Abstract
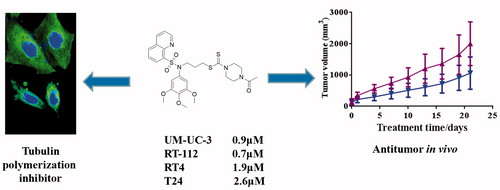
Novel sulfonamide-dithiocarbamate hybrids were designed and synthesised via the molecular hybridisation strategy. Among them, compound 13d displayed a potent activity with IC50 values of 0.9, 0.7, 1.9 and 2.6 µM against UM-UC-3, RT-112, RT4 and T24. Compound 13d inhibited the migration and regulated the migration-related markers (E-cadherin, N-cadherin, Vimentin, Snail and Slung) against RT-112 cells in a concentration dependent manner. By the tubulin polymerisation assay in vitro and immunostaining assay, compound 13d was identified as a novel tubulin polymerisation inhibitor. Intragastric administration of compound 13d could inhibit the growth of RT-112 cells in vivo in a xenograft mouse model with the low toxicity, indicating that it may be a leading candidate with antitumor properties to treat bladder cancer.
Graphical Abstract
Introduction
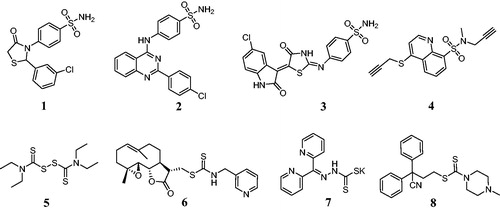
Sulfonamide derivatives were extensively studied due to a variety of biological activities such as antimicrobial, anticancer and antiviral propertiesCitation1–3. Benzenesulfonamide derivative 1 () induced nuclear condensation, cell shrinkage, and nuclear fragmentation to apoptosis against COLO-205 cell lineCitation4. Sulfonamide derivative 2 could decrease the cell viability of HT-29 cell line in a concentration dependent manner and with an IC50 value of 5.45 µMCitation5. Benzenesulfonamide 3 displayed a potent antiproliferative activity against MCF-7 cells with an IC50 value of 3.96 µM as an apoptosis inducerCitation6. Quinolinesulfonamide 4 exhibited the anticancer activity in vitro against T47D cells with an IC50 value of 0.27 µMCitation7. In addition, dithiocarbamates as organosulfur ligands were successfully applied as anticancer agentsCitation8. Disulfiram 5 could modulate ROS accumulation and overcome synergistically cisplatin resistance against breast cancer cellsCitation9. Dithiocarbamate 6 induced apoptosis by the mitogen-activated protein kinase signal pathwayCitation10. Dithiocarbamate 7 showed the potent anticancer effects against HER2-overexpressed SK-OV-3 and SK-BR-3 cellsCitation11. Dithiocarbamate 8 was developed as an antitumor candidate drug for the treatment of estrogen receptor-positive breast cancerCitation12.
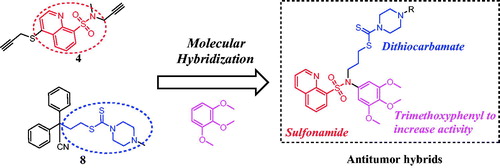
Over the last decade, a variety of molecules bearing the trimethoxyphenyl moiety have been designed and synthesised as anticancer agentsCitation13–15. In addition, trimethoxyphenyl derivatives have enjoyed great success due to their potent chemical stability and pharmacokinetics for cancer treatmentCitation16. These above interesting findings led us to perform the molecular hybridisation strategy of biologically active sulfonamide, dithiocarbamate and trimethoxyphenyl fragments to generate a novel scaffold with the aim of studying the impact of such modification on their antitumor activity. As shown in , a molecular hybridisation strategy based on bioactive compounds 4 and 8 produced a novel scaffold that has three parts: (1) a sulfonamide fragment as the central backbone; (2) a dithiocarbamate moiety and (3) a trimethoxyphenyl moiety to increase the antiproliferative activity. In this work, we synthesised and evaluated for the anticancer mechanisms in vitro and in vivo of sulfonamide-dithiocarbamate hybrids.
