Abstract
A series of compounds incorporating 3-(3-(2/3/4-substituted phenyl)triaz-1-en-1-yl) benzenesulfonamide moieties were synthesised and their chemical structure was confirmed by physico-chemical methods. Carbonic anhydrase (CA, EC 4.2.1.1) inhibitory effects of the compounds were evaluated against human isoforms hCA I and II. KI values of these sulphonamides were in the range of 21 ± 4–72 ± 2 nM towards hCA I and in the range of 16 ± 6–40 ± 2 nM against hCA II. The 4-fluoro substituted derivative might be considered as an interesting lead due to its effective inhibitory action against both hCA I and hCA II (KIs of 21 nM), a profile rarely seen among other sulphonamide CA inhibitors, making it of interest in systems where the activity of the two cytosolic isoforms is dysregulated.
1. Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are a superfamily of metalloenzymes that catalyse the interconversion reaction between carbon dioxide and bicarbonateCitation1,Citation2. CAs catalyse this reaction by using a metal hydroxide nucleophilic mechanismCitation3–5. The metal ions present within the active site of these enzymes (Zn(II), Cd(II), Co(II), Fe(II) or Mn(II) for the ι-CAs) are coordinated by three amino acid residues and a water molecule which is activated by the metal ion and the hydrophobic environment, becoming highly nucleophilicCitation3–5. This metal hydroxide species nucleophilically attacks CO2 and promotes its hydration to bicarbonate very efficiently at almost a neutral pHCitation3–5. There are eight genetically distinct CA families, namely α-, β-, γ-, δ-, ζ- η-, θ-, and ι-CAsCitation3,Citation4,Citation6,Citation7. In humans, there are 12 catalytically active α-CA isoforms which have different catalytic activitiesCitation6. These enzymes are distributed in many organs and tissues and they play important roles in many physiological and pathological processes such as acid-base regulation, biosynthetic reactions, electrolyte secretion, and calcification. Thus, human carbonic anhydrases (hCAs) are targets for the design of new drugs to use in the diagnosis and/or treatment of many diseases. For example, hCA II, IV, XII, and XIV inhibitors are used as diuretics, whereas hCA II, IV, and XII inhibitors are used as anti-glaucoma drugsCitation1,Citation2,Citation8,Citation9. hCA I plays a role in the regulation of retinal pathologic processes, and its inhibition is a strategy for the treatment of such conditions. hCA II inhibitors are used for the treatment of glaucoma, oedema, and epilepsyCitation5,Citation8. The cytosolic, highly abundant isoforms hCA I and II are in fact both drug targets for a multitude of diseases, as highlighted above, but due to their role in pH homeostasis and wide distribution in many tissues, are also frequently off-targets, when other isoforms (e.g. CA VA/B, VII, IX or XII) should be selectively inhibitedCitation7,Citation9. Although CA inhibitors (CAIs) have been often used in clinics, the first and second-generation drugs have several undesired side effects because of their low selectivities for various isozymes with medicinal chemistry applicationsCitation5,Citation8,Citation10,Citation11. Therefore, there is a need for compounds with higher selectivity to diverse hCAs compared to the available drugs used clinically nowadays. Compounds containing sulphonamide group (R-SO2NH2) and its isosteres sulfamide (R-NH-SO2NH2) and sulfamate (R-OSO2NH2) are among the most important classes of CAIsCitation12. Some of these derivatives have been used as drugs for many yearsCitation13. More recently, a compound bearing an ureido-substituted aryl-sulphonamide (SLC-0111) () was reported to show remarkable CA inhibitory effects and is presently in Phase Ib/II clinical trials as an antitumor/antimetastatic agentCitation14,Citation15.
