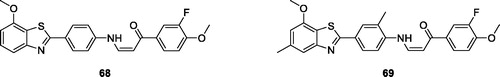?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Benzothiazole (BTA) belongs to the heterocyclic class of bicyclic compounds. BTA derivatives possesses broad spectrum biological activities such as anticancer, antioxidant, anti-inflammatory, anti-tumour, antiviral, antibacterial, anti-proliferative, anti-diabetic, anti-convulsant, analgesic, anti-tubercular, antimalarial, anti-leishmanial, anti-histaminic and anti-fungal among others. The BTA scaffolds showed a crucial role in the inhibition of the metalloenzyme carbonic anhydrase (CA). In this review an extensive literature survey over the last decade discloses the role of BTA derivatives mainly as anticancer agents. Such compounds are effective against various types of cancer cell lines through a multitude of mechanisms, some of which are poorly studied or understood. The inhibition of tumour associated CAs by BTA derivatives is on the other hand better investigated and such compounds may serve as anticancer leads for the development of agents effective against hypoxic tumours.
1. Introduction
Heterocyles are important pharmcophores and have significance to create privileged chemical structures possessing pharmacological activities. Five membered heterocyclic which incorporate oxygen, nitrogen and sulphur are found in broad spectrum therapeutic agents which have an enormous significance in drug discovery and drug development processesCitation1. Benzothaizole (BTA) is a fused benzoheterocyle which is present in many naturally occurring products and is responsible for the medicinal, pharmacological and pharmaceutical applications of such natural productsCitation2. BTA is present in terrestrial as well as marine compounds which exhibit various biological activitiesCitation3. The BTA nucleus is formed by the fusion of the thiazole ring with a benzene ringCitation4.
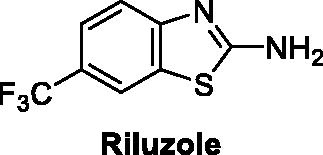
The pharmacological profile of the drug used for the management of amyotrophic lateral sclerosis Riluzole () attracted the attention of medicinal chemists towards biologically active benzothiazoleCitation5.
The BTA scaffold possesses a wide spectrum of biological activities such as anti-inflammatoryCitation6, fungicidalCitation7, anti-diabeticCitation8, analgesicCitation9, anti-microbialCitation10, antitumorCitation11, antileishmanialCitation12, antheltminticCitation13, antirheumaticCitation14 and CNS depressantCitation15 etc. BTA derivatives exhibit remarkable and prevalent biological and pharmacological activities against different types of tumours and cancer cell lines such as HeLa (human cervical cancer cell line), SW480 (human colon adenocarcinoma cell line), HepG2 (human liver carcinoma cells)Citation16, mammary and ovarian tumour cell linesCitation17, colon, nonsmall-cell lung and breast subpanels cell linesCitation18, and HCC (hepatocellular carcinoma)Citation19 etc.
Cancer is the most prominent, notably complex and lethal disease which became a serious concern of today’s medical science. It poses a great challenge to medical scientific community for development of drugs, medicines and procedures for safer treatment and cure of cancer diseaseCitation20. These neoplasm tumour cells are diversified, heterogeneous cells with rapid proliferative properties. These neoplasm malignant tumours, have potential to invade or spread to other parts of body through blood stream and lymphatic systemCitation21. The plethora of research mentioned in the present review of last decade on anticancer potential of BTA derivatives will be helpful in future drug discovery and drug development for the treatment of lethal cancer disease.
2. BTA derivatives as anticancer agents
2.1. Fluorinated derivatives of benzothiazole as anticancer agents
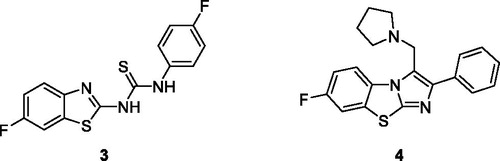
Aiello et al. synthesised fluorinated 2-aryl benzothiazole derivatives and evaluate them for anti-tumour activities against cancer cell lines such as MDA-MB-468 (mammary gland/breast tissues derived from metastatic site) and MCF-7 cell line (human breast adenocarcinoma). The fluorinated BTA derivatives 1 (3–(5-fluorobenzo[d]thiazol-2-yl)phenol) and 2 (4–(5-fluorobenzo[d]thiazol-2-yl)phenol) having hydroxyl substituents on the third and fourth position of phenyl exhibited the best activity having GI50 values of 0.57 and 0.4 µM respectively against MCF-cell line as compared to BTA derivatives containing alkoxy, methyl sulphonyl and ethyl substituents on the benzothiazole (). Kumbhare et al. afforded the N-bis-benzothiazole and benzothiazolyl thiocarbamide derivatives and screened for cytotoxic activities against two human cell lines U-937 (human macrophage cell line), THP-1 (human leukaemia monocytic cell line) and B16-F10 (mouse melanoma cell line). The thiourea containing benzothiazole derivative 3 () demonstrated the best antiproliferative activity against the U-937 cell line as compared to standard drug Etoposide. The IC50 values of compound 3 were higher (16.23 ± 0.81 µM)), (4847.73 ± 2.39 µM)) and (34.58 ± 1.73 µM)) as compared to standard compound etoposide IC50 values (17.94 ± 0.89), (18.69 ± 0.94) and (2.16 ± 0.11 µM)) against U-937, B16-F10 and THP-1 cell lines respectivelyCitation22.
Figure 3. Substituted thiourea containing benzothiazole derivative 3 and the substituted pyrrolidine based imidazo benzothiazole derivative 4.
Kumbhare et al. reported the synthesis of mannich base arylimidazo derivatives containing benzothiazole moiety and screened for their anticancer activities against HepG2, MCF -7 and HeLa cell lines. All these synthesised mannich bases BTA scaffolds showed cytotoxicity against all tested cell lines but the pyrrolidine based imidazo benzothiazole derivative 4 () demonstrated specific features of apoptosis as enhancement in the levels of caspase-3. The compound 4 exhibited anti cancer activity and proved to be the best antiproliferative agent as compared to other derivatives against HepG2, MCF-7 and HeLa cell line when screened at 4.0 µM concentrations. The SAR studies revealed that the incorporation of fluorine atom at the 7th position of derivative 4 enhanced the cytotoxicity. The compound 4 have potential to lead in the treatment of cancer especially against hepatocaricinoma. The anticancer activity potential of BTA scaffold 4 is encourging for the development of new anti-cancer therapeutic agents and this will be good addition in armamentarium that consists of paclitaxel, cisplatin and doxorubicin drugsCitation23.
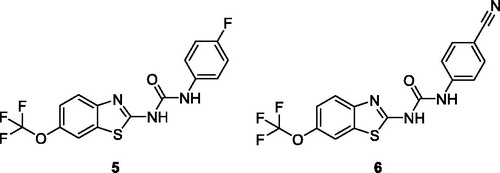
Caputo et al. afforded two types of five derivatives on the basis of an aryl amide and an aryl urea functionalities attached at C-2 of benzothiazole core and these scaffolds were screened against 60 human cancer cell lines. The urea moiety based fluorophenyl containing benzothiazole derivative 4 () and cyanophenyl containing benzothiazole derivative 5 () demonstrated remarkable anticancer activities. The BTA derivative 4 exhibited the anticancer activity at 10−5 M against different cell lines such as leukaemia cell lines (log GI50 value −5.48), non-small cell lung cell lines (log GI50 value −5.48), colon cancer cell lines (log GI50 value −5.51), central nervous system cancer cell lines (log GI50 value −5.49), melanoma cell lines (log GI50 value −5.48), ovarian cancer cell lines (log GI50 value −5.49), renal cancer cell lines (log GI50 value −5.53), prostate cancer cell lines (log GI50 value −5.50) and breast cancer cell lines (log GI50 value −5.56) in comparison with reference drug 5-fluorouracil NSC 19893. The BTA scaffold 5 showed remarkable growth inhibitory activities against different human tumour cell lines such as leukaemia cell lines (log GI50 value −5.93), non-small cell lung cell lines (log GI50 value −6.0), colon cancer cell lines (log GI50 value −5.89), central nervous system cancer cell lines (log GI50 value −5.73), melanoma cell lines (log GI50 value −5.89), ovarian cancer cell lines (log GI50 value −5.74), renal cancer cell lines (log GI50 value −5.90), prostate cancer cell lines (log GI50 value −5.72) and breast cancer cell lines (log GI50 value −6.0) as compared with reference drug 5-fluorouracil NSC 19893. The scaffolds 4 and 5 showed the best anticancer therapeutic potential due to presence of electron with drawing groups on para position of phenyl ringCitation23.
Figure 4. Substituted fluorophenyl containing benzothiazole urea derivative 5 and 2-substituted cyanophenyl containing benzothiazole urea derivatives 6.