Methods and materials
Reagents and chemicals
All the chemical reagents were purchased from Sigma Aldrich (Shanghai, China), Aladdin (Shanghai, China), and Ruida company (Shandong, China). 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were measured using CDCl3 and DMSO-d6 as solvents. High-resolution mass spectra (HRMS) spectrometry (Ruida company, China) was performed in this work.
Synthesis of compound 11
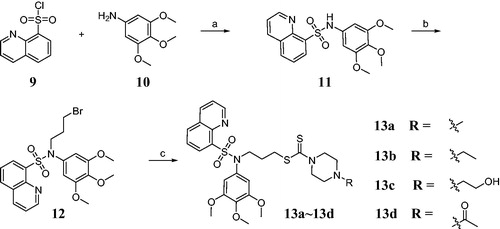
A mixture of quinoline-8-sulfonyl chloride 9 (5 mmol), 3,4,5-trimethoxyaniline 10 (5 mmol), and NaHCO3 (5 mmol) were dissolved in CH2Cl2 (15 ml). The mixture was stirred for 4 h at room temperature. The crude product was purified by column chromatography (n-hexane:ethyl acetate = 6:1) on silica gel to afford compound 11.
Synthesis of compound 12
Compound 11 (3 mmol), 1,3-dibromopropane (4.5 mmol) and NaOH (3 mmol) was dissolved in acetone (15 ml). The mixture was refluxed for 8 h. The crude product was purified by column chromatography (n-hexane:ethyl acetate = 9:1) on silica gel to afford compound 12.
Synthesis of sulfonamide hybrids 13a–13d
Compound 12 (2 mmol), triethylamine (3 mmol) and piperazine derivatives (2 mmol) were dissolved in MeOH (10 ml). CS2 (4 mmol) was added to this reaction mixture at 0 °C. The reaction mixture was stirred for 1 h at 0 °C. The crude product was purified by column chromatography (n-hexane:ethyl acetate = 8:1) on silica gel to afford hybrids 13a∼13d.
N-(3,4,5-trimethoxyphenyl)quinoline-8-sulfonamide (11)
White solid, yield: 94%, m.p.: 1 8 1 ∼ 183 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.87 (s, 1H), 9.16 (dd, J = 4.2, 1.7 Hz, 1H), 8.52 (dd, J = 8.4, 1.7 Hz, 1H), 8.39 (dd, J = 7.3, 1.3 Hz, 1H), 8.27 (dd, J = 8.2, 1.3 Hz, 1H), 7.81 – 7.60 (m, 2H), 6.35 (s, 2H), 3.51 (s, 6H), 3.47 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 152.6, 151.4, 142.7, 136.0, 135.1, 134.2, 133.9, 133.6, 132.3, 128.3, 125.6, 122.6, 98.0, 59.9, 55.6. HRMS (m/z): Calcd. C18H19N2O5S, [M + H]+m/z: 375.1015, found: 375.1018.
N-(3-bromopropyl)-N-(3,4,5-trimethoxyphenyl)quinoline-8-sulfonamide (12)
White solid, yield: 82%, m.p.: 1 2 9 ∼ 131 °C. 1H NMR (400 MHz, CDCl3) δ 9.09 (dd, J = 4.2, 1.7 Hz, 1H), 8.20 (td, J = 8.6, 1.5 Hz, 2H), 7.93 (dd, J = 8.2, 1.2 Hz, 1H), 7.55 – 7.39 (m, 2H), 6.12 (s, 2H), 4.22 (t, J = 6.8 Hz, 2H), 3.67 (d, J = 4.6 Hz, 3H), 3.47 (t, J = 6.8 Hz, 2H), 3.42 (d, J = 8.3 Hz, 6H), 2.09 (p, J = 6.8 Hz, 2H). 13 C NMR (100 MHz, CDCl3) δ 152.0, 150.1, 143.2, 136.6, 135.8, 135.6, 133.6, 133.1, 132.5, 127.7, 124.6, 121.0, 105.2, 59.8, 54.9, 51.0, 31.8, 29.5. HRMS (m/z): Calcd. C21H24BrN2O5S, [M + H]+m/z: 495.0589, found: 495.0593.
