = 7.9 Hz, 2H), 7.40 (d, J = 9.1 Hz, 1H), 7.37 (s, 1H), 7.34 (d, J = 6.3 Hz, 2H), 5.45 (s, 1H), 4.05 (d, J = 3.3 Hz, 2H), 3.97 (q, J = 7.7 Hz, 1H), 3.91–3.83 (m, 1H), 3.41 (s, 2H), 2.54 (s, 4H), 2.47–2.39 (m, 1H), 2.18 (d, J = 6.7 Hz, 1H), 1.51 (s, 6H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(pyrrolidin-1-y lmethyl)benzylidene)hydrazine-1-carboxamide (27). This compound was obtained as white solid in 83% yield. Mp 235.4–239.5 °C. ESI-MS m/z: [M + H]+ 603.2. 1H NMR (400 MHz, DMSO-d6) δ 11.18 (s, 1H), 9.86 (s, 1H), 9.14 (s, 1H), 9.01 (s, 1H), 8.50 (s, 1H), 8.09 (s, 1H), 8.01 (s, 1H), 7.78 (s, 1H), 7.67 (d, J = 7.8 Hz, 2H), 7.38 (dt, J = 21.0, 11.0 Hz, 4H), 5.45 (s, 1H), 4.05 (s, 2H), 3.97 (d, J = 7.3 Hz, 1H), 3.87 (s, 1H), 3.59 (s, 2H), 2.49 (s, 1H), 2.43 (s, 4H), 2.17 (s, 1H), 1.70 (s, 4H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(piperidin-1-yl methyl)benzylidene)hydrazine-1-carboxamide (28). This compound was obtained as yellow solid in 87% yield. Mp 236.4–238.5 °C. ESI-MS m/z: [M + H]+ 617.2. 1H NMR (400 MHz, DMSO-d6) δ 11.16 (s, 1H), 9.85 (s, 1H), 9.13 (s, 1H), 9.00 (s, 1H), 8.49 (s, 1H), 8.07 (d, J = 4.4 Hz, 1H), 8.00 (s, 1H), 7.75 (s, 1H), 7.66 (d, J = 8.0 Hz, 2H), 7.43–7.31 (m, 4H), 5.43 (s, 1H), 4.04 (s, 2H), 3.95 (dd, J = 15.7, 7.9 Hz, 1H), 3.85 (dd, J = 13.1, 8.3 Hz, 1H), 3.44 (s, 2H), 2.40 (dd, J = 14.1, 8.0 Hz, 1H), 2.31 (s, 4H), 2.19–2.10 (m, 1H), 1.48 (d, J = 5.2 Hz, 4H), 1.38 (s, 2H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(morpholinomethyl)benzylidene)hydrazine-1-carboxamide (29). This compound was obtained as white solid in 86% yield. Mp 232.4–236.8 °C. ESI-MS m/z: [M + H]+ 619.2. 1H NMR (400 MHz, DMSO-d6) δ 11.28 (s, 1H), 9.97 (s, 1H), 9.24 (s, 1H), 9.11 (s, 1H), 8.60 (s, 1H), 8.18 (d, J = 4.7 Hz, 1H), 8.11 (s, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.77 (d, J = 7.9 Hz, 2H), 7.47 (dd, J = 20.2, 12.3 Hz, 4H), 5.55 (s, 1H), 4.15 (s, 2H), 4.08–4.03 (m, 1H), 3.99 – 3.93 (m, 1H), 3.69 (s, 2H), 2.53 (s, 4H), 2.48 (d, J = 9.4 Hz, 1H), 2.30–2.18 (m, 1H), 1.80 (s, 4H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-((4-methylpipe razin-1-yl)methyl)benzylidene)hydrazine-1-carboxamide (30). This compound was obtained as white solid in 85% yield. Mp 233.4–237.6 °C. ESI-MS m/z: [M + H]+ 632.2. 1H NMR (400 MHz, DMSO-d6) δ 11.28 (s, 1H), 9.97 (s, 1H), 9.24 (s, 1H), 9.11 (s, 1H), 8.60 (s, 1H), 8.18 (d, J = 4.7 Hz, 1H), 8.11 (s, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.77 (d, J = 7.9 Hz, 2H), 7.47 (dd, J = 20.2, 12.3 Hz, 4H),δ 5.36 (s, 1H), 4.03 (d, J = 3.3 Hz, 2H), 3.96 (d, J = 7.8 Hz, 1H), 3.86 (dd, J = 8.4, 4.7 Hz, 1H), 3.48 (s, 2H), 2.66–2.51 (m, 4H), 2.38 (s, 4H), 2.15 (s, 3H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(2-(dimethylamino)ethoxy)benzylid ene)hydrazine-1-carboxamide (31). This compound was obtained as white solid in 76% yield. Mp 205.2–209.5 °C. ESI-MS m/z: [M + H]+ 551.18. 1H NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H), 9.80 (s, 1H), 9.02 (s, 1H), 8.88 (d, J = 4.0 Hz, 1H), 8.47 (d, J = 4.3 Hz, 1H), 8.06 (d, J = 4.2 Hz, 1H), 7.93 (d, J = 11.1 Hz, 1H), 7.74 (s, 1H), 7.61 (d, J = 8.5 Hz, 2H), 7.39 (d, J = 9.2 Hz, 1H), 7.27 (d, J = 9.0 Hz, 1H), 7.01 (d, J = 8.5 Hz, 2H), 4.07 (s, 3H), 4.06 (d, J = 5.3 Hz, 2H), 2.61 (d, J = 5.3 Hz, 2H), 2.18 (s, 6H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(2-(diethylamino)ethoxy)benzyliden e)hydrazine-1-carboxamide (32). This compound was obtained as white solid in 79% yield. Mp 208.5–210.2 °C. ESI-MS m/z: [M + H]+ 579.22. 1H NMR (400 MHz, CDCl3) δ 11.01 (s, 1H), 9.84 (s, 1H), 9.06 (s, 1H), 8.92 (s, 1H), 8.51 (s, 1H), 8.09 (s, 1H), 7.96 (s, 1H), 7.78 (s, 1H), 7.65 (d, J = 8.3 Hz, 2H), 7.40 (d, J = 9.1 Hz, 1H), 7.32 (s, 1H), 7.04 (d, J = 8.9 Hz, 2H), 4.12 (s, 3H), 4.06 (d, J = 6.0 Hz, 2H), 2.78 (s, 2H), 2.55 (d, J = 7.0 Hz, 4H), 0.97 (t, J = 7.0 Hz, 6H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(2-(pyrrolidin-1-yl)ethoxy)benzylid ene)hydrazine-1-carboxamide (33). This compound was obtained as yellow solid in 84% yield. Mp 203.2–208.8 °C. ESI-MS m/z: [M + H]+ 577.2. 1H NMR (400 MHz, DMSO-d6) δ 11.03 (s, 1H), 9.85 (s, 1H), 9.06 (s, 1H), 8.91 (s, 1H), 8.51 (s, 1H), 8.10 (dd, J = 7.0, 2.6 Hz, 1H), 7.96 (s, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.65 (d, J = 8.5 Hz, 2H), 7.42 (t, J = 9.2 Hz, 1H), 7.32 (s, 1H), 7.05 (d, J = 8.3 Hz, 2H), 4.12 (d, J = 6.7 Hz, 5H), 2.79 (d, J = 7.1 Hz, 2H), 2.52 (s, 4H), 1.68 (d, J = 5.5 Hz, 4H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(2-(piperidin-1-yl)ethoxy)benzylide ne)hydrazine-1-carboxamide (34). This compound was obtained as white solid in 75% yield. Mp 205.6–208.4 °C. ESI-MS m/z: [M + H]+ 591.22. 1H NMR (400 MHz, DMSO-d6) δ 11.01 (s, 1H), 9.84 (s, 1H), 9.07 (s, 1H), 8.92 (s, 1H), 8.52 (s, 1H), 8.09 (d, J = 2.8 Hz, 1H), 7.96 (s, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.65 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 6.6 Hz, 1H), 7.33 (s, 1H), 7.05 (d, J = 8.5 Hz, 2H), 4.12 (s, 3H), 4.04 (s, 2H), 2.66 (t, J = 5.8 Hz, 2H), 2.43 (s, 4H), 1.53–1.46 (m, 4H), 1.38 (d, J = 5.0 Hz, 2H).
