Abstract
Head and neck cancer (HNC) is one of the most common malignancies in the world. HNC is a group of cancers that starts in the mouth, nose, throat, larynx, sinuses, or salivary glands. According to this section of the body parts; induction of cancer can be associated with CO2 and oxidative stress. The aim of this study is to assess the activities of carbonic anhydrase (CA), catalase (CAT), paraoxonase1 (PON1), and xanthine oxidase (XO) activities in 89 HNC patients and 115 healthy volunteers. Paraoxonase1 activity was found lower in HNC cancer patients. There is no statistically significant difference between patients and controls for catalase, carbonic anhydrase, and xanthine oxidase enzyme levels. According to this results, paraoxonase1 levels could be a candidate as an oxidative marker in HNC patients, but further studies are needed to investigate the other type of cancer related PON1 and the other enzyme levels.
Introduction
Head and neck cancers (HNCs) are found in the oral cavity, pharynx, nasal cavity, paranasal sinuses, larynx, and salivary glandsCitation1. The five-year survival rate is only 30–35% in HNC typesCitation2.
Risk factors for HNCs are; using tobacco, high-volume alcohol, salt and processed food consumption, improper storage of food, nutritional deficiencies and inadequate oral hygieneCitation3,Citation4. All these risk factors related with inhalation may also affect enzyme denaturation since enzymes have numerous different functions in our bodies. Because of this reason we aimed to evaluate carbonic anhydrase (CA), catalase (CAT), paraoxonase1 (PON1) and xanthine oxidase (XO) levels in HNC patients. Another related effect of cancer is oxidative stressCitation5 which makes a wide contribution tissue destruction to give an imbalance between reactive oxygen species (ROS) and antioxidants to repair the resulting damage. ROS and antioxidants are very important in the development of treatment of various diseasesCitation6. On the other hand, the increase or decrease of the ROS level may affect the disease characteristics and the treatment effectCitation7.
Several functions such as cell proliferation, gene instability, invasion, and metastasis are affected by oxidative stress in cancer cellsCitation8. These cells are capable of producing too much ROS compared with normal cells and therefore they inhibit antioxidative defence system. In this mechanism, ROS plays an active role on the pathogenesis of different diseases such as cancer, and the processes of carcinogenesis. All can be induced by redox imbalance affected by ROSCitation9.
Cigarette smoke is a mixture of many chemical compounds, including various free radicals and toxic compoundsCitation10. That is why, smoking is a serious risk factor for the development of head and neck mucosal squamous cell carcinoma (SCC)Citation11. Smoking changes endothelial function, inflammation, the redox state and global DNA methylationCitation12. Smoking causes oxidative/antioxidant imbalance and causes oxidative stress. All of these are accompanied by lipid peroxidation, oxidative DNA damage, damage to macro- and micro-molecule cells, and antioxidant defensive disorders that can initiate a malignant process. Due to tobacco consumption, it influences the pH of body fluids as well as the formation and stabilisation of free radicalsCitation13.
Different alcoholic beverages are differentially associated with HNC riskCitation14. For a better understanding, alcohol-induced carcinogenesis is needed to adequately investigate the alcohol consumption which is a major risk factor. According to this, ethanol may additionally influence cancer risk, within this, HNC patients are directly affected by alcohol consumption. The risk is even greater in those people who smokeCitation15. Even small amounts of alcohol can increase cancer risk, especially the risk of cancers of the mouth, pharynx, larynx, oesophagus, breast, bowel, and liverCitation16.
Today’s concern is food safety for everybody since informal food production and marketing system is still a problem in many countriesCitation17. Diets in many countries have numerous increases in consumption of ultra-processed foodsCitation18. Definitely it is more crucial to examine the relative effect of the various dimensions of processing such as nutritional composition, food additives, and contact materials related with HNC riskCitation19,Citation20.
Oral hygiene is one of the many factors, which together with tobacco and alcohol have related effects on oral cancer such as having the result of HNCCitation21. Nutrition is critical to the oral health of the individual.