Triazenes are an interesting group of compounds which has many applications in the synthesis of various products, some of which possess pharmacological applicationsCitation16,Citation17. Dacarbazine and temozolomide (), both incorporating a triazene moiety, are in the clinical use for cancer treatment, as they possess acceptable toxicity and good pharmacokinetic propertiesCitation17,Citation18. Additionally, the triazene group is an isostere of the ureido group and was recently reported to lead to interesting CA inhibitory derivatives by Akocak et al.Citation19.
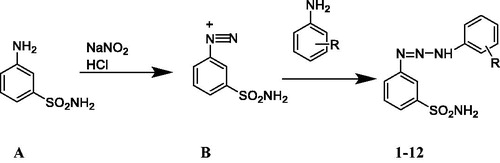
Some compounds bearing triazene substituted sulphanilamide or metanilamide (3-aminobenzene sulphonamide) were recently reported to possess CA inhibitory effects by one of our groupsCitation13,Citation20, but there are few studies on this class of CA inhibitors. In this study, we synthesised new 1,3-diaryltriazene sulphonamide compoundsCitation1–12 from the diazonium salt of metanilamide and different substituted aromatic amines and evaluated their inhibition profiles towards hCA I and II, in order to find out new drug candidates.
2. Experimental
2.1. Chemistry
All chemicals and solvents were purchased from Sigma-Aldrich and Merck. The nuclear magnetic resonance (NMR) spectra (1H NMR, 13 C NMR) were recorded on a Bruker AVANCE III 400 MHz (Bruker, Karlsruhe, Germany) spectrometer [400 MHz (1H) and 100 MHz (13 C)] in DMSO-d6. Chemical shifts are given as δ values in ppm. Tetramethylsilane was used as the internal standard and J values were expressed in Hz. Mass spectra of the compounds were recorded using a liquid chromatography ion trap-time of flight tandem mass spectrometer (Shimadzu, Kyoto, Japan) equipped with an electrospray ionisation (ESI) source, operating in both positive and negative ionisation mode. Shimadzu’s LCMS Solution software was used for data analysis. Melting points were determined using an Electrothermal 9100 instrument (IA9100, Bibby Scientific Limited, Staffordshire, UK) and are uncorrected. Reactions were monitored by Thin Layer Chromatography (TLC) [Silicagel 60 HF254 (Merck KGaA)].
2.1.1. Synthesis of 1,3-diaryltriazene sulphonamide derivatives
To the solution of 3-aminobenzene sulphonamide (5 mmol) in water (3 ml), concanrate HCl (1.5 ml) was added then the mixture was cooled to 0–5 °C and stirred for 5 min. To this mixture, sodium nitrite (7 mmol) in water (3 ml) was added dropwise during about 10–15 min at 0–5 °C. This mixture was stirred about 15–20 min at 0–5 °C. Then, this mixture (diazonium solution) was added to a suitable aniline (5 mmol) solution (in 5 ml methanol) by adjusting the pH between 6 and 7 with the simultaneous addition of saturated sodium acetate in water. The reaction mixture was stirred at 0–5 °C for 3 h and then overnight at room temperature in darkCitation13. The precipitated solid product was collected by filtration and washed several times with cold water. The crude compounds were air-dried then purified by crystallization from methanol. The chemical structures of the compoundsCitation1–12 were characterised by 1H NMR, 13 C NMR, and HRMS.
2.1.1.1. 3–(3-Pheynltriaz-1-en-1-yl)benzenesulfonamide (1)
Yield 72.3%. Mp: 146–147 °C. 1H NMR (DMSO-d6) δ (ppm) 12.71 (s, NH, 1H), 8.2 (s, 1H, Ar-H), 7.98-7.85 (m, 2H, Ar-H), 7.70 (t, 1H, J = 7.7 Hz, Ar-H), 7.58-7.50 (m, 2H, Ar-H), 7.49 (s, 2H, –SO2NH2), 7.20-6.99 (m, 1H, Ar-H), 6.71 (d, 1H, J = 7.6 Hz, Ar-H), 6.57 (d, 1H, J = 7.6 Hz, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 154.1, 145.8, 143.1, 130.5, 129.8, 129.3, 126.2, 117.8, 114.5, 113.9. HRMS (ESI-MS) m/z calculated [M + H]+ 277.0681; measured 277.06718.
