Abstract
Among the diagnostic techniques for the identification of tumour biomarkers, the liquid biopsy is considered one that offers future research on precision diagnosis and treatment of tumours in a non-invasive manner. The approach consists of isolating tumor-derived components, such as circulating tumour cells (CTC), tumour cell-free DNA (ctDNA), and extracellular vesicles (EVs), from the patient peripheral blood fluids. These elements constitute a source of genomic and proteomic information for cancer treatment. Within the tumour-derived components of the body fluids, the enzyme indicated with the acronym CA IX and belonging to the superfamily of carbonic anhydrases (CA, EC 4.2.1.1) is a promising aspirant for checking tumours. CA IX is a transmembrane-CA isoform that is strongly overexpressed in many cancers being not much diffused in healthy tissues except the gastrointestinal tract. Here, it is summarised the role of CA IX as tumour-associated protein and its putative relationship in liquid biopsyfor diagnosing and monitoring cancer progression.
1. Introduction
1.1. Carbonic anhydrases (CAs)
A crucial physiological reaction for the survival of all living organisms is the pivotal CO2 hydration/dehydration of the central metabolism. This reaction is connected with numerous metabolic pathways, such as photosynthesis and carboxylation reactions, and biochemical pathways including pH homeostasis, secretion of electrolytes, transport of CO2 and bicarbonate, and so onCitation1,Citation2. Moreover, the interconversion of CO2 and HCO3− is spontaneously and precisely balanced form the living organisms to maintain the equilibrium between dissolved inorganic carbon dioxide (CO2), carbonic acid (H2CO3), bicarbonate (HCO3−) and carbonate (CO32−)Citation3–6. The CO2 hydration/dehydration is catalysed by a superfamily of metalloenzymes, known as carbonic anhydrases (CAs, EC 4.2.1.1)Citation7–11, which are categorised into eight genetically distinct families (or classes), named with the Greek letters: α, β, γ, δ, ζ, η, ɵ, and ι. The last three classes were recently discoveredCitation12–14. Moreover, members of each class possess multiple transcript variants and protein isoforms, which are characterised by different biochemical properties and have specific tissue/organ and sub-cellular localizationsCitation11,Citation15–20. CAs present in animals belong to α-classCitation21,Citation22, plants and algae have α-, β-, γ-, δ- θ- and ι-classes; fungi encode for α- and β-CAs; protozoa for α-, β- and/or η-CAs; bacteria for α-, β-, γ- and ι-CA classesCitation11,Citation20,Citation23–27. Besides, a matrix protein called nacrein has been identified in the oyster Pinctada fucata. It participates in the formation of the nacreous layer and is characterised by a CA domain present at the N-terminus part of the polypeptide sequenceCitation28. In mammals, 16 α-CA isoforms have been identified: five of them are cytosolic (CA I, CA II, CA III, CA VII, and CA XIII), five are membrane-bound (CA IV, CA IX, CA XII, CA XIV, and CA XV), two are mitochondrial (CA VA and CA VB), and only one is secreted (CA VI), the last three (CA VIII, CA X, and CA XI) being devoid of catalytic activity and referred to as CA Related Proteins (CARPs)Citation8,Citation29,Citation30. CA active site includes a zinc ion (Zn2+), which plays a critical role in the catalytic enzyme function. In addition to the zinc, ζ-and γ-CAs reflect exceptions to this principle since they can use cadmium (ζ), iron (γ), or cobalt (γ)Citation31,Citation32.