(E)-N-(4-((4-chloro-3-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(2-morpholinoethoxy)benzylidene) hydrazine-1-carboxamide (35). This compound was obtained as white solid in 79 yield. Mp 210.2–215.3 °C. ESI-MS m/z: [M + H]+ 593.20. 1H NMR (400 MHz, DMSO-d6) δ 11.02 (s, 1H), 9.84 (s, 1H), 9.07 (s, 1H), 8.91 (s, 1H), 8.52 (s, 1H), 8.11 (s, 1H), 7.96 (s, 1H), 7.78 (s, 1H), 7.66 (d, J = 7.8 Hz, 2H), 7.45–7.38 (m, 1H), 7.33 (s, 1H), 7.06 (d, J = 8.0 Hz, 2H), 4.14 (s, 2H), 4.12 (s, 3H), 3.58 (s, 4H), 2.71 (s, 2H), 2.49–2.43 (m, 4H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(2-(4-methylpiperazin-1-yl)ethoxy) benzylidene)hydrazine-1-carboxamide (36). This compound was obtained as white solid in 91% yield. Mp 215.2–220.5 °C. ESI-MS m/z: [M + H]+ 606.2. 1H NMR (400 MHz, DMSO-d6) δ 10.99 (s, 1H), 9.80 (s, 1H), 9.01 (s, 1H), 8.86 (s, 1H), 8.46 (s, 1H), 8.04 (d, J = 4.9 Hz, 1H), 7.90 (s, 1H), 7.71 (s, 1H), 7.60 (d, J = 8.4 Hz, 2H), 7.36 (t, J = 9.3 Hz, 1H), 7.27 (s, 1H), 7.00 (d, J = 8.4 Hz, 2H), 4.07 (s, 2H), 4.05 (s, 3H), 2.68 (s, 2H), 2.44 (s, 8H), 2.28 (s, 3H).
(S,E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(2-(dimethylam ino)ethoxy)benzylidene)hydrazine-1-carboxamide (37). This compound was obtained as white solid in 90% yield. Mp 221.5–231.8 °C. ESI-MS m/z: [M + H]+ 607.2. 1H NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H), 9.87 (s, 1H), 9.14 (s, 1H), 9.01 (s, 1H), 8.51 (s, 1H), 8.10 (s, 1H), 7.97 (s, 1H), 7.78 (s, 1H), 7.67 (d, J = 8.3 Hz, 2H), 7.42 (t, J = 9.1 Hz, 1H), 7.33 (s, 1H), 7.00 (d, J = 8.4 Hz, 2H), 5.45 (s, 1H), 4.10 (s, 2H), 4.04 (s, 2H), 3.98 (s, 1H), 3.89 (s, 1H), 2.63 (s, 2H), 2.22 (s, 6H), 2.18–2.13 (m, 2H).
(S,E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(2-(diethylamin o)ethoxy)benzylidene)hydrazine-1-carboxamide (38). This compound was obtained as yellow solid in 89% yield. Mp 210.1–213.9 °C. ESI-MS m/z: [M + H]+ 635.2. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (s, 1H), 9.87 (s, 1H), 9.14 (s, 1H), 9.01 (s, 1H), 8.51 (s, 1H), 8.09 (dd, J = 6.8, 2.3 Hz, 1H), 7.97 (s, 1H), 7.80–7.75 (m, 1H), 7.67 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 9.1 Hz, 1H), 7.33 (s, 1H), 7.04–6.97 (m, 2H), 5.46 (s, 1H), 4.04 (s, 2H), 3.97 (d, J = 7.6 Hz, 2H), 3.88 (d, J = 4.4 Hz, 2H), 2.84 (s, 2H), 2.61 (s, 4H), 2.43 (dd, J = 13.8, 6.0 Hz, 1H), 2.20–2.12 (m, 1H), 1.00 (t, J = 6.7 Hz, 6H).
(S,E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(2-(pyrrolidin-1 -yl)ethoxy)benzylidene)hydrazine-1-carboxamide (39). This compound was obtained as white solid in 88% yield. Mp 216.2–218.9 °C. ESI-MS m/z: [M + H]+ 633.2. 1H NMR (400 MHz, DMSO-d6) δ 11.07 (s, 1H), 9.86 (s, 1H), 9.14 (s, 1H), 9.01 (s, 1H), 8.50 (s, 1H), 8.09 (dd, J = 6.8, 2.4 Hz, 1H), 7.97 (s, 1H), 7.80–7.75 (m, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 9.2 Hz, 1H), 7.33 (s, 1H), 6.99 (d, J = 8.5 Hz, 2H), 5.45 (s, 1H), 4.09–4.02 (m, 4H), 3.96 (t, J = 7.8 Hz, 1H), 3.91–3.84 (m, 1H), 3.63–3.53 (m, 4H), 2.43 (t, J = 7.2 Hz, 2H), 2.37 (s, 4H), 2.16 (d, J = 7.2 Hz, 1H), 1.91–1.87 (m, 1H).