2.1.1.2. 3-(3-(4-Fluorophenyl)triaz-1-en-1-yl) benzenesulfonamide (2)
Yield 47.2%. Mp: 162–163 °C. 1H NMR (DMSO-d6) δ (ppm) 12.75 (s, NH, 1H), 7.80 (s, 1H, Ar-H), 7.74-7.68 (m, 1H, Ar-H), 7.65-7.59 (m, 1H, Ar-H), 7.56-7.44 (m, 3H, Ar-H), 7.43 (s, 2H, –SO2NH2), 7.32-6.24 (m, 2H, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 146.2, 145.4, 130.0, 122.7, 119.1, 117.2, 116.2, 115.9, 110.85, 110.83. HRMS (ESI-MS) m/z calculated [M + H]+ 295.05867; measured 295.05786.
2.1.1.3. 3-(3-(4-Bromophenyl)triaz-1-en-1-yl) benzenesulfonamide (3)
Yield 53.0%. Mp: 159–160 °C. 1H NMR (DMSO-d6) δ (ppm) 7.89 (d, 1H, J = 1.86 Hz, Ar-H), 7.60-7.59 (m, 1H, Ar-H), 7.58 (d, 1H, J = 7.0 Hz, Ar-H), 7.56-7.55 (m, 3H, Ar-H), 7.54 (s, 2H, –SO2NH2), 7.42 (dd, 1H, J1 = 7.0 Hz, J2= 1.86 Hz, Ar-H), 7.38-7.36 (m, 1H, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 174.7, 146.5, 145.8, 134.2, 132.6, 130.5, 121.4, 120.1, 117.6, 114.4. HRMS (ESI-MS) m/z calculated [M + H]+ 354.97861; measured 354.97882.
2.1.1.4. 3-(3-(4-Ethoxyphenyl)triaz-1-en-1-yl) benzenesulfonamide (4)
Yield 42.7%. Mp: 152–154 °C. 1H NMR (DMSO-d6) δ (ppm) 12.45 (s, NH, 1H), 7.79 (s, 1H, Ar-H), 7.52 (d, 2H, J = 7.8 Hz, Ar-H), 7.45-7.43 (m, 3H, Ar-H), 7.00 (d, 2H, J = 7.8 Hz, Ar-H), 4.07-4.05 (m, 2H, –CH2–), 1.35 (t, 3H, J = 6.5 Hz, –CH3).13C NMR (DMSO-d6) δ (ppm) 158.6, 145.8, 143.4, 142.9, 130.5, 122.7, 119.0, 117.2, 115.4, 110.9, 63.8, 15.1. HRMS (ESI-MS) m/z calculated [M + H]+ 321.09431; measured 321.09354.
2.1.1.5. 3-(3-(4-Methoxyphenyl)triaz-1-en-1-yl) benzenesulfonamide (5)
Yield 40.0%. Mp: 133–134 °C. 1H NMR (DMSO-d6) δ (ppm) 12.46 (s, NH, 1H), 7.80 (s, 1H, Ar-H), 7.54 (d, 2H, J = 8.2 Hz, Ar-H), 7.53-7.43 (m, 3H, Ar-H), 7.42 (s, 2H, –SO2NH2), 7.02 (d, 2H, J = 8.2 Hz), 3.80 (s, 3H, –OCH3). 13 C NMR (DMSO-d6) δ (ppm) 159.3, 145.8, 143.6, 142.9, 130.5, 122.7, 119.0, 117.2, 114.9, 110.9, 55.8. HRMS (ESI-MS) m/z calculated [M + H]+ 307.07866; measured 307.07784.