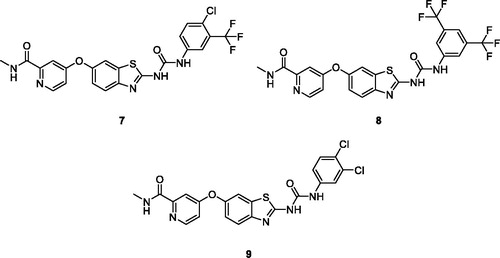
El-Damasy et al. synthesised the novel amide and urea based BTA series of 20 sorafenib analogues in which the pyridylamide privileged functionality was attached with an ether linkage at 6-position of the BTA ring. A selected group of 12 potent scaffolds were evaluated and appraised for anti-proliferative activities against sixty human cancer cell lines. These chlorotrifluoromethyl phenyl ureido picolinamide benzothiazoles 7 (), bis-trifluoromethyl phenyl ureido based picolinamide benzothiazole derivative 8 () and dichlorophenyl ureido based picolinamide benzothiazoles 9 () were more potent in the treatment of renal cell carcinoma than the standard drug sorafenib, used for the treatment of such tumours. The 3,5-bis-trifluoromethylphenylurea 8 showed good inhibitory activities against ACHN (renal cancer cells lines) and A-498 (human kidney carcinoma cell line) with GI50 values of 0.542 µM and 1.02 µM respectively. This compound also possess efficacy against UO-31 and RXF 393 cell lines. The derivatives 7 and 9 because of 3,4-disubstitutedphenyl moiety, exhibited excellent anti-proliferative activities with low IG50 values of 1.85, 2.10 µM against RCC and ACHN cell lines respectively. The SAR study revealed the fact that sorafenib analogues possesses anti proliferative activity due to the presence of both urea spacer and phenyl disubstitution. Compound 8 demonstrated the highest CLogP value being the most lipophilic and potent derivative, in the low µM rangeCitation24.
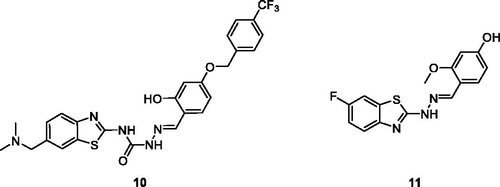
Ma et al. reported BTA derivatives containing an ortho-hydroxy-N-acyl hydrazone moiety for antiproliferative activities and procaspase-3 kinase activation activities against five different cell lines, namely MDA-MB-231 (human breast adenocarcinoma cell line), MNK-45 (gastric cancer cell line), NCI-H226 (human lung cancer cell line), HT-29 (human colorectal adenocarcinoma cell line) and SK-N-SH (neuroblastoma cell line). The substituted 2-hydroxybenzylidene containing semicarbazide 10 () showed inhibitory activities against all cell lines with IC50 and EC50 values ranging from 0.24 to 0.92 µM and 0.31 µM respectively. The SAR studies revealed the paharmacological activities of BTA scaffold 10 in in-vitro is due to introduction of phenyl and benzyloxyl substitutionsCitation25.
Gabr et al. obtained hydrazine derivatives by treating 2-amino-6-fluorobenzothiazole with hydrazine hydrate which was further treated with the suitable aldehydes to afford 27 different BTA Schiff base derivatives. These derivatives were screened for anti-tumour potential against Hela (cervical cancer) and COS-7 (kidney fibroblast cancer) cell lines. The hydrazine based benzothiazole 11 () exhibited IC50 of 2.41 µM and 4.31 µM against Hela and COS-7 cell lines as compared to reference doxorubicin having IC50 2.05 µM and 3.04 µM respectively. The SAR studies explained the effect of various substitutions on activities of all the synthesised derivatives. The scaffold present in 11 has the 2–(4-hydroxy-methoxy benzylidene)-hydrazino moiety at the C-2 position which remarkably enhances the anti-tumour potential, whereas replacing the 4-hydroxy moiety with 4-methoxy decreased the activities against both cell linesCitation26.
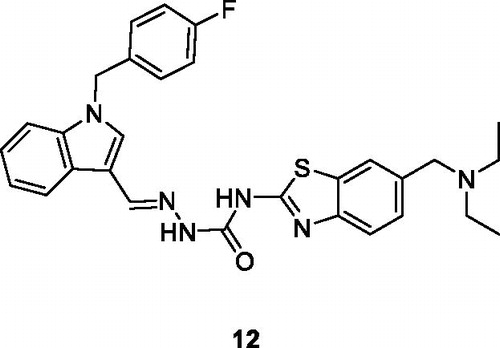
Junjie et al. reported the synthesis of semicarbazone containing BTA derivatives by the reaction of 4-nitrobenzyl bromide with substituted amines under different reaction conditions and evaluated their anticancer activity against four different cancer cell line such as human colon cancer cells (HT29), human lung cancer cell (H460), non-small cell lung cancer (A549) and human breast cancer (MDA-MB-231). Among these derivatives, the indole based hydrazine carboxamide scaffold 12 () showed potent antitumor activity with IC50 values of 0.015 µM for HT29, 0.28 µM for H460, 1.53 µM for A549 and 0.68 µM for MDA-MB-231. The structure – activity relationship explained that compound 12 exhibited the highest antitumor activity due to the presence of electron withdrawing groups in the 4 position of benzyl ringCitation27.
2.2. Imidazole based benzothiazole derivatives as anticancer agents
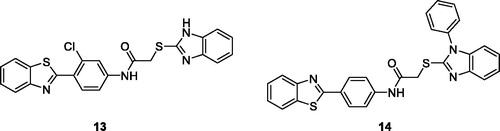
Yurttas et al. obtained 2–(4-aminophenyl)BTA derivatives substituted with different heterocyclic rings and tested their antitumor potential against 60 human tumour cell lines. The BTA derivatives 13 (2–(1H-benzo[d]imidazol-2-ylthio)-N-(4-(benzo[d]thiazol-2-yl)-3-chlorophenyl) acetamide) () and 14 (N-(4-(benzo[d]thiazol-2-yl)phenyl)-2-(1-phenyl-1H-benzo[d]imidazol-2-yl-thio)-acetamide) () showed remarkable antitumor potential against different cancer cell lines. The heterocylic substitutions affect the activity and antitumor potential of these BTA derivatives, with derivative 14 having comparable antitumor potential with the standard drugs whereas derivative 13 being less active compared to 14. The order overall antitumor potential of 2–(4-aminophenyl) benzothiazole derivatives with reference to the heterocyclic substitution was benzimidazole imidazole > benzothiazole > benzoxazoleCitation28.
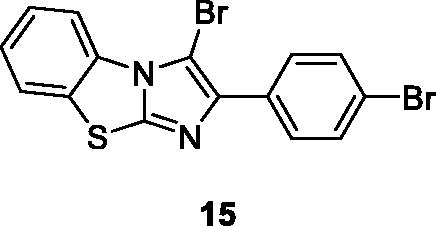
Singh et al. reported the synthesis of imidazole based benzothiazoles by treatment of substituted anilines with KSCN which afforded the desired benzothiazole derivatives, and studied their anticancer activities. Compound 15 () showed excellent anticancer activity possessing IC50 value 10 µM when compared with the standard drug doxorubicinCitation29.
2.3. Piperazine based benzothiazole derivatives as anticancer agents
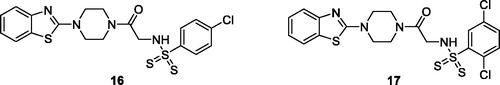
Al-Soud. et al. reported the synthesis of BTA derivatives incorporating sulphonamide, piperazino-arylsulfonamide and arylthiol scaffolds and determined their anti-proliferative potential against different cell lines, such as CCRF-SB (Human acute B-lymphoblastic leukaemia), DU-145 (human prostate cancer cell lines express androgen receptor), HepG-2 cell line (human liver cancer), WIL-2NS (Human splenic B-lymphoblastoid cells), MRC-5 (human lung fibroblast cell line), MCF-7 cell line (human breast adenocarcinoma), MT-4 (human T-cells containing an integrated HTLV-1 genome), SK-MES-1 cell line (human lung cancer) and SK-28 cell line (skin melanoma). Derivative 16 (N-(2–(4-(benzo[d]thiazol-2-yl)piperazin-1-yl)-2-oxoethyl)-4-chloro-benzenesulfonodithioamide) showed antiproliferative activity (CC50 = 8 ± 3 µM) against human derived DU-145 cell line () whereas derivative 17 (N-(2–(4-(benzo[d]thiazol-2-yl) piperazin-1-yl)-2-oxoethyl)-2,5-dichloro benzenesulfonodithio-amide) demonstrated remarkable activities against several human derived cell lines such as HepG2 and DU-145 (with CC50 of 8 ± 2 µM, and 9 ± 2 µM, respectively) (). Derivatives 16 and 17 exhibited atiproliferative potential due to the introduction of chloro and dichloro phenyl groups while their replacement of with hydrogen, methoxy, nitro, triflouromethyl and methyl groups lead to a decrease in the antiproliferative potential of BTA derivativesCitation30.