(S,E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(2-(piperidin-1-yl)ethoxy)benzylidene)hydrazine-1-carboxamide (40). This compound was obtained as yellow solid in 86% yield. Mp 213.4–218.4 °C. ESI-MS m/z: [M + H]+ 647.2. 1H NMR (400 MHz, DMSO-d6) δ 11.05 (s, 1H), 9.87 (s, 1H), 9.08 (s, 1H), 8.92 (s, 1H), 8.52 (s, 1H), 8.11 (d, J = 6.7 Hz, 1H), 7.96 (s, 1H), 7.78 (s, 1H), 7.66 (d, J = 8.4 Hz, 2H), 7.43 (t, J = 9.1 Hz, 1H), 7.33 (s, 1H), 7.06 (d, J = 8.5 Hz, 2H), 5.55 (s, 1H), 4.02–3.98 (m, 4H), 3.96–3.94 (t, J = 7.8 Hz, 1H), 3.91–3.84 (m, 1H), 2.89 (s, 2H), 2.53–2.48 (m, J = 7.2 Hz, 1H), 2.44 (s, 4H), 2.16–2.08 (m, J = 5.7 Hz, 1H),1.50 (s, 4H), 1.39 (s, 2H).
(S,E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(2-morpholinoe thoxy)benzylidene)hydrazine-1-carboxamide (41). This compound was obtained as white solid in 70% yield. Mp 214.2–216.5 °C. ESI-MS m/z: [M + H]+ 649.2. 1H NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H), 9.86 (s, 1H), 9.14 (s, 1H), 8.98 (d, J = 18.5 Hz, 1H), 8.50 (s, 1H), 8.09 (d, J = 7.0 Hz, 1H), 7.97 (s, 1H), 7.77 (d, J = 8.9 Hz, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.42 (t, J = 9.2 Hz, 1H), 7.33 (s, 1H), 7.01 (d, J = 8.4 Hz, 2H), 5.42 (d, J = 22.1 Hz, 1H), 4.15 (d, J = 5.3 Hz, 2H), 4.04 (s, 2H), 3.97 (d, J = 8.0 Hz, 1H), 3.88 (d, J = 5.1 Hz, 1H), 3.58 (s, 4H), 2.76–2.67 (m, 2H), 2.56–2.51 (m, J = 5.7 Hz, 1H),2.42 (d, J = 13.7 Hz, 4H), 2.26–2.18 (m, J = 5.7 Hz, 1H).
(S,E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(2-(4-methylpi perazin-1-yl)ethoxy)benzylidene)hydrazine-1-carboxamide (42). This compound was obtained as white solid in 78% yield. Mp 206.210.2 °C. ESI-MS m/z: [M + H]+ 662.2. 1H NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H), 9.86 (s, 1H), 9.14 (s, 1H), 8.98 (d, J = 18.5 Hz, 1H), 8.50 (s, 1H), 8.09 (d, J = 7.0 Hz, 1H), 7.97 (s, 1H), 7.77 (d, J = 8.9 Hz, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.42 (t, J = 9.2 Hz, 1H), 7.33 (s, 1H), 7.01 (d, J = 8.4 Hz, 2H), 5.42 (d, J = 22.1 Hz, 1H), 4.15 (d, J = 5.3 Hz, 2H), 4.04 (s, 2H), 3.97 (d, J = 8.0 Hz, 1H), 3.88 (d, J = 5.1 Hz, 1H), 3.58 (s, 4H), 2.76–2.67 (m, 2H), 2.58–2.56 (m, J = 5.7 Hz, 1H),2.42 (d, J = 13.7 Hz, 4H), 2.35–2.08 (m, 2H). 2.21–2.18 (m, J = 5.7 Hz, 1H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(3-(dimethylamino)propoxy)benzyl idene)hydrazine-1-carboxamide (43). This compound was obtained as yellow solid in 76% yield. Mp 209.3–213.5 °C. ESI-MS m/z: [M + H]+ 565.2. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (s, 1H), 9.93 (s, 1H), 9.16 (s, 1H), 9.01 (s, 1H), 8.61 (s, 1H), 8.19 (dd, J = 6.9, 2.7 Hz, 1H), 8.06 (s, 1H), 7.88 (dt, J = 9.1, 3.4 Hz, 1H), 7.74 (d, J = 8.1 Hz, 2H), 7.51 (t, J = 9.2 Hz, 1H), 7.42 (s, 1H), 7.13 (d, J = 8.3 Hz, 2H), 4.21 (s, 3H), 4.14 (t, J = 6.4 Hz, 2H), 2.46 (s, 2H), 2.24 (s, 6H), 2.01–1.93 (m, 2H).
(E)-N-(4-((4-chloro-3-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-2-(4-(3-(diethylamino)propoxy)benzylid ene)hydrazine-1-carboxamide (44). This compound was obtained as white solid in 79% yield. Mp 221.5–229.3 °C. ESI-MS m/z: [M + H]+ 593.2. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (s, 1H), 9.93 (s, 1H), 9.16 (s, 1H), 9.01 (s, 1H), 8.61 (s, 1H), 8.19 (dd, J = 6.9, 2.7 Hz, 1H), 8.06 (s, 1H), 7.88 (dt, J = 9.1, 3.4 Hz, 1H), 7.74 (d, J = 8.1 Hz, 2H), 7.51 (t, J = 9.2 Hz, 1H), 7.42 (s, 1H), 7.13 (d, J = 8.3 Hz, 2H), 4.21 (s, 3H), 4.14 (t, J = 6.4 Hz, 2H), 2.46 (s, 2H), 2.24 (s, 6H), 2.01–1.93 (m, 2H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(3-(pyrrolidin-1-yl)propoxy)benzyli dene)hydrazine-1-carboxamide (45). This compound was obtained as yellow solid in 77% yield. Mp 216.7–219.6 °C. ESI-MS m/z: [M + H]+ 591.2. 1H NMR (400 MHz, DMSO-d6) δ 10.91–10.70 (m, 1H), 9.87 (s, 1H), 8.96 (s, 1H), 8.77 (s, 1H), 8.49 (s, 1H), 8.07 (s, 1H), 7.94 (s, 1H), 7.80–7.69 (m, 1H), 7.34 (d, J = 42.0 Hz, 2H), 7.51 (t, J = 9.2 Hz, 1H), 7.42 (s, 1H), 7.13 (d, J = 32.2 Hz, 2H),4.35 (s, 2H), 4.06 (s, 3H), 2.38–2.32 (m, 4H), 1.83 (s, 2H), 1.05 (t, J = 7.0 Hz, 2H), 0.83 (t, J = 6.9 Hz, 6H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(3-(piperidin-1-yl)propoxy)benzylid ene)hydrazine-1-carboxamide (46). This compound was obtained as white solid in 84% yield. Mp 218.5–221.5 °C. ESI-MS m/z: [M + H]+ 605.2. 1H NMR (400 MHz, DMSO-d6) δ 11.02 (s, 1H), 9.85 (s, 1H), 9.06 (s, 1H), 8.92 (s, 1H), 8.52 (s, 1H), 8.10 (d, J = 6.9 Hz, 1H), 7.96 (s, 1H), 7.77 (s, 1H), 7.65 (d, J = 8.7 Hz, 2H), 7.42 (s, 1H), 7.33 (s, 1H), 7.04 (d, J = 8.7 Hz, 2H), 4.12 (s, 3H), 4.05 (t, J = 6.2 Hz, 2H), 2.38 (t, J = 6.7 Hz, 2H), 2.33 (s, 4H), 1.91–1.87 (m, 2H), 1.48 (d, J = 4.9 Hz, 4H), 1.37 (s, 2H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(3-morpholinopropoxy)benzylidene)hydrazine-1-carboxamide (47). This compound was obtained as white solid in 79% yield. Mp 226.5–230.2 °C. ESI-MS m/z: [M + H]+ 607.2. 1H NMR (400 MHz, DMSO-d6) δ 11.02 (s, 1H), 9.84 (s, 1H), 9.06 (s, 1H), 8.89 (d, J = 26.4 Hz, 1H), 8.52 (s, 1H), 8.10 (d, J = 6.7 Hz, 1H), 7.96 (s, 1H), 7.78 (s, 1H), 7.66 (d, J = 7.4 Hz, 2H), 7.41 (dd, J = 18.2, 9.1 Hz, 1H), 7.33 (s, 1H), 7.04 (d, J = 7.4 Hz, 2H), 4.12 (s, 3H), 4.07 (d, J = 5.4 Hz, 2H), 3.58 (s, 4H), 2.43 (t, J = 6.5 Hz, 2H), 2.37 (s, 4H), 1.94–1.85 (m, 2H).