All cancers have an appointment in the DNA sequence of the genomes of cancer cells. This indicates that chaperones and protein homeostasis play an important role in cancer formationCitation22, and thus, appreciating the role of enzymes may lead to having more opportunities to understand their effects for therapeutic intervention.
For the purposes of this study, HNC cases were evaluated by enzyme activity relations to have a better understanding of CO2 attraction by CAs and oxidative stress which is stimulated by CAT, PON1, and XO levels related with HNC. According to their importance a consequence summary of their roles are determined as follows.
When we use oxygen our bodies constantly produce free radicals. Free radicals are chemically unstable molecules or atoms. With this ability, they also make other molecules or atoms in the body very unstable resulting in damage of proteins, cell membranes, and even DNA structure. High levels of H2O2 may be a risk factor for damaged cells and various diseasesCitation23. Catalase transforms harmful superoxide radicals into hydrogen peroxide as an antioxidant enzyme, catalyses the reaction of hydrogen peroxide decomposition to water and oxygen.
PON 1, an ester hydrolase bound to calcium, is a member of free radical-scavenging system in metabolism. PON1 has high antioxidant capacity against lipid peroxidation resulting from free radicals. PON1 plays a role in the metabolism of oxidised, biologically active lipids and responsible for HDL-mediated reduction of LDL oxidationCitation8.
XO, an enzyme containing iron and molybdenum, catalyses the xanthine oxidation of hypoxanthine and the uric acid oxidation of xanthineCitation24. XO is usually found in the liver, lung, kidney, heart, brain, and plasmaCitation25,Citation26 and causes the formation of reactive oxygen species by the production of superoxide anion radicalsCitation27.
Different isoforms of CAs are known in human which belong to α-classCitation28 and CA-I and CA-II are two major cytosolic isoenzymes. CAs catalyse the convertible hydration of carbon dioxide to carbonic acid and place an active role for physiological and biological processes in human such as cell-cell adhesion and cell proliferationCitation29,Citation30. CA-II is associated with cancer and it modulates the regulation of pH balance between cancer cells and tumour microenvironmentCitation31.
According to their cancer relations, CAs, XO, PON1, and CAT levels were evaluated in this current study.
Materials and methods
Eighty nine HNC cases (77 male, 12 female) in the mean age of 59.3 (±9.2) and 115 healthy volunteers (96 male, 21 female) in the mean age of 57.6 (±11.3) were included in the study in the Department of Otolaryngology, Diskapi Yildirim Beyazit Research and Training Hospital, Ankara, Turkey. The patients had the following diagnoses: larynx (n = 74) and oral cavity (n = 15) cancer (). This study was also approved by Diskapi Yildirim Beyazit Research and Training Hospital Ethics Committee (30 May 2016, 30/37). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Table 1. Demographic findings of the HNC patients.
All chemicals were of analytical grade and obtained from Sigma-Aldrich (Taufkirchen, Germany) and Merck (Darmstadt, Germany).
Blood samples
Blood samples were taken from the Department of Otolaryngology, Diskapi Yildirim Beyazit Research and Training Hospital, Ankara, Turkey. Venous peripheral blood samples (5 ml) were collected and placed on ice.
Sample preparation
The collected blood samples were centrifuged at 2208 g for 20 min. and after the centrifugation, the serum and the buffy coat were collected. Serum samples were used for determining the activities of XO and PON1. The packed red cells were washed twice with NaCl (0.9%), the erythrocyte lysates were prepared by putting the cells into three freeze-thaw cycles in dry ice with the addition of five volumes of ice-cold distilled water. The hemolysates were used to determine the activity of CATCitation23.
Determination of enzyme levels
Cytosolic carbonic anhydrases (CA-I and CA-II) Activity
Carbonic anhydrase activity was measured by the hydration of CO2 according to the method of Wilbur and AndersonCitation32 which is based on the determination of the time required for the pH of solution decreasing from 10.0 to 7.4 owing to the hydration of CO2. Assays were performed at least twice on each lysateCitation23.
Catalase activity
CAT activity in erythrocytes was determined by using spectrophotometric UV assay method on a Biotek (Winooski, VT, USA). This method is based on monitoring CAT-catalyzed decomposition of H2O2 for 5 min. by measuring a decrease in absorbance at 240 nm, at 25 °C. The results were expressed as units per litre (U/L)Citation33.