3-(N-(3,4,5-trimethoxyphenyl)quinoline-8-sulfonamido)propyl-4-methylpiperazine-1-carbodithioate (13a)
White solid, yield: 76%, m.p.: 9 6 ∼ 98 °C. 1H NMR (400 MHz, CDCl3) δ 9.10 (dd, J = 4.2, 1.7 Hz, 1H), 8.37–8.08 (m, 2H), 7.93 (dd, J = 8.2, 1.2 Hz, 1H), 7.64–7.29 (m, 2H), 6.13 (s, 2H), 4.32 (s, 2H), 4.20 (t, J = 6.8 Hz, 2H), 3.96 (s, 2H), 3.67 (s, 3H), 3.42 (s, 6H), 3.40 (d, J = 7.3 Hz, 2H), 2.50 (s, 4H), 2.30 (s, 3H), 1.99–1.82 (m, 2H). 13 C NMR (100 MHz, CDCl3) δ 151.9, 150.2, 143.2, 136.5, 136.0, 135.5, 133.6, 133.1, 132.3, 127.7, 124.6, 121.0, 105.4, 59.8, 54.9, 53.2, 51.5, 44.3, 33.3, 27.8. HRMS (m/z): Calcd. C27H35N4O5S3, [M + H]+m/z: 591.1770, found: 591.1776.
3-(N-(3,4,5-trimethoxyphenyl)quinoline-8-sulfonamido)propyl-4-ethylpiperazine-1-carbodithioate(13b)
White solid, yield: 84%, m.p.: 1 3 1 ∼ 133 °C. 1H NMR (400 MHz, CDCl3) δ 9.21–8.97 (m, 1H), 8.20 (dd, J = 9.8, 8.1 Hz, 2H), 7.92 (d, J = 7.7 Hz, 1H), 7.72–7.32 (m, 2H), 6.14 (s, 2H), 4.24 (d, J = 26.0 Hz, 2H), 4.20 (t, J = 6.7 Hz, 2H), 3.89 (s, 2H), 3.67 (s, 3H), 3.43 (s, 6H), 3.39 (d, J = 7.3 Hz, 2H), 2.64–2.17 (m, 6H), 1.99–1.83 (m, 2H), 1.03 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 195.8, 151.9, 150.2, 143.3, 136.5, 136.0, 135.5, 133.6, 133.0, 132.3, 127.7, 124.6, 121.0, 105.5, 59.8, 54.9, 51.6, 51.2, 50.9, 33.1, 27.8, 11.0. HRMS (m/z): Calcd. C28H37N4O5S3, [M + H]+m/z: 605.1926, found: 605.1929.
3-(N-(3,4,5-trimethoxyphenyl)quinoline-8-sulfonamido)propyl-4–(2-hydroxyethyl)piperazine-1-carbodithioate(13c)
White solid, yield: 75%, m.p.: 1 0 6 ∼ 108 °C. 1H NMR (400 MHz, CDCl3) δ 9.10 (d, J = 2.1 Hz, 1H), 8.20 (dd, J = 6.8, 3.9 Hz, 2H), 7.93 (d, J = 8.1 Hz, 1H), 7.65–7.34 (m, 2H), 6.12 (s, 2H), 4.33 (s, 2H), 4.20 (t, J = 6.6 Hz, 2H), 3.96 (s, 2H), 3.67 (s, 3H), 3.66–3.57 (m, 2H), 3.42 (s, 8H), 2.73–2.49 (m, 6H), 1.93 (dd, J = 13.7, 6.8 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 196.3, 151.9, 150.2, 143.2, 136.3, 135.9, 135.5, 133.5, 133.1, 132.4, 127.6, 124.6, 121.0, 105.3, 59.8, 58.2, 56.7, 54.9, 51.5, 51.3, 33.2, 27.7. HRMS (m/z): Calcd. C28H37N4O6S3, [M + H]+m/z: 621.1875, found: 621.1878.