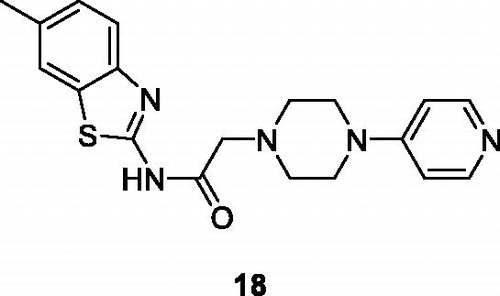
Gurdal et al. synthesised BTA derivatives which incorporate piperazine moieties and evaluated their cytotoxicity against different cancer cell lines such as HUH-7 (Heptacellular), MCF-7 (Breast) and HCT-116 (Colorectal). GI50 values of these derivatives indicated that all compounds exhibited good potential against the aforementioned cell lines but the pyridine containing derivative 18 () had a remarkable cytotoxic activity with GI50 value 7.9 µM, 9.2 µM and 3.1 µM for HCT-116, MCF-7 and HUH-7 respectively. Apoptosis caused by this derivative during cell cycle arrest at subG1 phase was confirmed by Hoechst staining and fluorescence activated cell sorting analysisCitation31.
2.4. Oxadiazole based benzothiazole derivatives as anticancer agents
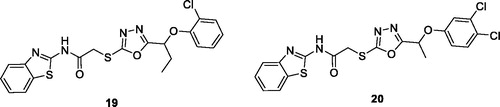
Akhtar et al. synthesised BTA and 1,3,4-oxadiazole-2-thione derivatives and determined their antitumor potential in in-vitro against different tumour cell lines. BTA derivatives 19 (N-(benzo[d]thiazol-2-yl)-2–(5-(1–(2-chlorophenoxy)propyl)-1,3,4-oxadiazol-2-ylthio)acetamide) and 20 (N-(benzo[d]thiazol-2-yl)-2–(5-(1–(3,4-dichlorophenoxy)ethyl)-1,3,4-oxadiazol-2-ylthio) acetamide) exhibited remarkable activities against CCRF-CEM (leukaemia) cell lines. The CC50 values of compounds 19 and 20 (CC50 = 12 2 µM and 8
1 µM respectively) were comparable to the standard drug Doxorubicin. The replacement of the chloro moiety with bromo in the hybrid structures of 19 and 20 decreased their anti-tumour potential ()Citation32.
2.5. Morpholine-thiourea based benzothiazole derivatives as anticancer agents
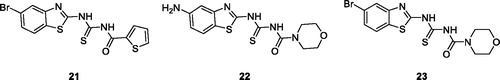
Saeed et al. reported the synthesis of benzothiazole moiety based thiourea derivatives. These novel derivatives were tested for anticancer potential against cancer cell lines MCF-7 and HeLa cells. The MTT assay (colorimetric assessment of cell metabolic activity) indicated that the thiophene based acetamide benzothiazole derivatives 21 (), morpholine based thiourea aminobenzothiazole derivative 22 () and morpholine based thiourea bromobenzothiazole 23 () were potent anticancer agents having IC50 values of 24.15, 26.43 and 18.10 µM against MCF-7 cell line, and of 46.46, 45.29 and 38.85 µM against HeLa cells lines respectivelyCitation33.
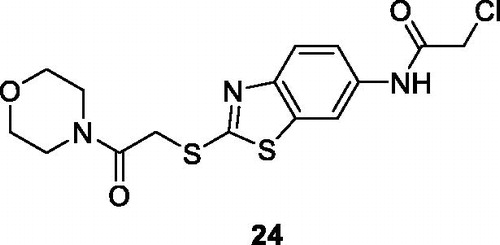
Lei et al. obtained the morpholine based acetamide benzothiazole derivative 24 () by the treatment of morpholine with 2-chloroacetyl chloride and studied its anticancer activity against HCC (human hepatocellular carcinoma) cell lines HepG2 and Bel7402, reporting IC50 value for HepG2 and Bel7402 in the millimolar rangeCitation34..
2.6. Thiophene based benzothiazole derivatives as anticancer agents
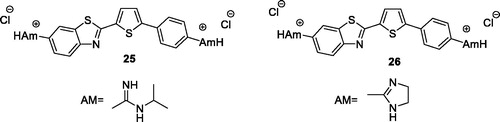
Racane et al. obtained the furyl and thienyl based diamidino substituted derivatives of phenyl-BTA and screened them for antiproliferative activities against different tumour cell line in vitro. Derivative 25 (diamidino-substituted thiophene based BTA, ) and 26 (imidazolinyl-substituted thiophene based BTA; ) exhibited low cytotoxic effects on normal human fibroblasts and strong antiproliferative effects on MiaPaCa-2 and MCF-7 cancer cell lines. The experimental data indicated that the benzothiazole derivatives having thiophene and imidazole substitions possessed interesting antiproliferative activitiesCitation35.
2.7. Thiadiazole based benzothiazole derivatives as anticancer agents
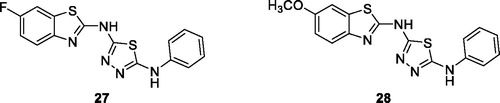
Sekar et al. reported the synthesis and anticancer activities of six noval BTA derivatives. All derivatives exhibited anticancer activities from a high to a moderate activity level with the substituted thiadiazole flourobenzothiazole 27 () and methoxybenzothiazole 28 () showing the best anti-cancer potential due to the presence of highly electronative and electron denoting pharmacophores (e.g. fluorine and methoxy moieties)Citation36.
2.8. Substituted pyridine based benzothiazole derivatives as anticancer agents
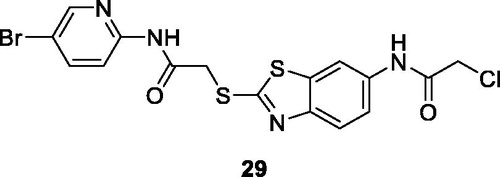
Shi et al. synthesised 20 BTA-2-thiol derivatives and investigated their anti-tumour potential against different cell lines such as SW480 (colon adenocarcinoma), HeLa, A549, HCT-116, HepG2 and SKRB-3 breast cancer cell line. The substituted bromopyridine acetamide benzothiazole derivative 29 () showed potent antitumor activity against SKRB-3, SW620, A549 and HepG2 cell lines with IC50 values of 1.2 nM, 4.3 nM, 44 nM and 48 nM, respectively. Apoptosis was the mechanism of cell death and was concentration dependent in HepaG2 cells. These results indicated that BTA-2-thiols exhibit broad spectrum anti-cancer activities which are worth to be further investigatedCitation16.
Xuejiao et al. reported the synthesis of a substituted pyridine based acetamide BTA derivative 30 () and screened its anti-cancer activity both in vitro and in vivo. Derivative 30 demonstrated anti-proliferative activities against a wide spectrum of human cell lines and induced the mitochondrial apoptotic pathway in HepaG2 cell lines. The BTA scaffold 30 proved to be a promising candidate for cancer chemotherapyCitation37.
Figure 18. Substituted pyridine based acetamide benzothiazole 30 and pyridine containing pyrimidine benzothiazole 31.
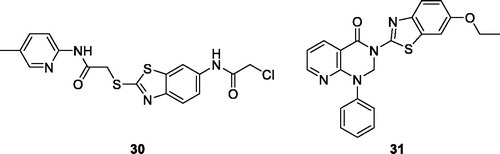
Kamal et al. reported the synthesis of novel phenyl pyridopyrimidinones based BTA derivatives which were examined against four different cancer cell lines such as ME-180, DU-145, MCF-7 and B-16. The pyridine containing pyrimidine benzothiazole 31 () exhibited interesting cytotoxicity with IC50 value of 4.01 µM against ME-180 (human cervical cancer cell line)Citation38.
2.9. Pyrazole based benzothiazole derivatives as anticancer agents
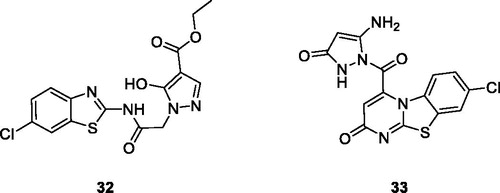
Gabr et al. reported the synthesis and evaluation of novel BTA scaffolds against 60 tumour cell lines at a single dose of 10 µM. The best derivatives were 32 () and 33 (), which were further screened at 5 doses. These derivatives demonstrated interesting anticancer activity at micro molar and sub micro molar concentrations, against all sixty tumour cell lines with GI50 in the low micro molar or submicromolar range. The SAR study revealed that introduction of the pyrazole moiety significantly enhanced the antitumor activity of both derivatives. Furthermore the presence of 2-hydroxy ester and 3-oxopyrazole within the pyrimidine moiety increased the anti-tumour activities of both derivatives. The simple BTA scafolds having pyrazole functionalities were more potent against different cell lines as compared to derivatives having the pyrazole ring within a pyrimidine moietyCitation39.