Paraoxonase activity
PON1 activity towards paraoxon as a substrate was quantified spectrophotometrically by the method described by Ganet alCitation34. The reaction was followed for 2 min. at 37 °C by monitoring the appearance of p-nitrophenol at 412 nm on a Biotek (Winooski, VT, USA). The final substrate concentration during enzyme assay was 2 mM, and all rates were determined in duplicate and corrected for the non-enzymatic hydrolysis. A molar extinction coefficient (ϵ) of 17,100 M−1 cm−1 for p-nitrophenol at pH 8.0 in 100 mMTris–base buffer was used for the calculation. One unit of PON1 activity is defined as 1 μmol of p-nitrophenol formed per minute under the above assay conditionsCitation35. Enzyme units were calculated from the absorbance changeCitation36.
Xanthine oxidase activity
XO activity was analysed essentially according to the method described by RoussosCitation37. The change in absorbance was recorded at 290 nm at 15 s interval for 1 min on a Biotek (Winooski, VT, USA). Suitable control was run simultaneously. RoussosCitation37 defined 1 U of activity as change in absorbance at 290 nm in 1 min by 1 ml enzyme preparationCitation33.
Statistical analysis
The distribution of enzyme levels were examined visually with Shapiro–Wilk test. The enzyme levels were presented as median (min-max: minimum-maximum). Enzyme levels of patient and control groups were compared with Mann–Whitney U test. The relationship between disease stages and enzyme levels in the patient group was investigated by Polyserial correlation coefficient. Statistical significance level was accepted as p < .05. All reactions were performed in triplicate. Statistical analysis was carried out with the use of IBM SPSS Statistics 21.0 IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp. Statistical significance was set at p ≤ .05.
Results
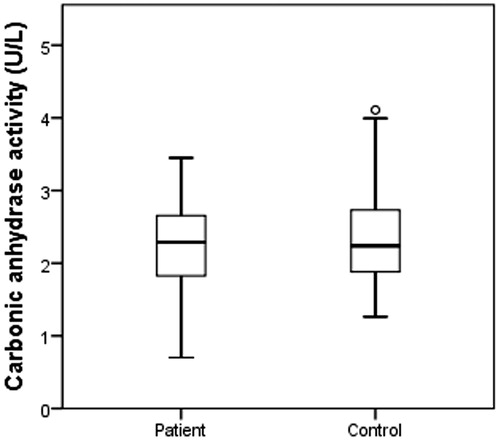
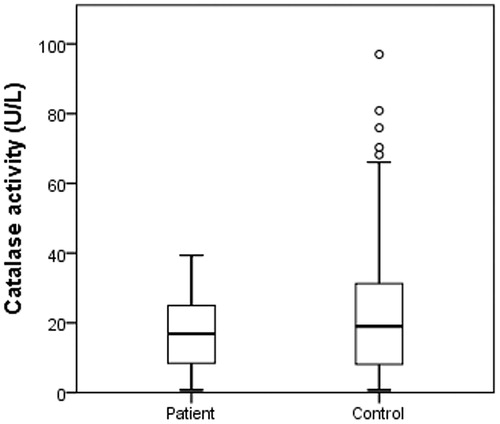
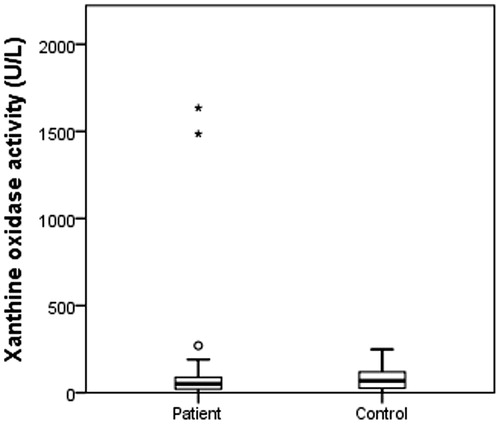
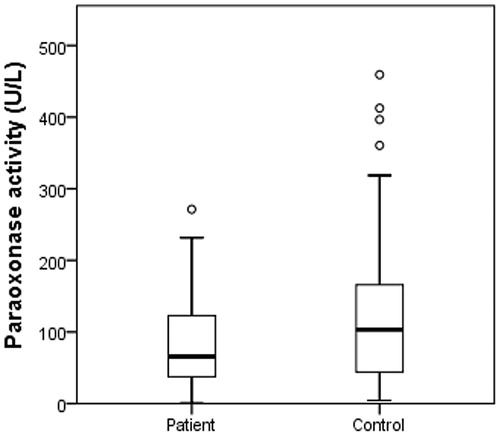
All above concerning lead us to evaluate their enzyme activity levels of cytosolic CAs, CAT, PON1, and XO on HNC patients. According to the results, there was no significant difference among CA, CAT, and XO when the enzyme levels of patients and controls were compared (p > .05) (). PON 1 levels were significantly higher in controls (p = .010, ) and there is a respectable difference for PON 1 levels ().