3-(N-(3,4,5-trimethoxyphenyl)quinoline-8-sulfonamido)propyl-4-acetylpiperazine-1-carbodithioate (13d)
White solid, yield: 90%, m.p.: 1 2 8 ∼ 129 °C. 1H NMR (400 MHz, CDCl3) δ 9.09 (dd, J = 4.2, 1.7 Hz, 1H), 8.27–8.09 (m, 2H), 7.93 (dd, J = 8.2, 1.1 Hz, 1H), 7.56–7.33 (m, 2H), 6.13 (s, 2H), 4.21 (t, J = 6.7 Hz, 2H), 4.19 (s, 4H), 3.66 (s, 3H), 3.65 (s, 2H), 3.55–3.49 (m, 2H), 3.45 (s, 2H), 3.42 (s, 6H), 2.06 (s, 3H), 1.94 (dd, J = 14.9, 7.7 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 197.0, 168.4, 152.0, 150.2, 143.2, 136.5, 135.9, 135.6, 133.5, 133.1, 132.4, 127.6, 124.6, 121.0, 105.4, 59.8, 54.9, 51.6, 44.2, 39.6, 33.2, 27.7, 20.4. HRMS (m/z): Calcd. C28H35N4O6S3, [M + H]+m/z: 619.1719, found: 619.1726.
Cell proliferation
Breast cancer cells (MDA-MB-453), liver cancer cells (SNU-423), and bladder cancer cells (UM-UC-3, RT-112, RT4, T24) were purchased from the Shanghai culture company, and cultured at 37 °C in an atmosphere containing 5% CO2. 4,000 cells per well were seeded into a 96-well plate for 24 h and compounds were added to cluture 48 h. Next, 6 mg/ml CCK8 (20 µl) per well was added to explore the cell survival.
Migration assay
Transwell chamber was used to do migration assay according to the reported referencesCitation17,Citation18. Ten thousand RT-112 cells containing compound 13d were seeded in the top chambers for 24 h incubation. The bottom chambers were fixed and stained with crystal violet for 2 h. Finally, the chambers were washed with water, and the top membrane was left to dry.
Western blot
RT-112 cells were treated with compound 13d and then lysed in a RIPA lysis buffer. The protein samples of RT-112 cells were electrophoresed on SDS-PAGE. Nextly, targeted proteins were transferred to PVDF membrane and incubated with antibodies (E-cadherin, N-cadherin, Vimentin, Snail and Slung). The markers were visualised by chemiluminescence detection reagents.
Tubulin polymerisation assay and immunostaining
An amount of 6 mg/ml tubulin was resuspended in the PEM buffer (80 mM PIPES (pH 6.9), 1 mM EGTA, 0.5 mM MgCl2, 1 mM ATP, 10.2% (v/v) glycerol) and then was preincubated with a sulfonamide-dithiocarbamate hybrid 13d on the ice. The reaction was monitored by a spectrophotometer (Beijng Tianhe Company) at 37 °C to obtain the results of tubulin polymerisation in vitro. RT-112 cells were treated with sulfonamide-dithiocarbamate hybrid 13d for 48 h. Then, slices were fixed by 4% paraformaldehyde for 20 min and washed by PBS for five times. 0.6% Triton-X-100 was added and 0.1% BSA was used to block. β-tubulin antibody was added to incubate overnight at 4 °C. DAPI was used to stain and then images were captured.
The in vivo antitumor effect of compound 13d
In vivo experiments were processed according to guidelines established by the ethics committee of the 4th affiliated hospital of CMU. Mice were implanted with RT-112 cells (1.5 × 107 cells per mouse) on the right flank of nude mice. The mice were divided into the saline group and sulfonamide-dithiocarbamate hybrid 13d (80 mg/kg) group (n = 5 mice for each group). Intragastric administration of hybrid 13d was performed every day for a period of 21 days.