(E)-N-(4-((3-chloro-4-fluoropheny)amino)-7-methoxyquinazolin-6-yl)-2-(4-(3-(4-methylpiperazin-1-yl)propoxy) benzylidene)hydrazine-1-carboxamide (48). This compound was obtained as white solid in 88% yield. Mp 216.5–218.2 °C. ESI-MS m/z: [M + H]+ 620.2. 1H NMR (400 MHz, DMSO-d6) δ 11.02 (s, 1H), 9.85 (s, 1H), 9.06 (s, 1H), 8.91 (s, 1H), 8.51 (s, 1H), 8.09 (d, J = 6.6 Hz, 1H), 7.96 (s, 1H), 7.77 (s, 1H), 7.65 (d, J = 8.6 Hz, 2H), 7.42 (t, J = 9.1 Hz, 1H), 7.32 (s, 1H), 7.04 (d, J = 8.7 Hz, 2H), 4.11 (s, 3H), 4.05 (s, 2H), 2.49–2.49 (m, 8H), 2.40 (d, J = 7.2 Hz, 2H), 2.13 (s, 3H), 1.88 (d, J = 7.2 Hz, 2H).
4.(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(3-(diethylamin o)propoxy)benzylidene)hydrazine-1-carboxamide (49)/NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H), 9.86 (s, 1H), 9.13 (s, 1H), 9.00 (s, 1H), 8.50 (s, 1H), 8.08 (d, J = 6.9 Hz, 1H), 7.98 (s, 1H), 7.78 (d, J = 10.0 Hz, 1H), 7.66 (d, J = 8.4 Hz, 2H), 7.41 (t, J = 9.1 Hz, 1H), 7.32 (s, 1H), 6.98 (d, J = 8.5 Hz, 2H), 5.44 (s, 1H), 4.04 (d, J = 4.8 Hz, 4H), 3.96 (t, J = 7.6 Hz, 1H), 3.89–3.85 (m, 1H), 2.44 (s, 1H), 2.37 (t, J = 7.1 Hz, 2H), 2.27 (s, 1H), 2.15 (s, 6H), 1.85 (d, J = 6.8 Hz, 2H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(3-(diethylamin o)propoxy)benzylidene)hydrazine-1-carboxamide (50) This compound was obtained as yellow solid in 86% yield. Mp 208.2–213.5 °C. ESI-MS m/z: [M + H]+ 649.2. 1H NMR (400 MHz, DMSO-d6) δ 11.06 (s, 1H), 9.85 (s, 1H), 9.13 (s, 1H), 9.01 (s, 1H), 8.50 (s, 1H), 8.09 (dd, J = 6.9, 2.6 Hz, 1H), 7.96 (d, J = 6.4 Hz, 1H), 7.78 (dt, J = 8.8, 3.4 Hz, 1H), 7.66 (d, J = 8.4 Hz, 2H), 7.41 (t, J = 9.1 Hz, 1H), 7.33 (s, 1H), 6.98 (d, J = 8.5 Hz, 2H), 5.45 (s, 1H), 4.05 (dd, J = 8.3, 4.5 Hz, 4H), 3.97 (q, J = 7.7 Hz, 1H), 3.87 (td, J = 8.4, 4.5 Hz, 1H), 2.54 (s, 2H), 2.46 (d, J = 7.0 Hz, 4H), 2.43–2.36 (m, 1H), 2.20–2.11 (m, 1H), 1.83 (q, J = 6.7 Hz, 2H), 0.94 (t, J = 7.1 Hz, 6H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(3-(pyrrolidin-1-yl)propoxy)benzylidene)hydrazine-1-carboxamide (51). This compound was obtained as white solid in 83% yield. Mp 223.1–230.5 °C. ESI-MS m/z: [M + H]+ 647.2. 1H NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H), 9.87 (s, 1H), 9.14 (s, 1H), 9.00 (s, 1H), 8.50 (s, 1H), 8.08 (d, J = 4.8 Hz, 1H), 7.96 (s, 1H), 7.77 (s, 1H), 7.66 (d, J = 8.5 Hz, 2H), 7.41 (t, J = 9.0 Hz, 1H), 7.32 (s, 1H), 6.98 (d, J = 8.3 Hz, 2H), 5.45 (s, 1H), 4.04 (s, 4H), 3.96 (d, J = 7.7 Hz, 1H), 3.87 (d, J = 4.3 Hz, 1H), 2.53 (s, 2H), 2.43 (s, 4H), 2.14 (s, 2H), 1.93–1.85 (m, 2H), 1.68 (s, 4H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(3-(piperidin-1-yl)propoxy)benzylidene)hydrazine-1-carboxamide (52). This compound was obtained as white solid in 81% yield. Mp 222.5–226.5 °C. ESI-MS m/z: [M + H]+ 661.2. 1H NMR (400 MHz, DMSO-d6) δ 11.07 (s, 1H), 9.86 (s, 1H), 9.13 (s, 1H), 8.99 (s, 1H), 8.49 (s, 1H), 8.07 (dd, J = 6.9, 2.6 Hz, 1H), 7.95 (s, 1H), 7.76 (s, 1H), 7.65 (d, J = 8.7 Hz, 2H), 7.41 (t, J = 9.1 Hz, 1H), 7.31 (s, 1H), 6.97 (d, J = 8.8 Hz, 2H), 5.45 (s, 1H), 4.04 (d, J = 6.6 Hz, 4H), 3.94 (s, 1H), 3.86 (d, J = 4.5 Hz, 1H), 2.41–2.33 (m, 4H), 2.31 (s, 2H), 2.14 (s, 2H), 1.87 (d, J = 7.9 Hz, 2H), 1.48 (s, 4H), 1.37 (s, 2H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(3-morpholino propoxy)benzylidene)hydrazine-1-carboxamide (53). This compound was obtained as white solid in 82% yield. Mp 226.5–229.5 °C. ESI-MS m/z: [M + H]+ 663.2. 1H NMR (400 MHz, DMSO-d6) δ 10.97 (s, 1H), 9.84 (s, 1H), 9.10 (s, 1H), 8.98 (s, 1H), 8.43 (s, 1H), 8.03 (s, 1H), 7.95 (s, 1H), 7.70 (s, 1H), 7.65 (d, J = 8.4 Hz, 2H), 7.36 (t, J = 9.1 Hz, 1H), 7.26 (s, 1H), 6.97 (d, J = 8.4 Hz, 2H), 5.41 (d, J = 8.2 Hz, 1H), 4.06 (d, J = 6.2 Hz, 2H), 4.02 (d, J = 3.4 Hz, 2H), 3.94 (t, J = 7.7 Hz, 1H), 3.87 (dd, J = 8.4, 4.7 Hz, 1H), 3.56 (t, J = 4.6 Hz, 4H), 2.43 (s, 1H), 2.41 (d, J = 7.1 Hz, 2H), 2.35 (s, 4H), 2.14 (d, J = 8.8 Hz, 1H), 1.90–1.84 (m, 2H).