Table 2. Comparison of enzyme levels.
HNCs are composed of heterogeneous groups of tumours and it is one of the most common causes of cancer related deathsCitation38. Free radicals have a crucial role in the pathogenesis of the carcinogenesisCitation39. ROS is produced as a cell-mediated response in normal cellular process; however, when ROS is produced in excessive amounts, it damages cellular components such as DNA, lipids, proteins and causes harmful effects on the bodyCitation9. ROS plays a dual role in the tumours; have the ability to support tumorigenesis and vascularisation. On the other hand, they may induce DNA damage, arrest cell cycle and induce apoptosisCitation40. Reactive oxygen species and other free radicals contribute to the development of cancer through chronic oxidative stress and associated chronic inflammation. ROS takes place in three major stages in the classical carcinogenesis model: initiation, promotion, and progressionCitation41.With the knowledge of the roles of ROS species and when we have look on the activity levels of CAT, PON1, and XO levels on HNC patients, the results have demonstrated a decreased PON 1 activity levels in patients. There were no statistically differences among CAT and XO enzymes according to the results.
CAs are important mediators of tumour cell pH by modulating the bicarbonate and proton concentrations for cell survival and proliferationCitation42.The pH of the tumour microenvironment drives to have a better understanding the relation between CO2 and tumour microenvironment with CAsCitation43. In the literature, CO2 insufflations influences the growth of cultured human tumour cellsCitation44.
Due to CAs broad roles in metabolism we have served as their ability to understand how they are effectively active on HNC patients. Thus CA activity levels on HNC patients weren’t adequately present as statistically.
Discussion
The main role of CAs in cancer may be to act as pH regulating mediatorsCitation45. According to the Frost’s group, CAs are the driving force in pH regulation in primary tumour cellsCitation46 and they also confirmed that the CAs have a tumour cell validity in the differential pH environment.
There are limited studies in the literature regarding to cytosolic carbonic anhydrases related with HNCs. Demir et al. found that there is no relation between of oesophageal cancer patients and control group for CA activity levelsCitation9. From this point; it is clearly shown that high CA activity levels are related with CO2levels due to smoking. Despite mounting preclinical evidence to support the efficacy of CO2 only a few clinical trials have been conducted on carcinogenesis. Supuran et al. have so many studies on tumour microenvironment related with CAsCitation47. In our study, 89 HNC patients were smokers and all healthy volunteers were non-smokers, due to the results we demonstrated high cytosolic CA activity in HNC patients but it was not statistically significant. Further studies are needed to evaluate the other type of CA isoenzymes to indicate the correlation on HNC patients.
Oxidative stress is related to all aspects of cancerCitation48. Cellular targets as DNA, phospholipids, proteins, and carbohydrates are affected by oxidative stress on the cell membrane. Insufficient antioxidant activity in the body is also considered a risk factor for developing cancer. That is why we aim to evaluate the CAT, PON1, and XO activity levels on HNC patients.
PON1 is sensitive to oxidative damage. Many studies have reported the correlation between PON1 enzyme and several cancer types such as bladderCitation49, prostateCitation50, lungCitation51, gastricCitation52, and ovarianCitation53.