1.2. Tumour-associated CA IX
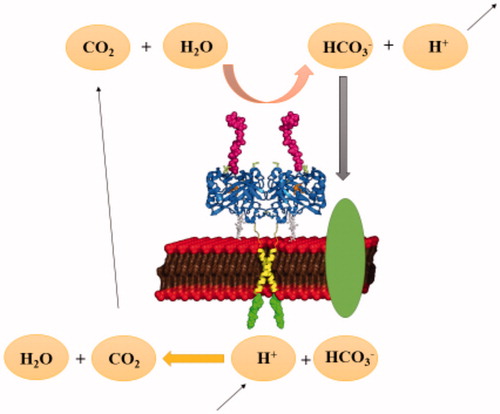
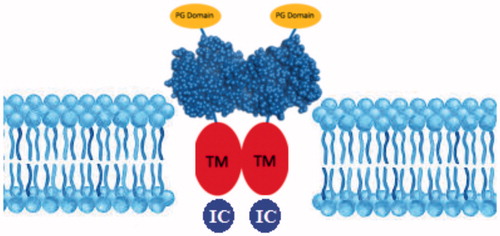
The glycolytic metabolism of cancer was evaluated for so many years to describe the fundamental role of tumour microenvironment and glycolysis in cancer growth and progressionCitation33. The transcription factors of the glycolytic pathway affect cell proliferation, which is an essential feature of carcinogenesisCitation34. Different types of enzymes are produced by tumours or by the body in response to malignancy and used as cancer biomarkersCitation35. It has been shown that the expression levels of certain enzymes can vary in various types of cancerCitation35. CA IX is a transmembrane CA isoform expressed in healthy tissues. shows the catalytically active CA IX on the cellular membrane surfaceCitation36–38. CA IX is highly overexpressed in many types of cancerCitation39,Citation40. For example, its expression is increased considerably in solid tumours of uterus, kidney, lung, colon, breast, brain, and ovaryCitation41–44. Tumour cells decrease their extracellular pH by lactic acid production and CO2 hydration, which is catalysed by CAs (). Since the tumour-associated CA IX is an efficient catalyst for the conversion of CO2 in bicarbonate and protons, they contribute to the acidification of the tumour environment. Moreover, its activity leads to the acquisition of metastatic phenotypes and chemoresistance to weakly basic anticancer drugsCitation21.
Figure 1. Structure of CA IX isoenzyme. (PG: Proteoglycan domain; TM: Transmembrane domain; IC: Intracellular domain).
Interestingly, CA IX has a metastatic activity related to the extracellular acidity since it has been shown to promote migration and invasion in tumour cellsCitation45,Citation46. Furthermore, oxygen has a crucial function in the regulation of redox balance and energy production in tumour tissues. An inadequate amount of oxygen in the tissues causes hypoxia, which is a characteristic marker of the tumour microenvironment. Hypoxia regulates the expression of many genes inducing a phenotypic alteration of stromal cells in the tumour microenvironment and promoting the survival of cancer cells. Low oxygen activates the HIF-1, the hypoxia-inducible factor 1 (HIF-1)Citation47,Citation48, which starts the transcription of several hypoxia-inducible genes, such as (Vascular Endothelial Growth Factor (VEGF), Glucose Transporter 1 (GLUT1), CA IX and CA XII))Citation49. CA IX is one of the most potent hypoxia-induced proteins, and the hypoxia-inducible proteins are important anti-cancer targetsCitation50. As a result, it is readily apparent that CA IX is associated with many tumours and are involved in the cancer processCitation34. The specific inhibition of CA IX activity with selective inhibitors, such as sulphonamide derivatives, represents a good strategy for establishing the driving role of this isoform, as well as other CAs, for example, CA XII, in tumorigenesisCitation51. Many CA inhibitors exist, which could be classified as inhibitors binding the metal ion, inhibitors anchoring to the water molecule/hydroxide ion coordinated to the metal, inhibitors occluding the active site entrance and inhibitors linking out of the active siteCitation52. Many of these inhibitors can be used to reduce the proliferation and invasion capacity of cancer cellsCitation53–55. Since pH-related cancer growth and metastasis might be dependent on the enzyme activity of the CA IX isoform, the identification of CA IX as tumour biomarker in the liquid biopsy is crucial for a new approach concerning the treatment of malignancy. The role of cancer markers in carcinogenesis is pivotal for early diagnosis, promotion of suitable procedures, and leading right management strategiesCitation56.
2. Tumour-derived components in liquid biopsy
Recently, liquid biopsy has begun an exciting approach in terms of early detection of tumorCitation57,Citation58. The technique, starting from the blood or body fluids, isolates tumour-derived components, such as circulating tumour cells (CTCs), circulating tumour DNA (ctDNA), and extracellular vesicles (EVs), as a source of genomic and proteomic information in patients with cancerCitation57. Liquid biopsy is an appropriate technique for correct treatments and figuring out the genetic changes in the tumour. It can be applied to many types of cancer, analysing the blood or body fluids of the patient taken in a non-invasive manner compared to the biopsy and without even detecting the symptomsCitation59.