Results and discussion
Synthesis of novel sulfonamide-dithiocarbamate hybrids
The sulfonamide-dithiocarbamate hybrids 13a–13d were synthesised according to Scheme 1. The desired compound 11 was obtained by the sulfonylation reaction of quinoline-8-sulfonyl chloride 9 and 3,4,5-trimethoxyaniline 10. For synthesis of compound 12, 1,3-dibromopropane was used in the presence of sodium hydroxide. Subsequently, intermediate 12 was treated with carbon disulfide and piperazine derivatives in the methanol solution to give sulfonamide-dithiocarbamate hybrids 13a–13d. The chemical structures of obtained compounds were confirmed by 1H NMR, 13C NMR and mass spectroscopic data which were consistent with the proposed molecular structures.
Anticancer activity in vitro of sulfonamide hybrids 13a–13d
CCK8 method was used to investigate the anticancer activity in vitro of sulfonamide-dithiocarbamate hybrids 13a–13d against cancer cells for 48 h. MDA-MB-453 (breast cancer cells), SNU-423 (liver cancer cells) and RT-112 (bladder cancer cells) were selected to explore the antiproliferative activity. In this work, 5-fluorouracil (5-Fu) was used as the control drug. The results of anticancer activity in vitro were listed in .
Table 1. Anticancer activity in vitro of sulfonamide hybrids 13a–13d.
Compound 11 and compound 12 displayed the weak antiproliferative activity against all three cancer cells with IC50 values of >40 µM. However, sulfonamide hybrids 13a–13d containing a dithiocarbamate moiety showed the potent activity with IC50 values from 0.7 to 8.7 µM. All these inhibitory results exhibited that the dithiocarbamate scaffold might play the pivotal effect for the anticancer activity in vitro of sulfonamide hybrids.
In addition, the effect of different piperazine units were explored. Replacement of the 1-methylpiperazine of compound 13a with a 1-ethylpiperazine of compound 13b led to an increase for the anticancer activity in vitro against SNU-423 and RT-112 cells. However, changing the 2-(piperazin-1-yl)ethan-1-ol of compound 13c to a 1-ethylpiperazine of compound 13b led to a decrease for the anticancer activity in vitro against all three cell lines. Importantly, sulfonamide hybrid 13d exhibited the best antiproliferative activity with an IC50 value of 0.7 µM against bladder cancer RT-112 cells among all sulfonamide-dithiocarbamate analogs.
Compound 13d inhibited the cell proliferation against bladder cancer cells
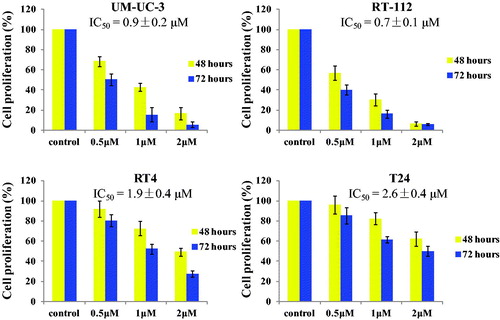
In order to explore the antiproliferation ability, four bladder cancer cell lines (UM-UC-3, RT-112, RT4 and T24) were selected and treated with the hybrid 13d in the 24-well plate for 48 h and 72 h. As shown in , compound 13d inhibited the cell proliferation against bladder cancer in a concentration dependent manner. After the treatment for 48 h, compound 13d displayed the potent activity with IC50 values of 0.9, 0.7, 1.9 and 2.6 µM against UM-UC-3, RT-112, RT4 and T24 cells. Compared with the cell proliferation of 48 h, the cell proliferation of 72 h was decreased. All these results indicated that compound 13d could inhibit the cell proliferation against bladder cancer cells in concentration-dependent and time-dependent manners.