(S,E)-N-(4-((3-chloro-4-fluorophenyl)amino)-7-((tetrahydrofuran-3-yl)oxy)quinazolin-6-yl)-2-(4-(3-(4-methylpi perazin-1-yl)propoxy)benzylidene)hydrazine-1-carboxamide (54). This compound was obtained as yellow solid in 81% yield. Mp 216.5–219.5 °C. ESI-MS m/z: [M + H]+ 676.2. 1H NMR (400 MHz, DMSO-d6) δ 11.07 (s, 1H), 9.85 (s, 1H), 9.12 (s, 1H), 8.99 (s, 1H), 8.48 (s, 1H), 8.06 (d, J = 7.1 Hz, 1H), 7.95 (s, 1H), 7.75 (s, 1H), 7.64 (d, J = 8.2 Hz, 2H), 7.40 (t, J = 9.0 Hz, 1H), 7.30 (s, 1H), 6.96 (d, J = 8.3 Hz, 2H), 5.43 (s, 1H), 4.13–4.00 (m, 4H), 3.95 (q, J = 7.5 Hz, 1H), 3.86 (dt, J = 12.2, 6.1 Hz, 1H), 2.60 (s, 2H), 2.45 (s, 1H), 2.43–2.35 (m, 4H), 2.35–2.22 (m, 4H), 2.15 (s, 1H), 2.12 (s, 3H), 1.96–1.77 (m, 2H).
Biological evaluation
Cytotoxicity assay in vitro
The cytotoxic activities of target compounds 9–54 were evaluated with HepG2, A549, MCF-7 and H1975 cell lines by the standard MTT assay in vitro, with compounds EGFR inhibitors afatinib as a positive control. The cancer cell lines were cultured in minimum essential medium (MEM) supplement with 10% fetal bovine serum (FBS). Approximately 4 × 103 cells, suspended in MEM medium, were plated onto each well of a 96-well plate and incubated in 5% CO2 at 37 °C for 24 h. The test compounds at indicated final concentrations were added to the culture medium and the cell cultures were continued for 72 h. Fresh MTT was added to each well at a terminal concentration of 5 μg/mL and incubated with cells at 37 °C for 4 h. The formazan crystals were dissolved in 100 μL DMSO each well, and the absorbency at 492 nm (for absorbance of MTT formazan) and 630 nm (for the reference wavelength) was measured with the ELISA reader. All of the compounds were tested three times in each of the cell lines. The results expressed as inhibition rates or IC50 (half-maximal inhibitory concentration) were the averages of two determinations and calculated by using the Bacus Laboratories Incorporated Slide Scanner (Bliss) software (Bacus Laboratories Inc, Lombard, IL, USA).
EGFR kinases assay in vitro
The selected compounds with excellent anti-proliferative activities were tested for their activity against EGFR kinases through the mobility shift assay. All kinases assays were performed in 96-well plates in a 50 μL reaction volume. The kinase buffer contains 50 mM HEPES, pH 7.5, 10 mM MgCl2, 0.0015% Brij-35 and 2 mM DTT. The stop buffer contains 100 mM HEPES, pH 7.5, 0.015% Brij-35, 0.2% Coating Reagent #3 and 50 mM EDTA. The compounds were diluted to 500 μM by 100% DMSO, then 10 μL of compound was transferred to a new 96-well plate as the intermediate plate, and 90 μL kinase buffer was added to each well. Then 5 μL of each well of the intermediate plate was transferred to 384-well plates. The following amounts of enzyme and substrate were used per well: kinase base buffer, FAM-labeled peptide, ATP and enzyme solution. Wells containing the substrate, enzyme, DMSO without compound were used as DMSO control. Wells containing just the substrate without enzyme were used as a low control. Incubate at room temperature for 10 min. Add 10 μL peptide solution to each well. Incubate at 28 °C for specified period of time and stop reaction by 25 μL stop buffer. At last data was collected on Caliper program and conversion values were converted to inhibition values. Percent inhibition = (max − conversion)/(max − min) × 100. “max” stands for DMSO control; “min” stands for low control.
Cell apoptosis assay by flow cytometry
A549 cells were seeded in 16-well plates at a density of 1 × 106 cells/well in RPMI 1640 medium and treated with 0.5, 1 µM 10 d for 48 h. Cultured cells were stained with Annexin V-FITC and propidium iodide (PI) in the dark at 4 °C for 30 min and analysed by FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) using Cell Quest software.
Flow cytometric analysis of cell cycle distribution assay
For flow cytometric analysis of DNA content, 5.0 × 105 cell/well A549 cells were grown in a costar 6-well cell culture cluster and grown for 24 h at 37 °C in 5% CO2, after the medium was removed and the cells were treated with specific concentrations of the test compounds 14 and 44 for 48 h. Blank wells treated with medium only were also included. After the incubation period, the A549 cells were collected, washed twice with ice-cold PBS, centrifuged and then fixed with ice-cold ethanol (70%) for at least 24 h. Cells were then collected, washed twice with ice-cold PBS, and treated with 30 ml RNase A (1 mg/mL) in PBS at 37 °C for about 30 min, and then stained with 50 ml propidium iodide (50 mg/mL) in PBS, the staining process lasted 30 min at 4 °C in darkness. The cellular DNA content of the stained cells was then analysed on BD-FACS Aria III flow cytometer and the cell cycle distribution was quantified.