2.1. Circulating tumour cells (CTCs)
Thomas Ashworth investigated CTCs in the 1860s, considering that tumour cells could have crossed the vessel wall and enter the bloodstreamCitation60. These cells are released from primary or metastatic cells. A few steps are required for the metastatic process. First, CTCs are separated from the tumour and incorporated into the bloodstream. The circulating CTCs are then protected from the immune cells to perform extravasation. After that, these cells adapt to the microenvironment of the new tissue and to form metastatic lesions. CTCs are found in circulation as single CTCs or CTC clusters. In the literature, it has been reported that tumour progression and patient survival is correlated with the numbers of CTCsCitation61. The cont of CTCs is also useful in the treatment response process. The technics known as immunophenotypic identification of cytokeratin, enzymatic methods, and RT-qPCR (reverse transcriptase quantitative polymerase chain reaction) are used to detect CTCsCitation62–66. The cell surface glycoprotein, EPCAM (Epithelial Cell Adhesion Molecule), which is highly expressed in epithelial cancer cells, serves as the primary antigen for CTCs detection. Moreover, with this method, prognostic information can be obtained in metastatic breast, colon, and prostate cancers. CTCsare subjected to genomic mappingCitation62–66, allowing a detailed analysis of the genes responsible for the uncontrolled growth of cells. Of course, the major drawbacks of this technique are (i) to distinguish cancer cells from millions of healthy cells; (ii) the detection of the kind of cancer.
2.2. Circulating tumour DNA (ctDNA)
The presence of ctDNA in the blood is dated back in 1948sCitation67. Although circulating cell-tumour DNA was first identified in 1948, it has only recently been investigated in “liquid biopsy” as cancer biomarkersCitation68. Tumours release fragments of DNA into the circulatory system, which are detectable and specific to cancer. There are several advantages to assessing ctDNA. Sampling is non-invasive and inexpensive compared to the tissue biopsy. Besides, ctDNA testing can be easily and frequently repeated to monitor changes that occur during treatment, serving as an early indicator of recurrence, resistance, or metastasisCitation69. Liquid biopsy is believed to exhibit tumour heterogeneity, as cells circulate from different regions of the tumour, and to obtain information in a shorter timeCitation69. CTCs and ctDNAs derived from primary or metastatic cells are abundant in blood. Both CTC and ctDNA provide prognostic information based on the number and level of events detectedCitation70–72 ().
Table 1. Comparison of CTCs and ctDNA.
2.3. Extracellular vesicles (EVs)
Extracellular Vesicles (EVs) are lipid structures released from cells. EVs contain proteins and nucleic acids and play a role in cellular communication, immune regulation, and microenvironmental modulationCitation73,Citation74. Nanosized exosomes (70–150 nm) are the most prominent members of these so-called extracellular vesicles (EVs) and are released from body fluids such as urine, ascites, and plasmaCitation73,Citation74. Moreover, they are liberated over all kinds of body cells (epithelium cells, haematopoietic cells, adipocytes, healthy and malignant cells)Citation75, released in almost all cell types under physiological and pathophysiological conditions and mediate intercellular contactsCitation76. A theranostic solution could be represented by the nanosized EVs, which may transmit biomarkers of diseases and/or vectors of therapeutic molecules, offering a unique opportunity to use a combination of different markers specifically expressed for tumour-derived EVsCitation74,Citation76,Citation77. For example, Prostate-Specific Antigen (PSA) does not differentiate between benign prostatic hyperplasia (BPH) and a Prostate Cancer (PC), resulting in large numbers of unnecessary biopsies and missed diagnosis of cancer. Since exosomes are directly detectable in patient plasma, the plasmatic exosomes expressing PSA have the potential in distinguishing healthy individuals, BPH, and PC77. Recently, it has been demonstrated that neurodegenerative disorders, including Alzheimer’s disease (AD), Parkinson, and amyotrophic lateral sclerosis, are correlated with extracellular vesicles. EVs have also been investigated in relation to infection caused by viruses, bacteria, fungi, protozoa, and helminthsCitation76. Such pathogens secrete EVs, and prions were even present in EVs.Finally, EVs seem to play key roles in autoimmune diseasesCitation76. EVs circulating in body fluids are valuable liquid biopsy biomarkers. Additionally, their protein concentration is higher in patients with advanced tumoursCitation73.