2.10. Pyrimidine based benzothiazole derivatives as anticancer agents
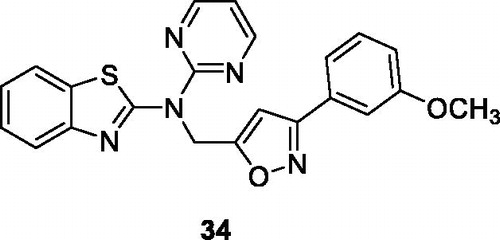
Kambhare et al. synthesised the isoxazole pyrimidine based BTAs and evaluated them for anticancer activity by the MTT assay against different cell lines such as A549, Colo205, MCF-7 and U937 cell lines, in comparison with the standard drug etoposide. The pyridine containing pyrimidine derivative 34 () demonstrated good anti-cancer potential with IC50 value 5.04 µM against colo205, 13.9 µM against U937, 30.67 µM against MCF-7 and 30.45 µM against A549 cell lines when compared with standard drug etoposide. The scaffold 34 activated p53 or TP53 (tumour protein) pathways, which regulate the equilibrium between apoptosis and cell proliferation. The SAR explained that the maximum cytotoxicity of derivative 34 against colon cancer cell line was due to the presence of methoxy group (–OCH3) in the phenylCitation40.
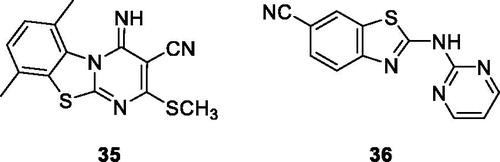
Waghmare et al. reported the synthesis of substituted pyrimidine containing benzothiazole derivative 35 () by refluxing BTAs with bis-methylthio methylene malononitrile, and tested their anticancer activity against 18 different cell lines. The scaffold 35 possessed excellent anticancer activity with a good percentage of growth inhibition against lung cancer, breast cancer and renal cancer cell lines. The good anticancer activity of derivative 35 is due to the presence of two methyl and one SCH3 groups in its structureCitation41.
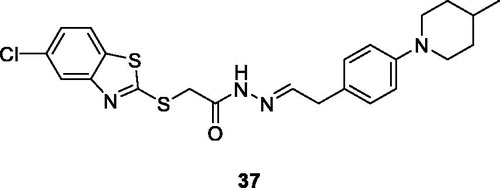
Caleta et al. synthesised cyano and amidinobenzothiazole substituted anilins which were treated with 2-bromo-6-cyanobenzothiazole under specific reaction conditions to afford compounds which have been studied for their anticancer activity against 6 cancer cell lines such as laryngeal carcinoma (Hep-2), breast carcinoma (MCF-7), cervical carcinoma (HeLa), pancreatic carcinoma (MiaPaCa-2), colon carcinoma (SW 620), lung carcinoma (H 460), and diploid fibroblasts (WI 38). Among them, the pyrimidine based carbonitrile benzothiazole derivative 36 () showed potent activity against all cancer cell lines used in the studyCitation42.
2.11. Piperidine based benzothiazole derivatives as anticancer agents
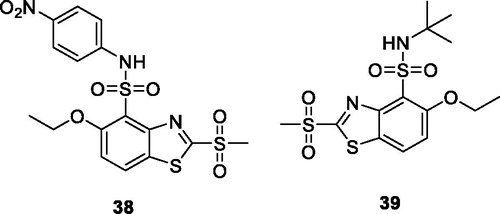
Osmaniye et al. prepared BTA acylhydrazone using 4-fluorobenzaldehyde refluxed with substituted amines, to afford the desired derivatives and studied their anticancer activities against rat brain glioma (carcinogenic C6) cell line, human lung adenocarcinoma epithelial (A549) cell line, human breast adenocarcinoma (MCF-7) cell line, human colorectal adenocarcinoma (HT-29) cell line and mouse embryo fibroblast (NIH3T3) cell line. The piperidine based acetohydrazide derivative 37 () showed modest activity having IC50 value 1 < mM, 0.03 mM, 0.10 mM, 0.30 mM and 1< mM for A549, HT-29, MCF-7, C6, and NIH3T3 cell lines respectively against reference drug cisplatinCitation43.
2.12. Secondary sulphonamide benzothiazole derivatives as anticancer agents
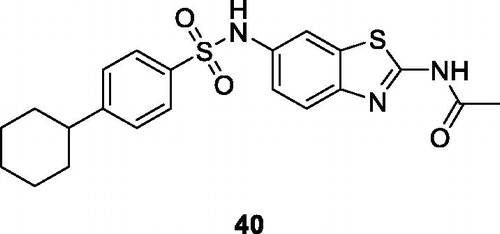
Lad et al. reported the synthesis of a series of methylsulfonyl benzothiazoles, obtained from 5-ethoxybenzothiazol-2-amine which were tested their anticancer activities. Among these derivatives, the nitrophenyl sulphonamide based methylsulfonyl benzothiazole 38 () and ter-butyl sulphonamide based methylsulfonyl benzothiazole 39 () exhibited the best anticancer activities against HeLa cell line with the IG50 value of 0.22 µM and 0.6 µM respectivelyCitation44.
Sadhasivam et al. reported the synthesis of 2, 6-disubstituted-BTA by reaction of 2-amino-6-nitrobenzothiazole and acetic anhydride and studied their anticancer activity against three cancer cell lines MCF-7, HeLa and MG63 (human osteosarcoma) The sulphonamide scaffold based BTA 40 () exhibited modest anti-cancer activity with IC50 of 34.5 µM for MCF-7, 44.15 µM for HeLa and 36.1 µM for the MG63Citation45.
2.13. Benzamide based benzothiazole derivatives as anticancer agents
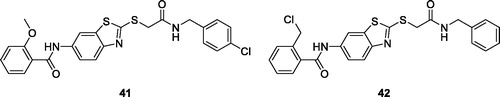
Wang et al. synthesised and evaluated 24 benzothiazole-2-thiol derivatives for antiproliferative activities against different human cancer cell lines such as A549, HCT-116, SW620, SW480, MDA-MB-468, SKRB-3, HeLa, SKOV-3, PC-3, BxPC-3, A431 and A375. Some derivatives exhibited better anticancer activities as compared to the standard drug cisplatin. The substituted methoxybenzamide benzothiazole 41 and the substituted chloromethylbenzamide benzothiazole 42 () showed good anti-tumour potential in vitro, with IC50 values ranging from 1.1 µM to 8.8 µM. The introduction of chloromethyl and methoxy functionalities in compounds 41 and 42 increased their anticancer activity compared to other synthesised analogsCitation46.
Figure 25. Substituted methoxybenzamide based benzothiazole derivative 41 and chloromethylbenzamide based benzothiazole 42.
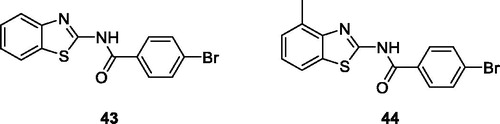
Bolelli et al. synthesised 2-substituted benzothiazoles by the reaction of carboxylic acid with thionyl chloride which afforded acyl chlorides, further treated with substituted benzothiazole to give 2-substituted benzothiazoles, which were studied for their inhibitory activities against human glutathione transferases (hGSTP1-1). The benzamide benzothiazole derivative 43 and benzamide methylbenzothiazole 44 () showed potent hGSTP1-1 inhibitory activities, useful in cancer chemotherapy. The SAR studies pinpointed that both these scaffold showed effective inhibition due to the presence of para-substitutions on the phenyl ring of the benzamide groupCitation47.
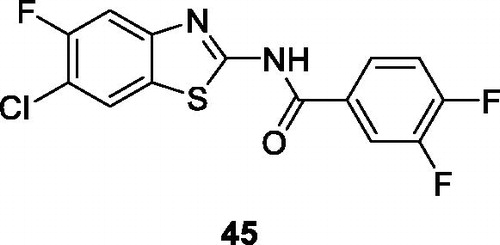
Corbo et al. reported 19 derivatives of benzamide based BTAs by the reaction of substituted aniline, potassium thiocyante and bromine, which afforded substituted compounds which were tested for anti-proliferative activity against MCF-7 and HepG2 cell lines. The substituted diflourobenzamide containing benzothiazole 45 () proved to be a potent anti-proliferative compound with the percentual inhibition of 64 ± 2 µM for MCF-7 cell line and of 64 ± 6 µM for HepG2 cell lineCitation48.