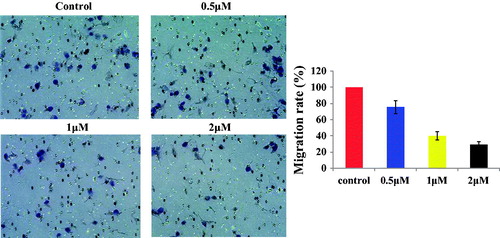
Compound 13d inhibited the migration against RT-112 cells
Based on the antiproliferative activity results, compound 13d was selected to investigate the anticancer mechanisms against the RT-112 cell line. Corning transwell plate was used to do migration assay against RT-112 cells for 48 h. With the treatment of sulfonamide-dithiocarbamate hybrid 13d at 0.5, 1 and 2 µM, the migration rate of RT-112 cells was 76%, 40% and 29%, respectively. The migration results in showed that compound 13d inhibited the migration against RT-112 cells in a concentration dependent manner.
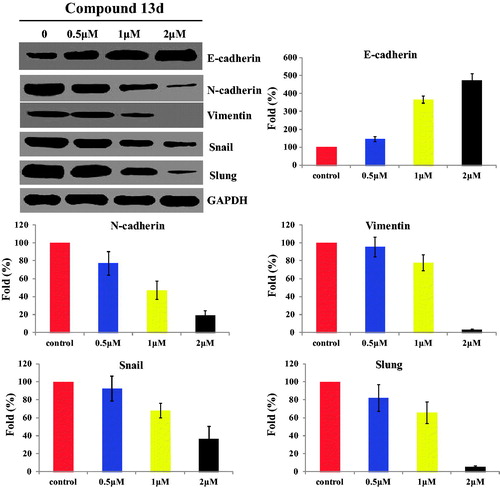
Compound 13d regulated the migration-related markers against RT-112 cells
To further characterise the migration effect of sulfonamide-dithiocarbamate hybrid 13d, the expression levels of migration-related proteins (E-cadherin, N-cadherin, Vimentin, Snail and Slung) were investigated against RT-112 cells. Compound 13d was treated for 48 h at concentrations of 0.5, 1 and 2 µM, respectively. As shown in , sulfonamide-dithiocarbamate hybrid 13d decreased the expression of N-cadherin, Vimentin, Snail and Slung in a concentration manner. In addition, hybrid 13d increased the expression of E-cadherin in a concentration manner. All western blotting results revealed that compound 13d could regulate the migration-related markers against RT-112 cells.
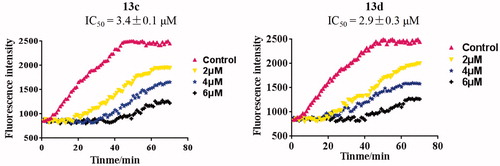
Tubulin polymerisation inhibitory activity in vitro of compound 13d
Because of the trimethoxyphenyl derivatives used as tubulin polymerisation inhibitorsCitation19, the most potent hybrids 13c and 13d containing the trimethoxyphenyl ring were evaluated for their tubulin polymerisation inhibitory activity in vitro. As shown in , the time-course analisis of tubulin polymerisation at the concentrations (control, 2, 4 and 6 µM) of 13c and 13d. The weak inhibition was observed at 2 µM and hybrids 13c and 13d significantly inhibited tubulin polymerisation at 6 µM. The IC50 values of hybrids 13c and 13d for tubulin polymerisation inhibitory activity in vitro were 3.4 and 2.9 µM, respectively. Tubulin polymerisation assay investigated that compound 13d inhibited tubulin polymerisation in a concentration dependent manner.
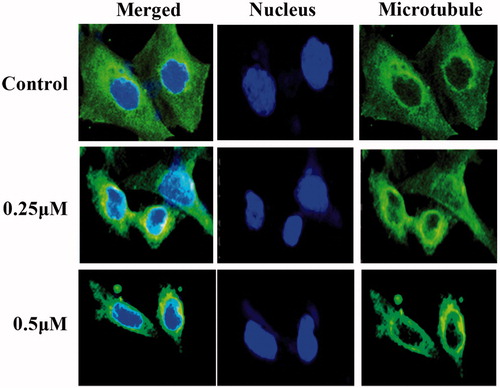
Compound 13d inhibited tubulin polymerisation by the immunostaining assay
Tubulin polymerisation inhibitory effects of the sulfonamide-dithiocarbamate hybrid 13d were further explored by the immunofluorescent staining in RT-112 cells. From the results in , the microtubule networks in RT-112 cells without the treatment of 13d had a normal arrangement with microtubules extending from the central regions of the cell to the cell periphery. However, after the exposure to 0.25 µM of compound 13d, the microtubule organisation in the cytosol were disrupted. The changes of the mitotic spindles were more clearly observed by increasing the concentration to 0.5 µM. All these results by the immunostaining assay indicated that compound 13d disrupted the microtubule organisation of RT-112 cells at low concentrations.