3. Tumour-associated CA IX as biomarker in liquid biopsy
The tumour-associated CA IX may be used as a cancer biomarker in the liquid biopsy technique. Carbonic anhydrase IX is a transmembrane enzymeCitation78, and it is involved in the growth and development of tumour cell adhesionCitation79,Citation80. There is an association between elevated serum levels of CA IX and CTCsCitation47. This suggests a relationship between hypoxia and tumour cell circulation in the bloodstream. In the peripheral venous blood, it is also possible to find the soluble form of CA IX, which is released by proteolytic cleavageCitation47,Citation50,Citation81. For example, a high level of soluble CA IX was found in the serum of patients with renal cancerCitation50. Müller et al. investigated the relationship between serum levels of CA IX and CTCs in metastatic breast cancerCitation47. Their findings suggested that the CA IX activity level was higher in cancer types with a high number of CTCs. In this condition, it is expected a decrease in the patient’s overall survival. Besides, CA IX disrupts cell-cell and cell-matrix interactions by triggering tumour acidification. As a consequence, CTCs separate from the primary tumour, and invasion occurs. Probably, CA IX inhibition could slow down the invasion process of CTCs.
During the tumour progression, exosomes and the metalloenzyme CA IX affect the growth and proliferation of the tumour. The relationship between exosomes and CA IX has been investigated using an in vitro cellular model of human prostate carcinoma cell line cultured in different pH conditions. The results showed that the acidic microenvironment increased both the expression and activity of CA IX in cancer cellsCitation82. Besides, the number of exosomes released by the cancer cells was raised together with the upregulation of the CA IXCitation82. These data strongly support that exosomes and CA IX are tumour-associated components and the enzyme CA IX is a cancer biomarker that could be used as valuable target of the liquid biopsyCitation82. Horie et al. demonstrated that CA IX exosomes were released from renal carcinoma cellsCitation49. The quantity of exosomal CA IX is increased in hypoxia response, promoting upregulation of MMP-2, migration and tube formation, and may induce angiogenesis in the tumour microenvironmentCitation49. Dorai and co-workers analysed the effect of increasing expression levels of CA IX in renal cancer cells. They showed that the level of released gangliocytes was positively correlated to that of exosomal CA IX expressionCitation83. Gangliosides play a critical role in cell adhesion, migration, and cell signallingCitation84. Since CA IX induces the release of gangliocyte-containing exosomes, the exosomal CA IX may be a valuable biomarker of the carcinogenesis process.
CA IX expression is regulated exclusively by HIF-1α, rapidly increases in response to hypoxia, and is very important for maintaining the acidic pH of the tumorCitation85. Brown-Glaberman proposed that the circulating CA IX could be considered as a biomarker for detecting the level of hypoxia and the upregulation of HIF-1αCitation86,Citation87. Circulating CA IX can be easily isolated from body fluids and considered a biomarker for different stages of cancers and the differentiation of local/advanced tumours. Malentacchi and coworkers to validate circulating CA IX as a tumour biomarker measuring the CA IX mRNA in the urine sediments of patients affected by kidney, prostate, and bladder cancersCitation88. As a result, they associated the mRNACA IX expression in the tumour of urogenital origin. Liu et al. have found that the combination of the CA IX/CD147 antibodies achieved higher efficiency in the NanoVelcro platform compared to EPCAM-based methods for capturing circulating cells coming from the renal carcinomaCitation89.