Docking studies
For docking purposes, the three-dimensional structure of the EGFR PDB code: 4G5P was obtained from RCSB Protein Data Bank. Autodock 4.2 (The Scripps Research Institute, La Jolla, CA, USA), Discover Studio 4.5 visualiser and Open Babel (Biovia Dassault System, Accelrys, San Diego, CA, USA) were used for the docking study. Firstly, using the Discover Studio 4.5 visualiser to prepare ligands (14 and 44) and acceptor protein (PDB code: 4G5P), then saved as pdb format after energy minimisation for ligand and clean protein, defined the activity site for acceptor protein. Secondly, the pdb file of ligand and the acceptor protein were converted to the pdbqt format by the Open Babel tool. Thirdly, docking was carried out by uploading the pdbqt file to the Autodock 4.5, and the result was named out.pdbq. Moreover, out.pdbpt file was uploaded to the Discover Studio 4.5 visualiser for analysing the result. All calculations were performed on Silicon Graphics workstation. Detailed user guidance and basic docking operations can be found on AutoDock official website http://autodock.scripps.edu/faqs-help/tutorial.
Results and discussion
Chemistry
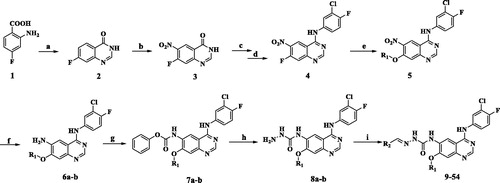
The structure and synthetic route of 9–54 are shown by and Scheme 1, respectively. The key intermediates 8a–b was synthesised from commercially available 2-amino-4-fluorobenzoic acid through eight steps. Finally, condensation of 8a–b with aromatic aldehydes in ethanol in the presence of a catalytic amount of glacial acetic acid yielded target compounds 9–54 respectively. Commercially available heterocyclic aldehydes reacted with 8a–b produced compounds 9–18, and side chains 57a-1 and 60a–f reacted with 8a–b produced compounds 18–54.
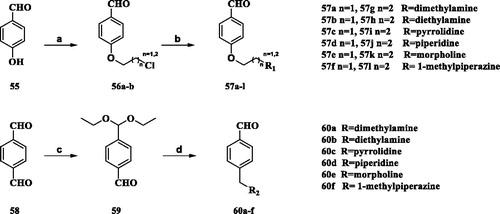
The general strategy for the synthesis of compounds 57a-1 and 60a–f is shown in Scheme 2. The reaction of the commercially available 4-hydroxybenzaldehyde (55) with 1-bromo-2-chloroethane or 1-bromo-2-chloropropane produced intermediates 56a–56b. Nucleophilic aromatic substitution of the position of chlorine in 56a–58b with small molecule amines in DMF at microwave 125 °C yielded compounds 57a-l. Commercially available terephthalaldehyde (58) reacted with triethyl orthoformate to obtain the crude product and purified by column chromatography (PE/EA, 5.95v/v) to afford the intermediate 59. Compound 59 was reductively aminated in the presence of sodium borohydride to give the crude product. Finally, a solution of the crude product in methanol reacted with a hydrochloric acid-methanol solution to afford the intermediate 60a–f.
Reagents and conditions: (a) EtOH, Formamidine acetate, 24h; (b) Con.H2SO4, fuming HNO3, 2h; (c) SOCl2, DMF(cat.), 4h; (d) 3-chloro-4-fluoroaniline, Isopropanol, Triethylamine, 1.5h; (e) (S)-tetrahydrofuran-3-ol, 60% NaH, THF, 3h; CH3OH, NaOH; (f) 80% hydrazine monohydrate, FeCl3, activated carbon, EtOH, 1h; (g) phenyl chloroformate, DIPEA, 1,4-dioxane, 10 °C to rt, 1.5h; (h) 80% hydrazine monohydrate, 1,4-dioxane, reflux; (i) commercially available Heterocyclic aldehydes, acetic acid(Cat.), EtOH, reflux.
Reagents and conditions: (a) acetonitriles, anhydrous, K2CO3, 1-bromo-2-chloroethane or 1-bromo-2-chloropropane, 1 h, rt; (b) DMF, microwave, Small molecule amines, 1 h, rt. (c) triethyl orthoformate, ammonium chloride, r.t.; (d) 1, methanol water, sodium borohydride; 2, diluted hydrochloric acid, heated. 3, hydrogen chloride in methanol, T = 0–20 °C.
In vitro anti-proliferative activity screening of compounds 9–54
Taking afatinib as reference compound, the target compounds (9–54) were evaluated for the anti-proliferative activity against a panel of four human cancer cell lines, belonging to different tumour types, namely human liver cancer cell lines (HepG2), human breast carcinoma cell lines (MCF-7), human lung carcinoma cell lines (A549), Human lung adenocarcinoma cell lines (H1975) at a concentration of 0.1–100 mM. HepG2, A549, and MCF-7 were selected to test the broad-spectrum anti-cell proliferative activity of compounds on tumour cells. H1975 was used to verify whether anti-cell proliferative activity was also observed for gefitinib-resistant cell lines. The results expressed as IC50 values were summarised in and the values were the average of at least two independent experiments. The IC50 values that were higher than 100 μM in all cell lines were not included.
Table 1. Chemical structures of the target compounds 9–54.
Table 2. In vitro anti-proliferative activities against different cancer cell lines for 72 h of the target compounds.
As shown in , the results of the cells showed that after the C-7 position of the quinazoline nucleus was modified, the anti-proliferative activity of H1975 against most of the target compounds was significantly increased. However, compounds 23, 24, 29, 38, 39, 43, 49 were showed no activity on some cell lines. The potential compounds well inhibited the growth of H1975 cells with IC50 values ranging from 0.83 ± 0.17 to 16.58 ± 1.39 μM. Notably, the anti-proliferative activity of compounds 14, 28, 44, 46, 50, 52 and 54 against H1975 cells was a single-digit level with IC50 values of 1.72 ± 0.85, 3.97 ± 0.64, 1.03 ± 0.17, 3.10 ± 0.49, 1.53 ± 0.52, 2.20 ± 0.37 and 6.23 ± 0.99 μM, respectively. Likewise, compounds 10, 12, 13, 19, 21, 24, 37, 42 and 48 showed moderate inhibitory activity. Unfortunately, compounds 9, 15, 16, 20, 25, 32, 34, 36, 38 and 49 had poorer cellular activity of H1975 despite their very good broad antitumour activity.
Interestingly, compound 14 showed a slight selectivity for H1975 cells. It is noteworthy that compound 44 have inhibitory activity against H1975 cells with an IC50 value of 1.03 ± 0.17 μM, which was lower than that of afatinib (0.49 ± 0.08 μM). However, the IC50 values of other cells were 0.02 ± 0.01, 0.41 ± 0.01 and 0.32 ± 0.02 μM, respectively, which were slightly higher than that of afatinib (1.40 ± 0.83, 1.33 ± 1.28, 2.63 ± 1.06 μM).