2.14. Quinolone based benzothiazole derivatives
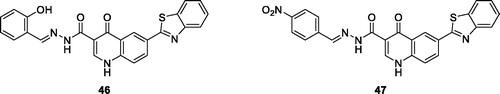
Abdelgawad et al. synthesised quinolone based benzothiazole derivatives by the treatment of substituted benzothiazoles with aromatic aldehydes. The nitrobenzylidene containing quinolone derivative 46 () and the hydroxybenzylidine containing derivative 47 () showed antitumor activities against the MCF-7 cell line, having IC50 values of 0.058 µM and 0.052 µM, respectivelyCitation49.
Figure 28. Hydroxybenzylidine containing quinolone benzothiazole derivative 46 and the nitrobenzylidene quinolone derivative 47.
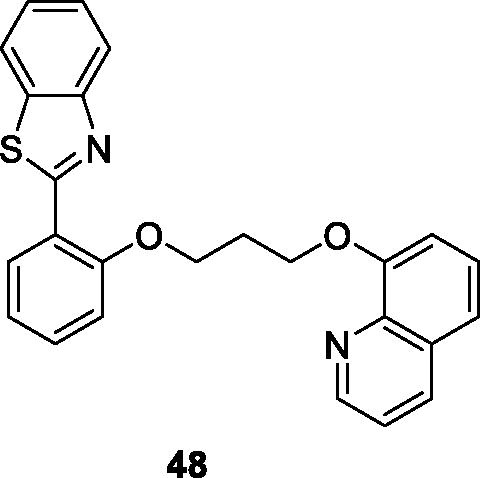
Sarkar et al. prepared benzothiazolyl quinoline derivatives by treatment of 2-aminobenzothiol with 2-hydroxy-benzaldehyde and studied their activities for the A1, A2A, A2B and A3 adenosine receptors. The A3 receptor is overexpressed in different cancer cell lines. The quinolone based derivative 48 () showed the maximum potency for the hA3 adenosine receptorCitation50.
2.15. Miscellaneous benzothiazole derivatives as anticancer agents
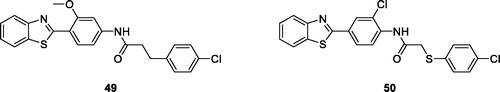
Tay et al. synthesised and evaluated N-[4-(benzothiazole-2yl) phenyl]-2-aryloxyacetamide derivatives for cytotoxicity and anticancer activity against sixty human cancer cell lines derived from 9 neoplastic diseases, among which L (leukaemia), M (melanoma), RC (renal cancer) NSCLC (non-small cell lung cancer) CC (colon cancer), OC (ovar ian cancer), BC (breast cancer), CNSC (central nervous system cancer) and PC (prostate cancer). Derivatives 49 (N-(4-(benzo[d]thiazol-2-yl)-3-methoxyphenyl)-3–(4-chlorophenyl)propanamide) and 50 (N-(4-(benzo[d]thiazol-2-yl)-2-chlorophenyl)-2–(4-chlorophenylthio)acetamide) () exhibited interesting anti-cancer activities. The SAR studies investigated that anticancer activities of these compounds were due to the substitutions (methoxy and chloro groups) present in their moleculesCitation51.
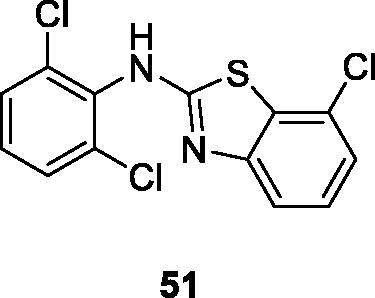
Noolvi et al. reported the synthesis of chloro substituted benzothiazole amines in order to produce isothiocyanates and thioureases. These BTA derivatives were tested for their anticancer activities. The dichlorophenyl containing chlorobenzothiazole 51 () showed good anticancer activity against 9 different cancer cell lines having GI50 values in the range of 1.60 µM–71.8 nM. The derivative 51 exhibited GI50 = 7.18 × 10−8 M against non-small cell lung cancer (HOP-92). The SAR studies showed that the highest activity of compound 51 was due to the presence of three chlorine atoms in the compound as compared to other derivativesCitation52.
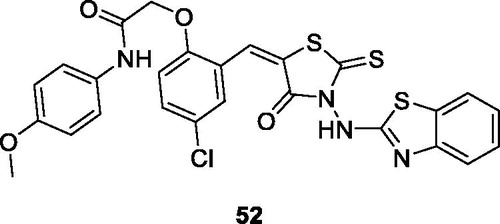
Havrylyuk et al. screened novel 4-thiazolidinone benzothiazole derivatives against ovarian, renal, prostate, leukaemia, melanoma, lung, colon, CNS and breast cancer cell lines. The thioxothiazolidine acetamide benzothiazole 52 () showed the most promising anti-cancer activity. The SAR studies pinpointed that the introduction of 4-chloro-phenoxy-N-(4-methoxyphenyl)-acetamide substitutions on position 5 of the 4-thiazolidinones ring enhanced the anti-cancer potentialCitation53.
Prabhu et al. synthesised and studied the anticancer activities of oxothiazolidine based BTA derivatives. The substituted chlorophenyl oxothiazolidine based benzothiazole 53 () showed the most effective anticancer activity against HeLa cell line, inducing 96.8% inhibition and IC50 value of 9.76 µM, when compared with the reference drug cisplatinCitation54.
Figure 33. Oxothiazolidine based benzothiazole derivative 53 and thiazolidine benzothiazole derivative 54.
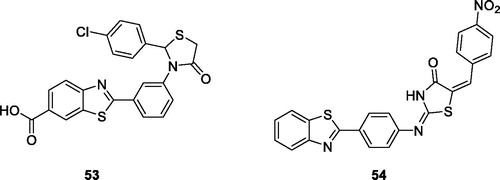
Abdelgawad et al. synthesised thiazolidinone containing benzoxazole derivatives by the treatment of aminobenzoic acid with substituted aniline that further reacted in different steps under specific conditions to afford final product and studied their anticancer activities against breast cancer (MCF7) and liver cancer (HEPG2) cell lines. Among all these derivatives, nitrobenzylidene containing thiazolidine derivative 54 () exhibited some anticancer activity, with an IC50 value of 36 nM and 48 nM against MCF7 and HEPG2, respectivelyCitation55.
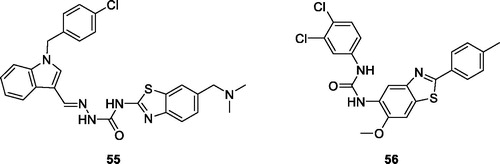
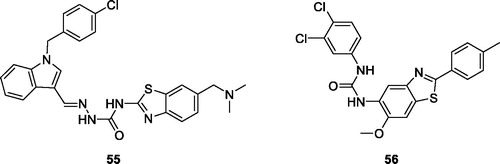
Ma et al. reported indole based BTA derivatives and studied their anticancer activities. Among all these derivatives, the chlorobenzyl indole semicarbazide benzothiazole 55 () exhibited anticancer activity against four cancer cell lines such as HT-29, H460, A549 and MDA-MB-231. Derivative 55 showed IC50 values of 0.024 µM for HT-29, 0.29 µM for H460, 0.84 µM for A549 and 0.88 µM for MDA-MB-231, respectivelyCitation56.
Xie et al. prepared substituted BTA derivatives and studied their anticancer activities. Among all derivatives, the urea benzothiazole 56 () exhibited interesting antitumor activity against 60 cancer cell lines. The average GI50 value for derivative 56 was 0.38 µMCitation57.
Uremis et al. synthesised BTA derivatives using substituted aldehyde with bicyclo[3.2.0]hept-2-en-6-one. The nitro-styryl containing benzothiazole derivative 57 and the fluorostyryl benzothiazole derivative 58 () were reported for their anticancer activity against pancreatic cancer cells having IC50 values of 27 ± 0.24 µM for 57 and of 35 ± 0.51 µM for derivative 58Citation58.
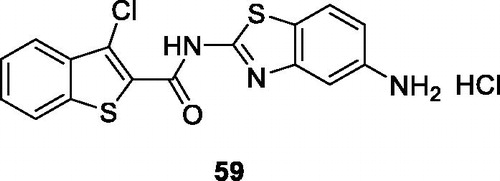
Cindric et al. obtained carboxamide containing benzothiazole by the condensation of substituted thiophenes and substituted amino-BTAs to prepare the desired product and studied their anticancer activity against MCF-7 cell line. The benzothiophene based carboxamide chloroaminobenzothiazole 59 () exhibited potent anticancer activity with an IC50 of 40 nMCitation59.