Metin et al. reported that the levels of PON1 activity were lower in oral squamous cell carcinoma than that of controls. Also, this group defended that there was no relation between serum PON1 activity level and tumour stageCitation54. Another group showed a decreased serum PON1 enzyme activity in oesophageal squamous cell carcinoma patientCitation8. Malik et.al also reported the low PON1 activities in oral squamous cell carcinoma patients. This group has suggested that PON1 levels may be a marker for oxidative stress in cancerCitation9. In our study, we evaluated that PON1 activity levels decreased in HNC patients via control group (p = .010) (). Therefore, according to our results PON1 may modulate an effective reaction to have a better understanding proper in carcinogenesis.
Cancer-associated XO induces oxidative stress, and XOR-derived ROS can activate genes associated with cancerCitation55. Kalcioglu et al. found an increased XO enzyme activity in HNC patientsCitation56.The hydrogen peroxide produced by superoxide dismutase (SOD) reacts with CAT to convert it into water and molecular oxygen. Gargouri et al. demonstrated the decreased CAT activity in erythrocytes of nasopharyngeal carcinoma patientsCitation57. Moreover, Demir et al showed that the activity levels of CAT were lower in the patients of oesophageal cancerCitation29 and Mrowicka et al. found a reduced CAT enzyme activity in HNC patients with a none significant differenceCitation58. In our study, XO and CAT enzyme levels were lower than control group enzyme levels and we found no significant value of these enzymes.
As a consequent of this part, the enzymes CAT, PON1, XO, and CA (I and II) have definitely different activities which also belong to the different groups. Thus, in the literature, all of these enzymes are related to ROS species. It is also well documented that ROS can activate cancer cellsCitation59. High levels of ROS suppress tumour growth and the interaction between ROS and enzyme levels through the inhibition of cell proliferation and induction of apoptosisCitation60 which is an important pathogenic factor for carcinogenesis. Within this, we aim to investigate the CAT, PON1, XO, and CA (I and II) enzyme levels according to their relations with cancer whether they have a common point or not.
Though there was no statistically significant difference among CAT, CA, and XO enzymes, there may be a relationship among the elevated level of oxidative stress on the lack of antioxidant protection and cancer development in HNC patients. Thus, further studies on mechanism and pathways of antioxidant defensive system will provide new insights into our future projects with the relation of CAs, XO, and CAT. Besides, our study has revealed significantly lower PON1 activity levels in HNC patients compared to control group. Decreased PON1 levels result may be a leading tool on oxidative stress pathways and it may be an effective agent in cancer-related complications. However, PON1’s activity alone is not an adequate parameter for understanding the cancer development process but we highly suggest that our data also support the major role of oxidative stress in cancer.
Disclosure statement
There is no conflict of interest of this study.
References
- Bose P, Brockton NT, Dort JC. Head and neck cancer: from anatomy to biology. Int J Cancer 2013;133:2013–23.
- Piętka G, Kukwa W, Bartnik E, et al. Mitochondrial DNA mutations in the pathogenesis in the head and neck squamous cell carcinoma. Otolaryngol Pol 2008;62:158–64.
- Suzuki T, Wakai K, Matsuo K, et al. Effect of dietary antioxidants and risk of oral, pharyngeal and laryngeal squamous cell carcinoma according to smoking and drinking habits. Cancer Sci 2006;97:760–7.
- Mutlu P, Mutlu M, Yalcin S, et al. Detection of XRCC1 gene polymorphisms in Turkish head and neck squamous cell carcinoma patients: a comparative analysis with different populations. J BUON 2015;20:540–7.
- Milonski J, Zielinska-Blizniewska H, Olszewski J, et al. DNA damage and oxidant-antioxidant status in blood of patients with head and neck cancer. DNA Cell Biol 2015;34:213–9.
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008;4:89–96.
- Gupta RK, Patel AK, Shah N, et al. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac J Cancer Prev 2014;15:4405–9.
- Sehitogulları A, Aslan M, Sayır F, et al. Serum paraoxonase-1 enzyme activities and oxidative stress levels in patients with esophageal squamous cell carcinoma. Redox Rep 2014;19:199–205.