2.1.1.6. 3-(3-(4-Ethylphenyl)triaz-1-en-1-yl) benzenesulfonamide (6)
Yield 19.3%. Mp: 151 °C. 1H NMR (DMSO-d6) δ (ppm) 12.66 (s, NH, 1H), 7.92 (s, 1H, Ar-H), 7.66 (d, 1H, J = 4.8 Hz, Ar-H), 7.59 (d, 1H, J = 7.7 Hz, Ar-H), 7.52-7.41 (m, 3H, Ar-H), 7.40 (s, 2H, -SO2NH2), 7.27 (d, 1H, J = 7.4 Hz, Ar-H), 7.19 (d, 1H, J = 7.7 Hz, Ar-H), 2.59 (q, 2H, J = 7.5 Hz, –CH2), 1.19 (t, 3H, J = 7.5 Hz, –CH3).13C NMR (DMSO-d6) δ (ppm) 148.1, 145.9, 143.5, 130.7, 129.3, 124.9, 121.6, 119.5, 117.6, 111.2, 28.5, 16.4. HRMS (ESI-MS) m/z calculated [M + H]+ 305.0994; measured 305.0985.
2.1.1.7. 3-(3-(3-Chlorophenyl)triaz-1-en-1-yl) benzenesulfonamide (7)
Yield 63.3%. Mp: 136–137 °C. 1H NMR (DMSO-d6) δ (ppm) 12.86 (d, NH, 1H, J = 23.1 Hz), 8.17 (d, 1H, J = 1.7 Hz, Ar-H), 8.00-7.83 (m, 1H, Ar-H), 7.80-7.70 (m, 1H, Ar-H), 7.62-7.47 (m, 2H, Ar-H), 7.49 (s, 2H, –SO2NH2), 6.80 (d, 1H, J = 2.3 Hz, Ar-H), 6.62 (dd, 1H, J1 = 8.9 Hz, J2= 2.3 Hz, Ar-H), 6.57 (s, 1H, Ar-H).13C NMR (DMSO-d6) δ (ppm) 154.8, 145.8, 134.4, 131.5, 130.6, 127.4, 124.4, 122.8, 120.7, 117.9, 113.5, 111.5. HRMS (ESI-MS) m/z calculated [M + H]+ 311.02912; measured 311.02855.
2.1.1.8. 3-(3-(3-Fluorophenyl)triaz-1-en-1-yl) benzenesulfonamide (8)
Yield 35.7%. Mp: 154–156 °C. 1H NMR (DMSO-d6) δ (ppm) 12.87 (d, NH, 1H, J = 16.7 Hz), 7.99 (s, 1H, Ar-H), 7.84 (s, 1H, Ar-H), 7.78-7.73 (m, 1H, Ar-H), 7.66-7.62 (m, 1H, Ar-H), 7.54-7.41 (m, 2H, Ar-H), 7.21-7.13 (m, 1H, Ar-H), 6.90-6.86 (m, 1H, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 151.9, 150.4, 145.7, 131.6, 130.5, 125.1, 124.4, 120.0, 118.4, 117.8, 111.5, 111.0. HRMS (ESI-MS) m/z calculated [M + H]+ 295.05867; measured 295.05832.
2.1.1.9. 3-(3-(3-Methoxyphenyl)triaz-1-en-1-yl) benzenesulfonamide (9)
Yield 43%. Mp: 201 °C. 1H NMR (DMSO-d6) δ (ppm) 8.11 (s, 1H, Ar-H), 7.92 (d, 1H, J = 7.6 Hz, Ar-H), 7.82 (d, 1H, J = 7.2 Hz, Ar-H), 7.71-7.54 (m, 2H, Ar-H), 7.49 (s, 2H, –SO2NH2), 6.40-6.26 (m, 3H, Ar-H), 3.89 (s, 3H, –OCH3). 13 C NMR (DMSO-d6) δ (ppm) 160.1, 155.6, 153.0, 145.1, 132.5, 129.9, 126.1, 125.2, 117.9, 117.1, 106.9, 95.9, 55.4. HRMS (ESI-MS) m/z calculated [M + H]+ 307.07866; measured 307.07828.