Structure-activity relationship analysis
In combination with the activity of all compounds on the four cells, we found that most of the compounds’ activity is reduced in the b strategy. Affected by the 4 position of the aromatic ring, the density of the electron cloud of the aromatic ring is reduced. In strategy a, the cell activity of the three carbon chains is apparently stronger than that of the two carbons. Among them, compounds 44 and 50 show the most prominent activity, they all have the characteristics of a tricarbon chain linker and diethylamine. In the c strategy, the activity on H1975 is significantly increased and the activity on other cells remained since the hydroxyl group is replaced by an amine, such as compounds 9–12. When the aromatic ring is replaced with an aromatic heterocycle, the activity against all cells slightly decreases. Same as we expected, compounds 13 and 14 in which the aromatic rings contain two heteroatoms, exhibit very prominent inhibitory activity against H1975.
Kinase inhibitory activity
In order to find inhibitors targeting L858R/T790M, some compounds with significant inhibitory activity against H1975 cells were selected. The in vitro enzymatic inhibitory activities against EGFRL858R/T790M and EGFRWT were evaluated by using the well-established ELISA-based assay, and afatinib was employed as positive controls (). Notably, compound 14 (IC50 = 8.4 nM) with a 2- (pyrrolidin-1-yl) pyrimidine side chain at the 7-position of the quinazoline ring had almost the same inhibitory activity as afatinib (IC 50 = 3.8 nM). Among the compounds tested, most of the compounds showed single-digit nanomolar levels of wild-type kinase activity. However, most compounds had low inhibitory activity against the L858R/T790M mutant. It is noteworthy that when the C-7 side chain introduced a flexible hydrophobic chain, the inhibitory activity of these compounds on L858R/T709M kinase was significantly enhanced. Among them, compound 44 was the best representative. And the most potent compound 14 exhibited excellent inhibition against EGFRWT and EGFRL858R/T790M with an IC50 at 6.3 nM and 8.4 nM, respectively.
Table 3. Inhibitory activity of selected compounds against different types of EGFRs in vitro.
Morphologic changes of A549 cells under inverted microscopy and fluorescence microscopy
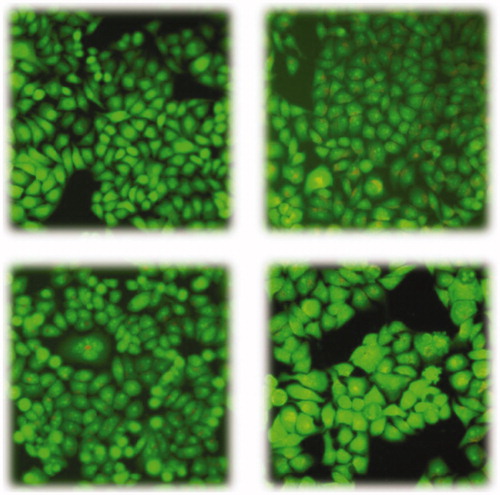
To explain the inhibition of cell growth, the apoptotic experiment by acridine orange (AO) single staining would be carried out to exam the effect of compounds 14 and 44 on A549 cell. As shown in , the control group cell () was stained with acridine orange (AO) and the shape of the cell was full and the edge was clear. But in the other pictures (), the cell showed an identical phenomenon, the shape of which was abnormal with cell shrinkage, chromatin condensation or decomposition into fragments of varying sizes. This phenomenon indicated that compounds 14 and 44 can induce A549 cell apoptosis.
Figure 3. Morphologic changes of A549 cells under inverted microscopy and fluorescence microscopy. 3(a) the control group cell treated with nothing; 3(b) Experimental group treated with 1.33 μM concentration of afatinib. 3(c) Experimental group treated with 1.59 μM concentration of 14. 3(d) Experimental group treated with 0.41 μM concentration of 44.
Induction of apoptosis assay on A549 cells
Apoptosis refers to the autonomous and orderly death of cells controlled by genes. Furthermore, its startup is affected by many external factors. To further elucidate the relationship between apoptosis and compounds 14 and 44, Annexin V-FITC and propidium iodide (PI) double staining flow cytometry was used to evaluate the effect of compounds 14 and 44 on apoptosis of A549 cells at specific concentrations influences. The results are shown in .
Figure 4. Cell apoptosis analysis on A549 cells treated with compounds 14 and 44 at 2.5 μM detected by FCM.
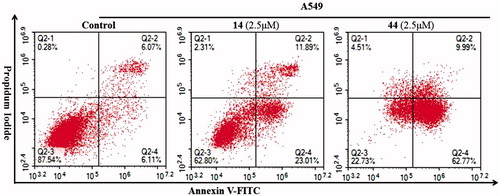
According to the results of , compounds 14 and 44 effectively induced cell apoptosis at a concentration of 2.5 μM. Treatment of A549 cells with compounds 14 and 44 for 48 h resulted in 34.9% and 72.76% of apoptotic cells (early + late), respectively, compared to 12.18% of apoptotic cells in the untreated control. These results revealed that compounds 14 and 44 inhibited cell growth through cell apoptosis induction.
Cell cycle analysis on A549 cells by flow cytometry
Aiming to better elucidate the relationship between the mechanism of inhibition of proliferation and cell cycle arrest, cells cycle distribution on A549 cells by treating with 2.5 μM concentrations compounds 14 and 44 were performed. The results are shown in and . The region marked with different colours represents % population at different phases of the cell cycle. As can be seen, compound 14 caused an increase in the proportion of cells in S phase (from 40.03% in the control to 50.68%) with a concomitant decrease of cells in G0/G1 phase of the cell cycle (from 26.57% in the control to 23.62%) and cells in G2/M phase of the cell cycle (from 29.4% in the control to 25.7%). Interestingly, the effects of compound 44 and compound 14 on cell cycle were quite different. As shown in , compound 44 caused an increase in the proportion of cells that cause G0/G1 phase cells (from 26.57% to 50.01% of controls), accompanied the decrease of S phase cell ratio (23.62% from 26.57% of controls) and G2/M phase cell ratio (from 29.4% in the control group to 25.7%). These results indicated that compounds 14 and 44 can inhibit tumour cell proliferation and lead to apoptosis by blocking the cell cycle.
Figure 5. Cell cycle distribution of A549 cells treated at 2.5 μM compound 14 and 44 for 48 h detected by FCM.
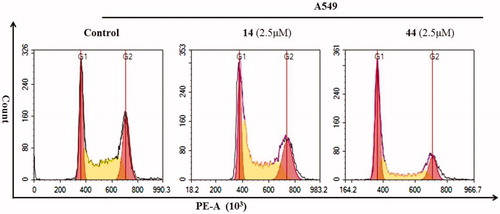
Table 4. Analysis of cell cycle on A549 cells treated at 25 μM compound 14 and 44 for 48 h by flow cytometry.
Molecular docking study
To better understand how the 14 and 44 contributed to the EGFR kinase inhibitory activities, molecular docking simulation studies were carried out by using AutoDock 4.2, Open Babel and Discover Studio 4.5 visualizer. And based on the in vitro inhibition results, we selected compounds 14 and 44, our best EGFR inhibitors in this study, as the ligand examples. And the co-crystal structure of EGFRT790M in complex with afatinib was obtained from the RSC Protein Data Bank (PDB code: 4G5P). The four selected compounds (afatinib, BMC201725-9o, 14 and 44) occupied the ATP site of EGFR kinase (). The binding patterns of compounds 14 and 44 to EGFR kinase are shown in detail in .