- Malik UU, Siddiqui IA, Hashim Z, Zarina S. Measurement of serum paraoxonase activity and MDA concentrations in patients suffering with oral squamous cell carcinoma. Clin Chim Acta 2014;430:38–42.
- Shin JM, Park JH, Kim HJ, et al. Cigarette smoke extract increases vascular endothelial growth factor production via TLR4/ROS/MAPKs/NF-kappaB pathway in nasal fibroblast. Am J Rhin Allergy 2017;31:78–84.
- Reuther WJ, Brennan PA. Is nicotine still the bad guy? Summary of the effects of smoking on patients with head and neck cancer in the postoperative period and the uses of nicotine replacement therapy in these patients. Br J Oral Maxillofac Surg 2014;52:102–5.
- Taskin MI, Bilen C, Ergun A, et al. In vitro effects of estrogen and progesterone containing drugs on human erythrocyte carbonic anhydrase I and II isozymes in women smokers and nonsmokers. J Chin Med Assoc 2015;78:513–9.
- Katakwar P, Metgud R, Naik S, Mittal R. Oxidative stress marker in oral cancer: a review. J Cancer Res Ther 2016;12:438–46.
- Kawakita D, Matsuo K. Alcohol and head and neck cancer. Cancer Metastasis Rev 2017;36:425–34.
- Purdue MP, Hashibe M, Berthiller J, et al. Type of alcoholic beverage and risk of head and neck cancer–a pooled analysis within the INHANCE Consortium. Am J Epidemiol 2009;169:132–42.
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 2001;25:263–70.
- World Health Day: [Food Safety: What you should know]. WHO publications: Worh Health Organization;2015 [cited 5 November 2018]. Avaliable from: http://www.searo.who.int/entity/world_health_day/2015/whd-what-you-should-know/en/
- Fiolet T, Srour B, Sellem L, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ 2018;360:k322.
- Edefonti V, Hashibe M, Parpinel M, et al. Natural vitamin C intake and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Int J Cancer 2015;137:448–62.
- Edefonti V, Hashibe M, Ambrogi F, et al. Nutrient-based dietary patterns and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Ann Oncol 2012;23:1869–80.
- Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann Oncol 2016;27:1619–25.
- Van Drie JH. Protein folding, protein homeostasis, and cancer. Chin J Cancer 2011;30:124–37.
- Ozensoy O, Kockar F, Arslan O, et al. An evaluation of cytosolic erythrocyte carbonic anhydrase and catalase in carcinoma patients: an elevation of carbonic anhydrase activity. Clin Biochem 2006;39:804–49.
- Kaya MO, Kaya Y, Çelik G, et al. Differential in vitro inhibition studies of some cerium vanadate derivatives on xanthine oxidase. J Enzyme Inhib Med Chem 2015;30:286–9.
- Sathisha KR, Khanum SA, Chandra JNNS, et al. Synthesis and xanthine oxidase inhibitory activity of 7-methyl-2-(phenoxymethyl)-5H-[1,3,4]thiadiazolo[3,2-a] pyrimidin-5-one derivatives. Bioorg Med Chem 2011;19:211–20.
- Bytyqi-Damoni A, Genc H, Zengin M, et al. In vitro effect of novel β-lactam compounds on xanthine oxidase enzyme activity. Artif Cells Blood Substit Immobil Biotechnol 2012;40:369–77.
- Isık H, Aynıoglu O, Tımur H, et al. Is xanthine oxidase activity in polycystic ovary syndrome associated with inflammatory and cardiovascular risk factors? J Reprod Immunol 2016;116:98–103.
- Guler OO, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2015;29:1–6.
- Demir H, Akkus ZA, Cebi A, et al. Catalase, carbonic anhydrase and other biochemical parameters in esophageal cancers in Turkey. Asian Pac J Cancer Prev 2010;11:1029–32.
- Kose LP, Gulcin İ, Yıldırım A, et al. The human carbonic anhydrase isoenzymes I and II inhibitory effects of some hydroperoxides, alcohols, and acetates. J Enzyme Inhib Med Chem 2016;31:1248–53.