2.1.1.10. 3-(3-(2-Chlorophenyl)triaz-1-en-1-yl) benzenesulfonamide (10)
Yield 48.4%. Mp: 149–150 °C. 1H NMR (DMSO-d6) δ (ppm) 13.12 (s, NH, 1H), 7.89 (s, 1H, Ar-H), 7.68 (d, 1H, J = 8.0 Hz, Ar-H), 7.58-7.42 (m, 3H, Ar-H), 7.46 (s, 2H, –SO2NH2), 7.45-7.39 (m, 2H, Ar-H), 7.34-7.27 (m, 1H, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 145.9, 142.5, 130.74, 130.73, 130.6, 129.4, 128.8, 128.4, 120.2, 119.7, 118.0, 111.6. HRMS (ESI-MS) m/z calculated [M + H]+ 311.02912; measured 311.02941.
2.1.1.11. 3-(3-(2-Fluorophenyl)triaz-1-en-1-yl) benzenesulfonamide (11)
Yield 34.2%. Mp: 167–168 °C. 1H NMR (DMSO-d6) δ (ppm) 13.06 (s, NH, 1H), 7.82 (s, 1H, Ar-H), 7.72-7.67 (m, 1H, Ar-H), 7.56-7.46 (m, 3H, Ar-H), 7.43 (s, 2H, –SO2NH2), 7.33-7.31 (m, 2H, Ar-H), 7.28-7.25 (m, 1H, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 145.9, 142.3, 130.7, 129.1, 129.0, 125.4, 120.0, 119.7, 117.8, 117.3, 117.1, 111.4. HRMS (ESI-MS) m/z calculated [M + H]+ 295.05867; measured 295.05799.
2.1.1.12. 3-(3-(2-Bromophenyl)triaz-1-en-1-yl) benzenesulfonamide (12)
Yield 13%. Mp: 157 °C. 1H NMR (DMSO-d6) δ (ppm) 13.08 (s, NH, 1H), 7.87 (s, 1H, Ar-H), 7.72 (d, 1H, J = 7.9 Hz, Ar-H), 7.63 (d, 1H, J = 7.9 Hz, Ar-H), 7.54-7.45 (m, 4H, Ar-H), 7.44 (s, 2H, –SO2NH2), 7.25 (d, 1H, J = 7.4 Hz, Ar-H). 13 C NMR (DMSO-d6) δ (ppm) 147.7, 145.9, 142.3, 133.8, 130.6, 129.2, 129.0, 120.2, 119.9, 119.8, 118.0, 111.6. HRMS (ESI-MS) m/z calculated [M + H]+ 354.97861; measured 354.97882.
2.2. Carbonic anhydrase inhibition assay
CA inhibition assay was done as described in our previous studies by using an esterase assay with 4- nitrophenyl acetate as standardCitation21–31. The enzymes were purified from human blood as described earlierCitation27,Citation28.
3. Discussion
3.1. Chemistry
Compounds 1–12, 3-(3-(2/3/4-substituted phenyl)triaz-1-en-1-yl) benzenesulfonamide, were synthesised and purified successfully for the first time (except 2 and 5 reported eralierCitation13) as shown in Scheme 1. The diazonium salt obtained from the 3-aminobenzenesulfonamide A was reacted with sodium nitrite (in the presence of a strong acid) generating the diazonium salts B, which were treated with the suitable aniline derivative, leading to triazenes 1–12. The anilines used were: unsubstituted aniline (1), 4-fluoroaniline (2), 4-bromoaniline (3), 4-ethoxyaniline (4), 4-methoxyaniline (5), 4-ethylaniline (6), 3-chloroaniline (7), 3-fluoroaniline (8), 3-methoxyaniline (9), 2-chloroaniline (10), 2-fluoroaniline (11), and 2-bromoaniline (12) in the series. Compound 1, the non-substituted derivative, was synthesised with the highest yield (% 72.3) whereas the 2-bromo substituted derivative compound 12 was synthesised with the lowest yield (% 13) in the series.