4. Conclusion
Liquid biopsy technology allows the detection of solid tumours, such as those involving lung, breast, and pancreatic, using the blood or other body fluids. Liquid biopsy can detect cancer-specific markers even in lesions that are too small to be recognised by other available methods, indicating that this method can be used early in cancer diagnosis. Among the known biomarkers of the liquid biopsy, the CA IXisoenzyme could be a promising candidate for tumour detection. In fact, the CA IX activity level is higher in cancer types with a high number of CTCs; the quantity of exosomal CA IX is increased in hypoxia response; the release of the exosomal gangliocyte is correlated to the exosomal CA IX expression increase; the circulating CA IX is associated to tumors of urogenital origin; CA IX disrupts cell-cell and cell-matrix interactions by triggering tumour acidification. In this context, the tumour-associated CA IX could be considered a valid biomarker of the non-invasive liquid biopsy, which is viewed as a technique that offers future research on precision diagnosis and treatment of tumours in a non-invasive manner. Moreover, CA IX could be a valid molecular target in the treatment of cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Johnson X, Alric J. Interaction between starch breakdown, acetate assimilation, and photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. J Biol Chem 2012;287:26445–52.
- Tcherkez G, Boex-Fontvieille E, Mahe A. Respiratory carbon fluxes in leaves. Curr Opin Plant Biol 2012;15:308–14.
- Smith KS, Ferry JG. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 2000;24:335–66.
- Maeda S, Price GD, Badger MR, et al. Bicarbonate binding activity of the CmpA protein of the cyanobacterium Synechococcus sp. strain PCC 7942 involved in active transport of bicarbonate. J Biol Chem 2000;275:20551–5.
- Joseph P, Ouahrani-Bettache S, Montero JL, et al. A new beta-carbonic anhydrase from Brucella suis, its cloning, characterization, and inhibition with sulfonamides and sulfamates, leading to impaired pathogen growth. Bioorg Med Chem 2011;19:1172–8.
- Joseph P, Turtaut F, Ouahrani-Bettache S, et al. Cloning, characterization, and inhibition studies of a beta-carbonic anhydrase from Brucella suis. J Med Chem 2010;53:2277–85.
- Annunziato G, Angeli A, D’Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. ChemMedChem 2016;11:1904–14.
- Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94.
- Del Prete S, Vullo D, De Luca V, et al. Sulfonamide inhibition studies of the beta-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:1115–20.
- Del Prete S, De Luca V, De Simone G, et al. Cloning, expression and purification of the complete domain of the eta-carbonic anhydrase from Plasmodium falciparum. J Enzyme Inhib Med Chem 2016;31:54–9.
- Capasso C, Supuran CT. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68.
- Jensen EL, Clement R, Kosta A, et al. A new widespread subclass of carbonic anhydrase in marine phytoplankton. Isme J 2019;13:2094–106.
- Kikutani S, Nakajima K, Nagasato C, et al. Thylakoid luminal theta-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc Natl Acad Sci USA 2016;113:9828–33.
- Del Prete S, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum–the eta-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96.
- Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54.
- Supuran CT. Carbonic anhydrase activators. Future Med Chem 2018;10:561–73.
- Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:56–74.
- Supuran CT, Capasso C. Carbonic anhydrase from Porphyromonas gingivalis as a drug target. Pathogens 2017;6:30–42.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- Capasso C, Supuran CT. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin Ther Targets 2015;19:1689–704.
- Aspatwar A, Tolvanen ME, Ortutay C, et al. Carbonic anhydrase related proteins: molecular biology and evolution. Subcell Biochem 2014;75:135–56.
- Supuran CT. Carbonic anhydrases as drug targets–an overview. Curr Top Med Chem 2007;7:825–33.
- Supuran CT, Capasso C. The eta-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin Ther Targets 2015;19:551–63.
- Capasso C, Supuran CT. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr Med Chem 2015;22:2130–9.
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32.
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs – antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87.
- Capasso C, Supuran CT. Anti-infective carbonic anhydrase inhibitors: a patent and literature review. Expert Opin Ther Pat 2013;23:693–704.
- Miyamoto H, Miyashita T, Okushima M, et al. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc Natl Acad Sci USA 1996;93:9657–60.
- Supuran CT. Carbonic anhydrases–an overview. Curr Pharm Des 2008;14:603–14.
- Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:48–60.
- Lane TW, Saito MA, George GN, et al. Biochemistry: a cadmium enzyme from a marine diatom. Nature 2005;435:42.
- Ferry JG. The gamma class of carbonic anhydrases. Biochim Biophys Acta 2010;1804:374–81.