- Supuran CT, De Simone G, editors. Carbonic anhydrases as biocatalysts: from theory to medical and industrial applications. Berlin: Elsevier; 2015.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–54.
- Cengiz FP, Beyaztas S, Gokce B, et al. Catalase, carbonic anhydrase and xanthine oxidase activities in patients with mycosis fungoides. J Enzyme Inhib Med Chem 2015;30:212–5.
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–6.
- Arslan M, Gencer N, Arslan O, Guler OO. In vitro efficacy of some cattle drugs on bovine serum paraoxonase 1 (PON1) activity. J Enzyme Inhib Med Chem 2012;27:722–9.
- Bilen C, Beyaztaş S, Arslan O, Güler OO. Investigation of heavy metal effects on immobilized paraoxanase by glutaraldehyde. J Enzyme Inhib Med Chem 2013;28:440–6.
- Roussos GG, Methods in enzymology. New York: Academic Press. 1967; p. 5–16.
- Manifar S, Abbassi F, Dizgah IM, et al. Alteration of lipid peroxidation and total antioxidant capacity in patients with head and neck cancers following radiotherapy. J Arc Mil Med 2015;3:e30431.
- Malathi M, Vijay M, Shivashankara AR. The role of oxidative stress and the effect of radiotherapy on the plasma oxidant-antioxidant status in head and neck cancer. J Clin Diag Res 2011;5:249–51.
- Assi M, Rébillard A. The Janus-faced role of antioxidants in cancer cachexia: new insights on the established concepts. Oxid Med Cell Longev 2016;2016:9579868.
- Kesarwala AH, Krishna MC, Mitchell JB. Oxidative stress in oral diseases. Oral Dis 2016;22:9–18.
- Cianchi F, Vinci MC, Supuran CT, et al. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther 2010;334:710–9.
- McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012;3:84–97.
- Gutt CN, Kim ZG, Hollander D, et al. CO2 environment influences the growth of cultured human cancer cells dependent on insufflation pressure. Surg Endosc 2001;15:314–8.
- Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:25.
- Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol 2013;4:370–80.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Noda N, Wakasugi H. Cancer and oxidative stress. JMAJ 2001;44:535–9.
- Aydin O, Yacinkaya S, Eren E, et al. Diminished arylesterase enzyme activity and total thiol levels in bladder cancer patients. Clin Lab 2013;59:1231–7.
- Eroglu M, Yilmaz N, Yalcinkaya S, et al. Enhanced HDL-cholesterol-associated anti-oxidant PON-1 activity in prostate cancer patients. Kaohsiung J Med Sci 2013;29:368–73.
- Elkiran ET, Mar N, Aygen B, et al. Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 2007;7:48–55.
- Akçay MN, Yilmaz I, Polat MF, Akçay G. Serum paraoxonase levels in gastric cancer. Hepatogastroenterology 2003;50: cclxxiii–cclxxv.
- Camuzcuoglu H, Arioz DT, Toy H, et al. Serum paraoxonase and arylesterase activities in patients with epithelial ovarian cancer. Gynecol Oncol 2009;112:481–5.
- Metin ZB, Aydin S, Unur M, et al. Oral squamous cell carcinoma and serum paraoxonase 1. J Laryngol Otol 2013;127:1208–13.
- Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid Med Cell Longev 2016;2016:1.
- Kalcioğlu MT, Kizilay A, Yilmaz HR, et al. Adenosine deaminase, xanthine oxidase, superoxide dismutase, glutathione peroxidase activities and malondialdehyde levels in the sera of patients with head and neck carcinoma. J Ear, Nose, Throat 2003;12:16–22.
- Gargouri B, Lassoued S, Ayadi W, et al. Lipid peroxidation and antioxidant system in the tumour and in the blood of patients with nasopharyngeal carcinoma. Biol Trace Elem Res 2009;132:27–34.
- Mrowicka M, Zielińska-Bliźniewska H, Pietkiewicz P, et al. Evaluation of selected indicators of antioxidant status in patients with head and neck cancer. Otolaryngol Pol 2015;69:44–50.
- Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 2006;10:175–6.
- Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Rad Res 2010;44:479–96.