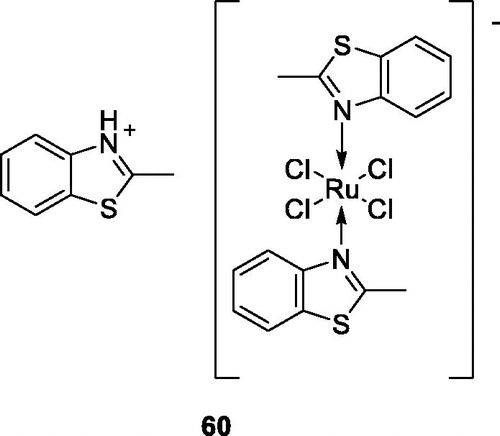
Nikolova et al. obtained Ru(III) complexes containing benzothiazole derivatives and tested their anticancer activity against K-562 and KE-37 (human leukemic) cell lines. Among all these derivatives, the Ru(III) containing methylbenzothiazole 60 () exhibited the highest cytotoxic activity against K-562 and KE-37, having IC50 values of 7.74 ± 2.50 for KE-37 and of 16.21 ± 2.33 for K-562 when compared with the standard drug cisplatinCitation60.
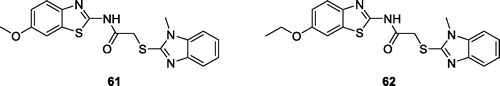
Yurttas et al. obtained benzothiazole derivatives by acetylation of substituted benzothiazole with chloroacetyl chloride that was further treated in series of reactions to obtain specific derivatives which were tested for their anticancer activities against lung carcinoma (A549) cell line. Derivatives 61 and derivative 62 () exhibited good anticancer activities against A549 with IC50 values of 10.67 ± 2.02 µg/mL and 9.0 ± 1.0 µg/mL respectively as compared to the reference compound cisplatinCitation61.
Figure 38. Benzimidazole based acetamide methoxybenzothiazole derivative 61 and acetamide ethoxybenzothiazole derivative 62.
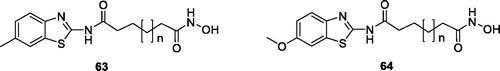
Oanh et al. reported the synthesis of hydroxamic acids containing benzothiazole by the reaction of 2-aminobenzothiazole with adipic acid to produce esters that were converted into the desired BTA hydroxamates, and tested for their anticancer activities against five different cell lines such MCF-7, AsPC-1, SW620, PC3 and NCI-H460. Among the synthesised derivatives, hydroxamic acids 63 and 64 () exhibited good anticancer activities, with the average IC50 value of 0.81 µg/mL and 1.28 µg/mL respectivelyCitation62.
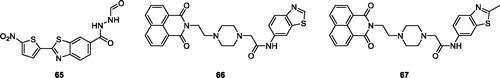
Rodrigues et al. synthesised carbohydrazide containing BTA derivatives and studied their antitumor activities against human prostate cancer cell lines. N′-formyl-2–(5-nitrothiophen-2-yl)benzothiazole-6-carbohydrazide 65 () exhibited potent anticancer activity against PC-3 and LNCaP having IC50 values 19.9 ± 1.17 and 11.2 ± 0.79 µg/m respectivelyCitation63.
Rao et al. reportyed DNA-intercalating naphthalimide-benzothiazole derivatives and evaluated their cytotoxicities against three different cancer cell lines such as HT29, A549 and MCF-7. Among all tested derivatives, the naphthalimide derivative 66 () possessed good antitumor activity against HT-29, A549 and MCF-7 cell lines having IC50 values of 3.72 ± 0.3 µM, 4.074 ± 0.3 µM and 07.91 ± 0.4 µM respectively. The naphthalimide 67 () showed antitumor activity with IC50 values of 03.47 ± 0.2 µM for HT-29, 03.89 ± 0.3 µM for A549 and 05.08 ± 0.3 µM for MCF-7 cell linesCitation64.
Rao et al. reported the synthesis of 2-arylaminobenzothiazole-arylpropeonones and studied their cytotoxic activities against different human cancer cell lines. Among all synthesised derivatives, substituted phenylamino based methoxybenzothiazole 68 () and substituted phenylamino based methoxy methylbenzothiazole 69 () showed potent cytotoxic activity against HeLa cell lines possessing IC50 values of 0.5 ± 0.02 and 0.6 ± 0.29 µMCitation65.
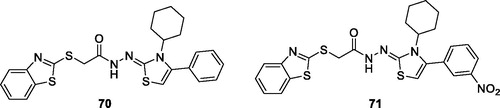
Osmaniye et al. synthesised benzothiazole-thiazolidine derivatives and studied their anticancer activities against C6 and healthy NIH3T3 cell lines. Among the synthesised derivatives, substituted phenylthizolidene based benzothiazole 70 and substituted nitrophenylthizolidene benzothiazole 71 () exhibited some cytotoxic activities against C6 cell line showing IC50 value 0.03 mM. The SAR studies showed that the presence of a phenyl group on the thiazolidine part of the structure increased the selectivity while substitution with an electron withdrawing or donating groups decreased the selectivityCitation66.
Benzothiazole derivatives with carbonic anhydrase inhibitory and antitumor action
Carbonic anhydrases (CAs, EC 4.2.1.1) are zinc enzymes which catalyse the reversible interconversion between CO2 and bicarbonateCitation67–71. CO2 is efficiently hydrated through a zinc hydroxide intermediate from the enzyme active site with generation of the weak base bicarbonate and the strong acid H+. As a consequence, CAs are involved in pH regulation, electrolyte secretion and metabolism, in normal and tumour tissuesCitation70–72. Fifteen α-CA isoforms are present in humans, with at least two of tyhem overexporessed in hypoxic tumours (CA IX and XII)Citation67,Citation71,Citation72, as a consequence of the hypoxia inducible factor (HIF-1α) transcription factor cascade activationCitation67,Citation70. CAs are efficiently inhibited by a range of compounds, such as the sulphonamides and their isosteresCitation73–76, and inorganic anionsCitation77, which constitute the main zinc-binding CA inhibitor (CAI) classesCitation67,Citation76,Citation77. Some of the CAIs belonging to the sulphonamideCitation78, sulfocoumarinCitation79, saccharinCitation80 or other structurally related chemotypesCitation81,Citation82, were shown to possess significant antitumor effects, with one such derivative (SLC-0111) in Phase I/IIb clinical trials for the management of hypoxic metastatic tumoursCitation78,Citation83. Indeed, by inhibiting the tumour-associated isoforms CA IX and XII, such CAIs interfere with the pH regulation and metabolism of tumours, leading to the inhibition of growth of the primary tumour, metastases and reducing the population of cancer stem cellsCitation78. As a consequence, many CAIs belonging to various classes are nowadays investigated for their antitumor/antimetastatic effects, including many BTA derivatives ()Citation84–91.
Figure 43. Benzothiazoles with potent CA IX inhibitory actionCitation84–91.
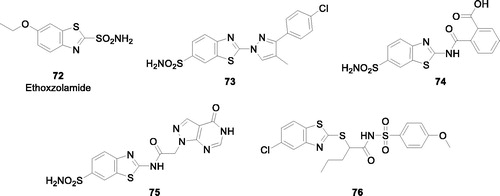
.Indeed, using the ethoxzolamide 72, a CAI in clinical use for decades as lead molecule, a multitude of primary sulphonamides (e.g. compounds 73–75) as well as the secondary siulfonamide 76 were reported to act as highly efficient, frequently low nanomolar inhibitors against the tumour-associated isoforms CA IX and XIICitation84–91. No ex vivo or in vivo studies are available so far with these potent CA IX/XII inhibitors, but compounds belonging to other classes of sulphonamides were proved to possess significant antitumor effects in vivo when they act as potent inhibitors of these two CA isoformsCitation78,Citation92–98. Thus future studes may address this issue, considering the fact that the BTA scaffold present in these compounds may induce interesting phisico-chemical and pharmacologic properties to the CA IX/XII inhibitors, of which many chemical families are alredy reportedCitation99–103.
4. Conclusions
Benzothiazole is a pharmacophore widely used in medicinal chemistry. This review points out to a growing interest in the development of lead or hybrid structures bearing the BTA moiety as antiproliferative and anticancer agents. The present work describes the potential of BTA scaffolds in the management of various types of cancers such as ovarian, prostate, central nervous system, renal, gastric, pancreatic, liver, breast and colon cancers. SAR studies reaveled that the anticancer activity of BTA scaffolds depends upon the nature of substituents present in these molecules, being multifactorial and not always easy to rationalise. The plethora of research on the anticancer profile of BTA derivatives mentioned in this review and their rationalisation based on the drug targets of these derivatives, when this was possible, may be useful for the development of novel such agents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Akhtar J, Khan AA, Ali Z. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem 2017;5:143–89.
- Keri SR, Patil RM, Patil AS, Budagumpi S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur J Med Chem 2015;89:207–51.
- Gunawardana GP, Koehn FE, Lee AY, et al. Pyridoacridine alkaloids from deep water marine sponges of the family Pachastrellidae: structure revision of dercitin and related compounds and correlation with the Kuanoniamines. J Org Chem 1992;57:1523–6.
- Jaiswal S, Mishra PA, Srivastava A. The different kinds of reaction involved in synthesis of 2-substituted 2enzothiazole and its derivatives: a review. Res J Pharm Biol Chem Sci 2012;3:631–41.