Figure 6. The protonated state of several important residue were adjusted by using AutoDock 4.5 in favour of forming reasonable hydrogen bond with the ligand and Molecular docking analysis was carried out by the Discover Studio 4.5 visualizer to explore the binding model for the active site of EGFR with its ligand. Docking simulations showed that the four selected compounds (afatinib, BMC201725-9o, 14 and 44) occupied the ATP site of EGFR kinase 6(a). Then we employed 3 D interaction map 6(b), 2 D diagram of the compound 14 6(c) and 2 D diagram of the compound 44 6(d) to display the interaction with the targeted protein (4G5P).
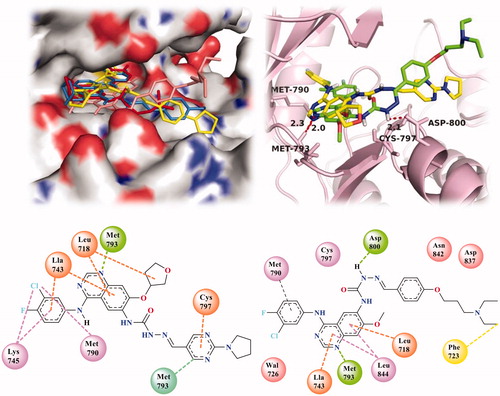
As clearly shown in , the two compounds 14 and 44 all formed a hydrogen bond from the N1 of quinazoline core to the main chain NH of Met793, and the length of hydrogen bond were 2.0 and 2.3 Å, respectively. Compared to afatinib and 44, another hydrogen bond existed between 44 and EGFR from NH in the Hydrazine carboxamide of 44 to the residue Asp800 of EGFR with the length of 2.1 Å (). Furthermore, several interactions existed between 14 and the residues of protein, such as pi-Alkyl interactions of the 3-Chloro-4-fluoroaniline with Met790 and Lys745. Alkyl interactions of the quinazoline ring with Leu718 and Ala743. There was a hydrogen-carbon interaction between the pyrimidine ring and Asp800 (). Interestingly, compound 44 had pi-sigma interactions between its hydrophobic side-chain ends and Phe723 in addition to the above several interactions (). These all indicated that the introduction of Heterocyclic aldehydes and flexible hydrophobic side chains to C-7 of TYB-65 led to a new binding interaction with the EGFR-TK domain, which contributed to its inhibitory activity against EGFR.
Conclusion
Herein, we used three different modification methods to structurally modify our previously studied compound BMC201725-9o, which led to the design and synthesis of a series of new derivatives and increased activity on EGFR.L858R/T790M. These compounds were subjected to biological activities evaluation including anti-proliferative effects against four tumour cell lines (HepG2, A549, MCF-7 and H1975). Most of the synthesised compounds exhibited moderate to excellent anti-proliferative activities against the tested tumour cell lines. The hit 44 exhibited excellent inhibition of the tested tumour cell lines with the IC50 values of 0.02 ± 0.01 μM, 0.41 ± 0.013 μM, 0.32 ± 0.02 μM and 0.83 ± 0.17 μM, respectively. An EGFR kinase inhibition assay indicated that some of the tested derivatives displayed good inhibition against EGFRWT and EGFRL858R/T790M. Notably, compound 14 not only exhibited excellent anti-proliferative activity against the tumour cells, but also showed potent inhibitory activity toward EGFRWT (IC50 6.3 nM) and EGFRL858R/T790M (IC50 8.4 nM), similar to that of afatinib (IC50 4.0 and 3.8 nM). According to the result of AO single staining and Annexin V/PI staining, the 14 and 44 could induce remarkable apoptosis of A549 cells. Compound 14 caused S phase arrest and induced apoptosis to inhibit cell proliferation. Compound 44, on the other hand, caused arrest in G0/G1 phase to induce apoptosis. In general, compounds 14 and 44 exerted potential antitumour activity through several mechanisms, including anti-proliferation and cell cycle arrest, and may serve as model molecule to help us to further design and develop more potent anticancer agents. These findings presented herein show the noncovalent inhibitor 14 and 44 have the potential to target EGFR mutants. The results also provide more insights for designing new classes of mutant-selective EGFR inhibitors.
IENZ_1518957_Supplementary Material
Download PDF (641.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Song Z, Ge Y, Wang C, et al. Challenges and perspectives on the development of small-molecule EGFR inhibitors against T790M-mediated resistance in non-small-cell lung cancer. J. Med. Chem 2016;59:6580–94.
- Zhang H-Q, Gong F-H, Ye J-Q, et al. Design and discovery of 4-anilinoquinazoline-urea derivatives as dual TK inhibitors of EGFR and VEGFR-2. Eur J Med Chem 2017;125:245–54.
- Han J, Henriksen S, Nørsett KG, et al. Balancing potency, metabolic stability and permeability in pyrrolopyrimidine-based EGFR inhibitors. Eur J Med Chem 2016;124:583–607.
- Amin KM, Barsoum FF, Awadallah FM, et al. Identification of new potent phthalazine derivatives with VEGFR-2 and EGFR kinase inhibitory activity. Eur J Med Chem 2016;123:191–201.
- Chen L, Fu W, Feng C, et al. Structure-based design and synthesis of 2,4-diaminopyrimidines as EGFR L858R/T790M selective inhibitors for NSCLC. Eur J Med Chem 2017;140:510–27.
- Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786–92.
- Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2008;14:2895–9.
- Ou SHI. Second-generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a better mousetrap? A review of the clinical evidence. Crit Rev Oncol Hematol 2012;83:407–21.
- Ward RA, Anderton MJ, Ashton S, et al. Structure- and reactivity-based development of covalent inhibitors of the activating and gatekeeper mutant forms of the epidermal growth factor receptor (EGFR). J Med Chem 2013;56:7025–48.
- Liu Q, Sabnis Y, Zhao Z, et al. Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol 2013;20:146–59.
- Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009;462:1070–4.
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nature Med 2015;21:560.
- Bryan MC, Burdick DJ, Chan BK, et al. Pyridones as highly selective, noncovalent inhibitors of T790M double mutants of EGFR. ACS Med Chem Lett 2016;7:100–4.
- Heald R, Bowman KK, Bryan MC, et al. Noncovalent mutant selective epidermal growth factor receptor inhibitors: a lead optimization case study. J Med Chem 2015;58:8877–95.
- Shao J, Chen E, Shu K, et al. 6-Oxooxazolidine-quinazolines as noncovalent inhibitors with the potential to target mutant forms of EGFR. Bioorg Med Chem 2016;24:3359–70.
- Tu Y, Wang C, Xu S, et al. Design, synthesis, and docking studies of quinazoline analogues bearing aryl semicarbazone scaffolds as potent EGFR inhibitors. Bioorg Med Chem 2017;25:3148–57.