- Jang M, Kim SS, Lee J. Cancer cell metabolism: implications for therapeutic targets. Exp Mol Med 2013;45:e45.
- Petrou A, Geronikaki A, Terzi E, et al. Inhibition of carbonic anhydrase isoforms I, II, IX and XII with secondary sulfonamides incorporating benzothiazole scaffolds. J Enzyme Inhib Med Chem 2016;31:1306–11.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Supuran CT, Di Fiore A, Alterio V, et al. Recent advances in structural studies of the carbonic anhydrase family: the crystal structure of human CA IX and CA XIII. Curr Pharm Des 2010;16:3246–54.
- Hilvo M, Baranauskiene L, Salzano AM, et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–809.
- Di Fiore A, De Simone G, Menchise V, et al. Carbonic anhydrase inhibitors: X-ray crystal structure of a benzenesulfonamide strong CA II and CA IX inhibitor bearing a pentafluorophenylaminothioureido tail in complex with isozyme II. Bioorg Med Chem Lett 2005;15:1937–42.
- Winum JY, Rami M, Scozzafava A, et al. Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med Res Rev 2008;28:445–63.
- Winum JY, Pastorekova S, Jakubickova L, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with bis-sulfamates. Bioorg Med Chem Lett 2005;15:579–84.
- Eldehna WM, Abo-Ashour MF, Berrino E, et al. SLC-0111 enaminone analogs, 3/4-(3-aryl-3-oxopropenyl) aminobenzenesulfonamides, as novel selective subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform IX. Bioorg Chem 2019;83:549–58.
- Mboge MY, Chen Z, Wolff A, et al. Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: Disconnect between activity and growth inhibition. PLoS One 2018;13:e0207417.
- Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70.
- Eldehna WM, Nocentini A, Al-Rashood ST, et al. Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in vitro antitumor activity towards colon cancer. Bioorg Chem 2018;81:425–32.
- Liao SY, Lerman MI, Stanbridge EJ. Expression of transmembrane carbonic anhydrases, CAIX and CAXII, in human development. BMC Dev Biol 2009;9:22.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Muller V, Riethdorf S, Rack B, et al. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res 2011;13:R71.
- Aspatwar A, Tolvanen ME, Ortutay C, et al. Carbonic anhydrase related protein VIII and its role in neurodegeneration and cancer. Curr Pharm Des 2010;16:3264–76.
- Horie K, Kawakami K, Fujita Y, et al. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun 2017;492:356–61.
- Zavada J, Zavadova Z, Zat’ovicova M, et al. Soluble form of carbonic anhydrase IX (CA IX) in the serum and urine of renal carcinoma patients. Br J Cancer 2003;89:1067–71.
- Pan PW, Waheed A, Sly WS, et al. Carbonic anhydrases in the mouse harderian gland. J Mol Histol 2010;41:411–7.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Parkkila S, Rajaniemi H, Parkkila AK, et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA 2000;97:2220–4.
- Guler O, Simone G, Supuran C. Drug design studies of the novel antitumor targets carbonic anhydrase IX and XII. Curr Med Chem 2010;17:1516–26.
- Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 2013;23:737–49.
- Nishiumi S, Yoshida M. [Possibility of metabolite biomarkers for early detection of cancer]. Gan to Kagaku Ryoho 2018;45:894–8.
- Han X, Wang J, Sun Y. Circulating tumor DNA as biomarkers for cancer detection. Genomics Proteomics Bioinformatics 2017;15:59–72.
- Zhang W, Xia W, Lv Z, et al. Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell Physiol Biochem 2017;41:755–68.
- Offin M, Chabon JJ, Razavi P, et al. Capturing genomic evolution of lung cancers through liquid biopsy for circulating tumor DNA. J Oncol 2017;2017:1.
- Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Austr Med J 1869;14:146–7.
- Sheng W, Ogunwobi OO, Chen T, et al. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014;14:89–98.
- Suo Y, Gu Z, Wei X. Advances of in vivo flow cytometry on cancer studies. Cytometry A 2019. [Epub ahead of print]. doi:10.1002/cyto.a.23851
- Khetani S, Mohammadi M, Nezhad AS. Filter-based isolation, enrichment, and characterization of circulating tumor cells. Biotechnol Bioeng 2018;115:2504–29.