- Bryson M, Fulton B, Benfield P. Riluzole a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs 1996;52:549–63.
- Venkatesh P, Pandeya NS. Synthesis, characterisation and anti-inflammatory activity of some 2-amino benzothiazole derivatives. Int J ChemTech Res 2009;1:1354–8.
- Liu Y, Wang Y, Dong G, et.al. Novel benzothiazole derivatives with a broad antifungal spectrum: design, synthesis and structure–activity relationships. Med Chem Commun 2013;4:1551–61.
- Mats ME, Shani BG, Pasternak L, et al. Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives. J Med Chem 2013;56:5335–50.
- Bele DS, Singhvi I. Synthesis and analgesic activity of some Mannich bases of 6-substituted-2-aminobenzothiazole. Res J Pharm and Tech 2008;1:22–4.
- Padalkar SV, Gupta DV, Phatangare RK, et al. Synthesis of novel dipodal-benzimidazole, benzoxazole and benzothiazole from cyanuric chloride: structural, photophysical and antimicrobial studies. J Saudi Chem Soc 2014;18:262–8.
- Cai J, Sun M, Wu X, et al. Design and synthesis of novel 4-benzothiazole amino quinazolines Dasatinib derivatives as potential anti-tumor agents. Eur J Med Chem 2013;63:702–12.
- Delmas F, Avellaneda A, Giorgio DC, et al. Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives. Eur J Med Chem 2004;39:685–90.
- Munirajasekhar D, Himaja M, Sunil VM. Synthesis and anthelmintic activity of 2-amino-6- substituted benzothiazoles. Int Res J Pharm 2011;2:114–7.
- Yadav SP, Devprakash D, Senthilkumar GP. Benzothiazole: different methods of synthesis and diverse biological activities. Int J Pharm Sci Drug Res 2011;3:01–7.
- Siddiqui N, Rana A, Khan AS, et al. Synthesis of benzothiazole semicarbazones as novel anticonvulsants—the role of hydrophobic domain. Bioorg Med Chem Lett 2007;17:4178–82.
- Shi HX, Wang Z, Xia Y, et al. Synthesis and biological evaluation of novel benzothiazole-2-thiol derivatives as potential anticancer agents. Molecules 2012;17:3933–44.
- Leong CO, Suggitt M, Swaine DJ, et al. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles. Mol Cancer Ther 2004;3:1565–75.
- Mortimer CG, Wells G, Crochard JP, et al. Antitumor benzothiazoles. 26.1 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective Inhibitory activity against lung, colon, and breast cancer cell lines. J Med Chem 2006;49:179–85.
- Baffy G. Hepatocellular carcinoma in type 2 diabetes: more than meets the eye. Am J Gastroenterol 2012;107:53–5.
- McCutcheon M. Where have my eyebrows gone? Cengage learning. 2019. ISBN 07668393462001;5.
- Anand P, Kunnumakara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008;25:2097–116.
- Kumbhare MR, Dadmal T, Kosurkar U, et al. Synthesis and cytotoxic evaluation of thiourea and N-bis-benzothiazolederivatives: a novel class of cytotoxic agents. Bioorg Med Chem Lett 2012; 22:453–5.
- Kumbhare MR, Kumar VK, Ramaiah JM, et al. Synthesis and biological evaluation of novel Mannich bases of 2-arylimidazo[2,1- b]benzothi azoles as potential anti-cancer agents. Eur J Med Chem 2011;46:4258–66.
- El-Damasy KA, Lee HJ, Seo HS, et al. Design and synthesis of new potent anticancer benzothiazole amides and ureas featuring pyridylamide moiety and possessing dual B-RafV600E and C-Raf kinase inhibitory activities. Eur J Med Chem 2016;115:201–16.
- Ma J, Zhang G, Han X, et al. Synthesis and biological evaluation of benzothiazole derivatives bearing theortho hydroxy- N –acylhydrazone moiety as potent antitumor agents. Arch Pharm Chem Life Sci 2014;347:1–14.
- Gabr TM, El-Gohary SN, El-Bendary RE, et al. Synthesis, in vitro antitumor activity and molecular modeling studies 4 of a new series of benzothiazole Schiff bases. Chin Chem Lett 2016;27:380–6.
- Junjie AM, Gang UH, Lijun EIX, et al. Design, synthesis and biological evaluation of novel benzothiazole derivatives bearing semicarbazone moiety as antitumor agents. Chem Res Chin Univ 2015;31:958–63.
- Yurttas L, Tay F, Demirayak S. Synthesis and antitumor activity evaluation of new 2-(4-aminophenyl)benzothiazole derivatives bearing different heterocyclic rings. J Enzyme Inhib Med Chem 2014;30:458–65.
- Singh Y, Kaur B, Kaur A, et al. spectral studies and biological activity of 2, 3-disubstituted imidazo [2, 1-b] benzothiazole derivatives. Indian J Pharmaceut Biol Res 2018;6:1–8.
- Al-Soud AY, Al-Sa’doni HH, Saeed B, et al. Synthesis and in vitro anti-proliferative activity of new benzothiazole derivatives. ARKIVOC 2008;2008:225–238.
- Gurdal EE, Durmaz I, Atalay CR, Yarim M. Cytotoxic activities of some benzothiazole-piperazine derivatives. J Enzyme Inhib Med Chem 2015;30:649–54.
- Akhtar T, Hameed S, Al-Masoudi AN, et al. In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta Pharm 2008;58:135–49.
- Saeed S, Rashid N, Jones GP, et al. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur J Med Chem 2010;45:1323–31.
- Lei Q, Zhang L, Xia Y, et al. A novel benzothiazole derivative SKLB826 inhibits human hepatocellular carcinoma growth via inducing G2/M phase arrest and apoptosis. RSC Adv 2015;5:41341.
- Racane L, Kulenovic TV, Pavelic KS, et al. Novel diamidino-substituted derivatives of phenyl benzothiazolyl and dibenzothia zolyl furans and thiophenes: synthesis, anti-proliferative and DNA binding properties. J Med Chem 2010;53:2418–32.
- Sekar V, Perumal P, Arunachalam , Gandhimathi S. Screening of anticancer activity in newly synthesized benzothiazole derivatives. J Pharm Sci Res 2011;3:1520–4.
- Xuejiao S, Yong X, Ningyu W, et al. A novel benzothiazole derivative YLT322 induces apoptosis via the mitochondrial apoptosis pathway in vitro with anti-tumor activity in solid malignancies. PLoS One 2013;8:e63900.
- Kamal A, Ashraf M, Vardhan VSP, et al. Synthesis and anticancer potential of benzothiazole linked phenylpyridopyrimidinones and their diones as mitochondrial apoptotic inducers. Bioorg Med Chem Lett 2014;24:147–51.
- Gabr TM, El-Gohary SN, El-Bendary RE, El-Kerdawy MM. Synthesis and in vitro antitumor activity of new series ofbenzothiazole and pyrimido[2,1-b]benzothiazole derivatives. Euro J Med Chem 2014;85:576–92.
- Kumbhare MR, Dadmal LT, Devi AT, et al. Isoxazole derivatives of 6-fluoro-N-(6-methoxybenzo[d]thiazol-2-yl)benzo[d]thiazol-2-amine and N-(pyrimidin-2-yl)benzo[d]thiazol-2-amine: regulation of cell cycle and apoptosis by p53 activation via mitochondrial-dependent pathways. Med Chem Commun 2014;5:1744.
- Waghmare SG, Chidrawar BA, Bhosale NV, et al. Synthesis and in-vitro anticancer activity of 3-cyano-6,9-dimethyl-4-imino 2-methylthio 4H-pyrimido [2,1-b] [1,3] benzothiazole and its 2-substituted derivative. J Pharm Res 2013;7:823–7.
- Caleta I, Kralj M, Marjanovic M, et al. Novel cyano- and amidinobenzothiazole derivatives: synthesis, antitumor evaluation, and X-ray and quantitative structure-activity relationship (QSAR) analysis. J Med Chem 2009;52:1744–56.
- Osmaniye D, Levent S, Karaduman BA, et al. Synthesis of new benzothiazole acylhydrazones as anticancer agents. Molecules 2018;23:1054.
- Lad NP, Manohar Y, Mascarenhas M, et al. Methylsulfonyl benzothiazoles (MSBT) derivatives: search for new potential antimicrobial and anticancer agents. Bioorg Med Chem Lett 2017; 27:1319–24.
- Sadhasivam G, Kulanthai K, Natarajan A. Synthesis and anti-cancer Studies of 2, 6-disubstituted benzothiazole derivatives. Orient J Chem 2015;31:819–26.
- Wang Z, Shi HX, Wang J, et al. Synthesis, structure–activity relationships and preliminary antitumor evaluation of benzothiazole-2-thiol derivatives as novel apoptosis inducers. Bioorg Med Chem Lett 2011;21:1097–101.