Compound 13d inhibited the tumor growth of RT-112 cells
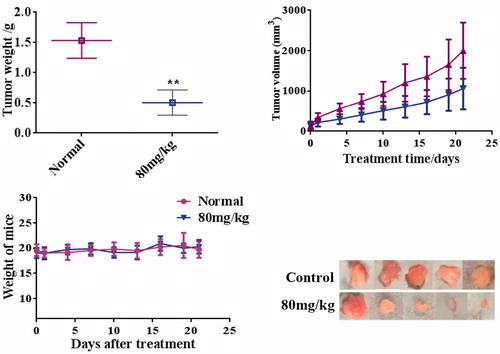
In order to investigate the tumor growth effects of compound 13d, the xenograft nude mouse of RT-112 cells were established. From the antitumor results in , there is no significant difference in body weights between control and 13d treated groups (80 mg/kg). The tumor volume of 13d treated mice was obviously less than that of the control group. The average tumor weights of control and 80 mg/kg 13d groups were 1.53 ± 0.30 g and 0.51 ± 0.21 g (inhibitory rate: 66.67%). These results showed that compound 13d inhibited the tumor growth of RT-112 cells with the low toxicity.
Conclusion
Bladder cancer is one of the most costly cancers to treat due to the considerable costs associated with the life-long clinical management and bladder tumors are not easy to cure because of their high metastasis rates. Therefore, it is very necessary to develop novel antitumor agants to treat bladder cancer. In this work, we designed novel sulfonamide-dithiocarbamate hybrids by the molecular hybridisation strategy based on reported bioactive scaffolds. Among all sulfonamide-dithiocarbamate hybrids, compound 13d displayed the potent activity with IC50 values of 0.9, 0.7, 1.9 and 2.6 µM against UM-UC-3, RT-112, RT4 and T24 cells. In addition, 13d inhibited the cell proliferation against bladder cancer cell lines by concentration-dependent and time-dependent manners.
From the migration assay, we found that compound 13d inhibited the migration against RT-112 cells in a concentration dependent manner. With the treatment of compound 13d at 2 µM, the migration rate of RT-112 cells was obviously decreased to 29% compared with control groups. By the western blotting experiments, 13d decreased the expression of N-cadherin, Vimentin, Snail and Slung and increased the expression of E-cadherin in a concentration manner.
Furthermore, the microtubule organisation in the cytosol of RT-112 cells was disrupted with the treatment of compound 13d. The IC50 value of hybrid 13d for tubulin polymerisation inhibitory activity in vitro was 2.9 µM. By the tubulin polymerisation in vitro assay and immunofluorescent staining experiments, compound 13d was identified as a novel tubulin polymerisation inhibitor. More importantly, compound 13d inhibited the tumor growth of RT-112 cells in vivo with the low toxicity. In summary, compound 13d may be a leading candidate with antitumor properties to treat bladder cancer.
Author contributions
Jia Liu, Chunlai Liu, Xiling Zhang, Liu Yu and Xue Gong performed the experiments and analysed the data. Ping Wang wrote the manuscript. All authors read and approved the manuscript.
Supplemental Material
Download PDF (908.8 KB)Acknowledgements
The authors thank the supports from the 4th affiliated hospital of CMU.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8.
- Wang X-L, Wan K, Zhou C-H. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur J Med Chem 2010;45:4631–9.