- Werbin JL, Nordberg JJ, Tzucker J, et al. RareCyte® CTC analysis step 2: detection of circulating tumor cells by CyteFinder® automated scanning and semiautomated image analysis. Methods Mol Biol 2017;1634:173–80.
- Hassan EM, Willmore WG, DeRosa MC. Aptamers: promising tools for the detection of circulating tumor cells. Nucleic Acid Ther 2016;26:335–47.
- Ghossein RA, Bhattacharya S. Molecular detection and characterization of circulating tumor cells and micrometastases in prostatic, urothelial, and renal cell carcinomas. Semin Surg Oncol 2001;20:304–11.
- Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l'homme. C R Seances Soc Biol Fil 1948;142:241–3.
- Kanwar N, Hu P, Bedard P, et al. Identification of genomic signatures in circulating tumor cells from breast cancer. Int J Cancer 2015;137:332–44.
- Li H, Jing C, Wu J, et al. Circulating tumor DNA detection: a potential tool for colorectal cancer management. Oncol Lett 2019;17:1409–16.
- Heitzer E, Auer M, Ulz P, et al. Circulating tumor cells and DNA as liquid biopsies. Genome Med 2013;5:73.
- Lim M, Kim CJ, Sunkara V, et al. Liquid biopsy in lung cancer: clinical applications of circulating biomarkers (CTCs and ctDNA). Micromachines (Basel) 2018;9. doi:10.3390/mi9030100
- Neumann MHD, Bender S, Krahn T, et al. ctDNA and CTCs in liquid biopsy - current status and where we need to progress. Comput Struct Biotechnol J 2018;16:190–5.
- Shang M, Ji JS, Song C, et al. Extracellular vesicles: a brief overview and its role in precision medicine. Methods Mol Biol 2017;1660:1–14.
- Campanella C, Caruso Bavisotto C, Logozzi M, et al. On the choice of the extracellular vesicles for therapeutic purposes. Int J Mol Sci 2019;20. doi:10.3390/ijms20020236
- Colombo M, Giannandrea D, Lesma E, et al. Extracellular vesicles enhance multiple myeloma metastatic dissemination. Int J Mol Sci 2019;20. doi: 10.3390/ijms20133236
- Fais S, O’Driscoll L, Borras FE, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano 2016;10:3886–99.
- Logozzi M, Angelini DF, Giuliani A, et al. Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers (Basel) 2019;11. doi:10.3390/cancers11101449
- De Simone G, Supuran CT. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim Biophys Acta 2010;1804:404–9.
- Svastova E, Witarski W, Csaderova L, et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem 2012;287:3392–402.
- Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med Res Rev 2018;38:1799–836.
- Ilie M, Mazure NM, Hofman V, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer 2010;102:1627–35.
- Logozzi M, Capasso C, Di Raimo R, et al. Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. J Enzyme Inhib Med Chem 2019;34:272–8.
- Dorai T, Sawczuk IS, Pastorek J, et al. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer 2005;41:2935–47.
- Murai T. The role of lipid rafts in cancer cell adhesion and migration. Int J Cell Biol 2012;2012:1.
- Kaluz S, Kaluzova M, Liao SY, et al. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochim Biophys Acta 2009;1795:162–72.
- Brown-Glaberman U, Marron M, Chalasani P, et al. Circulating carbonic anhydrase IX and antiangiogenic therapy in breast cancer. Dis Markers 2016;2016:1.
- Finkelmeier F, Canli O, Peiffer KH, et al. Circulating hypoxia marker carbonic anhydrase IX (CA9) in patients with hepatocellular carcinoma and patients with cirrhosis. PLoS One 2018;13:e0200855.
- Malentacchi F, Vinci S, Melina AD, et al. Urinary carbonic anhydrase IX splicing messenger RNA variants in urogenital cancers. Urol Oncol 2016;34:292 e9–292 e16.
- Liu S, Tian Z, Zhang L, et al. Combined cell surface carbonic anhydrase 9 and CD147 antigens enable high-efficiency capture of circulating tumor cells in clear cell renal cell carcinoma patients. Oncotarget 2016;7:59877–91.