- Bolelli k, Musdal Y, Aki-Yalcin E, et al. Synthesis and activity mechanism of some novel 2-substituted benzothiazoles as hGSTP1-1 enzyme inhibitors. SAR QSAR Environ Res 2017;28:927–40.
- Corbo F, Carocci A, Armenise D, et al. Antiproliferative activity evaluation of a series of N-1,3-benzothiazol-2-ylbenzamides as novel apoptosis inducers. J Chem 2016;5:4267564.
- Abdelgawad AM, Lamie FP, Ahmed MO. Synthesis of new quinolone derivatives linked to benzothiazole or benzoxazole moieties as anticancer and anti-oxidant agents. Med Chem 2016;6: 31–9.
- Sarkar B, Maiti S, Jadhav RG, Paira P. Benzothiazolylquinoline conjugates as novel human A3 receptor antagonists: biological evaluations and molecular docking studies. R Soc Open Sci 2018;5:171622.
- Tay F, Yurttaş L, Demirayak Ş. Synthesis of some N-[4-(benzothiazole-2yl) phenyl]-2-aryloxyacetamide derivatives and their anticancer activities. J Enzyme Inhib Med Chem 2012;27:515–20.
- Noolvi NM, Patel MH, Kaur M. Benzothiazoles: search for anticancer agents. Eur J Med Chem 2012;54:447–62.
- Havrylyuk D, Mosula L, Zimenkovsky B, et al. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur J Med Chem 2010;45:5012–21.
- Prabhu PP, Panneerselvam T, Shastry SC, et al. Synthesis and anticancer evaluation of 2-phenyl thiaolidinone substituted 2-phenyl benzothiazole-6-carboxylic acid derivatives. J Saudi Chem Soc 2015;19:181–5.
- Abdelgawad AM, Belal A, Ahmed MO. Synthesis, molecular docking studies and cytotoxic screening of certain novel thiazolidinone derivatives substituted with benzothiazole or benzoxazole. J Chem. pharm Sci 2013;5:318–27.
- Ma J, Bao G, Wang L, et al. Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur J Med Chem 2015;96:173–86.
- Xie X, Yan Y, Zhu N, Liu G. Benzothiazoles exhibit broad-spectrum antitumor activity: their potency, structure-activity and structure-metabolism relationships. Eur J Med Chem 2014;76:67–78.
- Uremis N, Uremis MM, Tolun IF, et al. Synthesis of 2-substituted benzothiazole derivatives and their in vitro anticancer effects and antioxidant activities against pancreatic cancer cells. Anticancer Res 2017;37:6381–9.
- Cindric M, Peric M, Kralj M, et al. Antibacterial and antiproliferative activity of novel 2-benzimidazolyland 2-benzothiazolyl-substituted benzo[b]thieno-2-carboxamides. Mol Divers 2018;22:637–46.
- Nikolova Momekov G, Bakalova A, et al. Novel Ru(III) complexes with some benzothiazole derivatives: synthesis, physicochemical and pharmacological investigations. Drug Res 2015;65:317–22.
- Yurttas L, Cavusoglu KB, Sever A, Ciftci AG. A preliminary investigation of anticanceractivity of novel benzothiazole derivatives against A549 lung carcinoma cell line. Turk J Biochem 2017;42: 217–24.
- Oanh KTD, Hai VH, Park HS, et al. Benzothiazole-containing hydroxamic acids as histone deacetylase inhibitors and antitumor agents. Bioorg Med Chem Lett 2011;21:7509–12.
- Rodrigues RJ, Charris J, Camacho J, et al. N-Formyl-2-(5-nitrothiophen-2-yl)benzothiazole-6-carbohydrazide as a potential anti-tumour agent for prostate cancer in experimental studies. J Pharm Pharmacol 2013;65:411–22.
- Rao NS, Nagesh N, Nayak LV, et al. Design and synthesis of DNA-intercalative naphthalimide- benzothiazole/cinnamide derivatives: cytotoxicity evaluation and topoisomerase-IIα inhibition. Med Chem Comm 2019;10:72–9.
- Rao SVA, Rao BB, Sunkari S, et al. 2-Arylaminobenzothiazole-arylpropenone conjugates as tubulin polymerization inhibitors. Med Chem Commun 2017;8:924–41.
- Osmaniye D, Levent S, Ardıc MC, et al. Synthesis and anticancer activity of some novel benzothiazolethiazolidine derivatives. Phosphorus Sulfur 2018;193:249–56.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:168–81.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:pii:E25.
- Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:pii:E48.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12.
- Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21.
- Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97.
- De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70.
- Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300.
- Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–9.
- Supuran CT, Ilies MA, Scozzafava A. Carbonic anhydrase inhibitors. Part 29. Interaction of isozymes I, II and IV with benzolamide-like derivatives. Eur J Med Chem 1998;33:739–52.
- Sentürk M, Gülçin I, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: a patent review (2008–2018). Expert Opin Ther Pat 2018;28:729–40.
- Di Fiore A, Pedone C, Antel J, et al. Carbonic anhydrase inhibitors: the X-ray crystal structure of ethoxzolamide complexed to human isoform II reveals the importance of thr200 and gln92 for obtaining tight-binding inhibitors. Bioorg Med Chem Lett 2008;18:2669–74.
- SitaRam CM, Khloya P, et al. 4-Functionalized 1,3-diarylpyrazoles bearing 6-aminosulfonylbenzothiazole moiety as potent inhibitors of carbonic anhydrase isoforms hCA I, II, IX and XII. Bioorg Med Chem 2014;22:6945–52.
- Ibrahim DA, Lasheen DS, Zaky MY, et al. Design and synthesis of benzothiazole-6-sulfonamides acting as highly potent inhibitors of carbonic anhydrase isoforms I, II, IX and XII. Bioorg Med Chem 2015;23:4989–99.
- Küçükbay FZ, Buğday N, Küçükbay H, et al. Synthesis, characterization and carbonic anhydrase inhibitory activity of novel benzothiazole derivatives. J Enzyme Inhib Med Chem 2016;31:1221–5.
- Petrou A, Geronikaki A, Terzi E, et al. Inhibition of carbonic anhydrase isoforms I, II, IX and XII with secondary sulfonamides incorporating benzothiazole scaffolds. J Enzyme Inhib Med Chem 2016;31:1306–11.
- Payaz DÜ, Küçükbay FZ, Küçükbay H, et al. Synthesis carbonic anhydrase enzyme inhibition and antioxidant activity of novel benzothiazole derivatives incorporating glycine, methionine, alanine, and phenylalanine moieties. J Enzyme Inhib Med Chem 2019;34:343–9.
- Ammazzalorso A, Carradori S, Angeli A, et al. Fibrate-based N-acylsulphonamides targeting carbonic anhydrases: synthesis, biochemical evaluation, and docking studies. J Enzyme Inhib Med Chem 2019;34:1051–61.
- Abdoli M, Angeli A, Bozdag M, et al. Synthesis and carbonic anhydrase I, II, VII, and IX inhibition studies with a series of benzo[d]thiazole-5- and 6-sulfonamides. J Enzyme Inhib Med Chem 2017;32:1071–8.
- Alterio V, Esposito D, Monti SM, et al. Crystal structure of the human carbonic anhydrase II adduct with 1-(4-sulfamoylphenyl-ethyl)-2,4,6-triphenylpyridinium perchlorate, a membrane-impermeant, isoform selective inhibitor. J Enzyme Inhib Med Chem 2018;33:151–7.
- Supuran CT. Carbon-versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018;33:485–95.
- Pustenko A, Stepanovs D, Žalubovskis R, et al. 3H-1,2-benzoxathiepine 2,2-dioxides: a new class of isoform-selective carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2017;32:767–75.
- Ramya PVS, Angapelly S, Angeli A, et al. Discovery of curcumin inspired sulfonamide derivatives as a new class of carbonic anhydrase isoforms I, II, IX, and XII inhibitors. J Enzyme Inhib Med Chem 2017;32:1274–81.
- Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1,3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhib Med Chem 2018;33:1575–80.
- El-Gazzar MG, Nafie NH, Nocentini A, et al. Carbonic anhydrase inhibition with a series of novel benzenesulfonamide-triazole conjugates. J Enzyme Inhib Med Chem 2018;33:1565–74.
- D'Ascenzio M, Guglielmi P, Carradori S, et al. Open saccharin-based secondary sulfonamides as potent and selective inhibitors of cancer-related carbonic anhydrase IX and XII isoforms. J Enzyme Inhib Med Chem 2017;32:51–9.
- Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: perfluoroalkyl/aryl-substituted derivatives of aromatic/heterocyclic sulfonamides as topical intraocular pressure-lowering agents with prolonged duration of action. J Med Chem 2000;43:4542–51.
- Scozzafava A, Briganti F, Mincione G, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–700.
- Supuran CT, Clare BW. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50.
- Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8.
- Sarikaya SBÖ, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.