- Hou Z, Lin B, Bao Y, et al. Dual-tail approach to discovery of novel carbonic anhydrase IX inhibitors by simultaneously matching the hydrophobic and hydrophilic halves of the active site. Eur J Med Chem 2017;132:1–10.
- Suthar SK, Bansal S, Lohan S, et al. Design and synthesis of novel 4-(4-oxo-2-arylthiazolidin-3-yl)benzenesulfonamides as selective inhibitors of carbonic anhydrase IX over I and II with potential anticancer activity. Eur J Med Chem 2013;66:372–9.
- Alafeefy a. M, Ahmad R, Abdulla M, et al. Development of certain new 2-substituted-quinazolin-4-yl-aminobenzenesulfonamide as potential antitumor agents. Eur J Med Chem 2016;109:247–53.
- Eldehna WM, Abo-Ashour MF, Nocentini A, et al. Novel 4/3-((4-oxo-5-(2-oxoindolin-3-ylidene)thiazolidin-2-ylidene)amino) benzenesulfonamides: synthesis, carbonic anhydrase inhibitory activity, anticancer activity and molecular modelling studies. Eur J Med Chem 2017;139:250–62.
- Marciniec K, Pawełczak B, Latocha M, et al. Synthesis, anti-breast cancer activity, and molecular docking study of a new group of acetylenic quinolinesulfonamide derivatives. Molecules 2017;22:300.
- Odularu a. T, Ajibade PA. Dithiocarbamates: challenges, control, and approaches to excellent yield, characterization, and their biological applications. Bioinorg Chem Appl 2019;2019:8260496.
- Yang Z, Guo F, Albers a. E, et al. Disulfiram modulates ROS accumulation and overcomes synergistically cisplatin resistance in breast cancer cell lines. Biomed Pharmacother 2019;113:108727.
- Ding Y, Yang Z, Ge W, et al. Synthesis and biological evaluation of dithiocarbamate esters of parthenolide as potential anti-acute myelogenous leukaemia agents. J Enzyme Inhib Med Chem 2018;33:1376–91.
- Yang Y, Liu Y, Guo R, et al. The novel dithiocarbamate, DpdtC suppresses HER2-overexpressed cancer cells by up-regulating NDRG1 via inactivation of HER2-ERK 1/2 signaling. Sci Rep 2018;8:3398.
- Ji X-W, Chen G-P, Song Y, et al. Intratumoral estrogen sulfotransferase induction contributes to the anti-breast cancer effects of the dithiocarbamate derivative TM208. Acta Pharmacol Sin 2015;36:1246–55.
- Ali KA, Abdel Hafez NA, Elsayed MA, et al. Synthesis, anticancer screening and molecular docking studies of new heterocycles with trimethoxyphenyl scaffold as combretastatin analogues. Mini-Rev Med Chem 2018;18:717–27.
- Yang F, He C-P, Diao P-C, et al. Discovery and optimization of 3,4,5-trimethoxyphenyl substituted triazolylthioacetamides as potent tubulin polymerization inhibitors. Bioorg Med Chem Lett 2019;29:22–7.
- Xin Y-B, Li J-J, Zhang H-J, et al. Synthesis and characterisation of (Z)-styrylbenzene derivatives as potential selective anticancer agents. J Enzyme Inhib Med Chem 2018;33:1554–64.
- Li L, Jiang S, Li X, et al. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur J Med Chem 2018;151:482–94.
- Thayanithy V, O’Hare P, Wong P, et al. A transwell assay that excludes exosomes for assessment of tunneling nanotube-mediated intercellular communication. Cell Commun Signal 2017;15:46.
- Curet MA, Watters JJ. P2Y14 receptor activation decreases interleukin-6 production and glioma GL261 cell proliferation in microglial transwell cultures. J Neuro-Oncol 2018;137:23–31.
- Zhang Y-L, Li B-Y, Yang R, et al. A class of novel tubulin polymerization inhibitors exert effective anti-tumor activity via mitotic catastrophe. Eur J Med Chem 2019;163:896–910.