The chemical structures of the compounds 1–12 were characterised by 1H NMR, 13 C NMR, and HRMS (see the experimental part for details).
3.2. Carbonic anhydrase inhibitory effects
CA inhibition with compounds 1–12 on hCA I and hCA II are shown in . Acetazolamide (AZA) was used as a reference drug. According to , KI values (inhibitory potency) of the compounds 1–12 were in the range of 21 ± 4–72 ± 2 nM towards hCA I while they were in the range of 16 ± 6–40 ± 2 nM towards hCA II. KI values of AZA were 19 ± 2 nM and 17 ± 4 nM towards hCA I and hCA II, respectively (by an esterase method, using 4-nitrophenyl acetate as substrate).
Table 1. Inhibitory effects of the compounds 1–12 on hCA I and II isoenzymes by an esterase, 4-nitrophenyl acetate assay.
It can be seen from the results in that all compounds in the series had a higher KI compared to AZA towards hCA I. According to the , 4-fluoro substituted derivative compound 2 and non-substituted derivative compound 1, had the lowest KI values (21 ± 4 nM and 28 ± 6 nM, respectively) in the series towards hCA I. The 4-fluoro substituted derivative compound 2 had the most effective inhibitory activity with a similar KI value (21 ± 4 nM) with AZA against hCA I.
According to the KI values of the compounds against hCA II, the most active compounds in the series were the 4-ethoxy substituted derivative compound 4 and the 4-ethyl substituted derivative compound 6 with their lower KI values than AZA (16 ± 6 nM and 16 ± 2 nM, respectively). On the other hand, the non-substituted derivative 1 and the 4-bromo substituted derivative 3 had similar KI values, comparable to those of the reference drug, AZA (18 ± 5 nM and 19 ± 4 nM, respectively). When the KI values towards hCA II in are evaluated, it was observed that substitution at the para position of the phenyl ring leads to more effective inhibitors compared to the ortho or meta substitutions. Probably, this is due to the steric impairments in which the latter two substitution patterns participate within the costrained active site of the enzyme, as compared to the less sterically impaired para substitution, as observed for many other types of CAIsCitation32–41.
4. Conclusions
We report a series of 1,3-diaryltriazene substituted metanilamide derivatives 1–12, acting as CA inhibitors against the widely spread cytosolic isoforms hCA I and II. According to the inhibition results, the 4-fluoro substituted derivative compound 2 can be considered as a lead molecule due to its interesting inhibition profile against both hCA I and hCA II, making it of interest in systems where the activity of the two cytosolic isoforms is dysregulated.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References:
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:168–81.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Supuran CT. Editorial. Carbonic anhydrases as drug targets. Curr Pharm Des 2008;14:601–2.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:48.
- Langella E, Alterio V, D'Ambrosio K, et al. Exploring benzoxaborole derivatives as carbonic anhydrase inhibitors: a structural and computational analysis reveals their conformational variability as a tool to increase enzyme selectivity. J Enzyme Inhibit Med Chem 2019;34:1498–505.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Exp Opin Drug Discov 2017;12:61–88.
- Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12.
- Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97.
- Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–9.
- Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70.
- Carta F, Supuran CT, Scozzafava A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med Chem 2014;6:1149–65.
- Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1, 3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhibit Med Chem 2018;33:1575–80.
- Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902.
- Pacchiano F, Aggarwal M, Avvaru BS, et al. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem Communicat 2010;46:8371–3.
- Kimball DB, Haley MM. Triazenes: a versatile tool in organic synthesis. Angew Chem Int Ed 2002;41:3338–51.
- Marchesi F, Turriziani M, Tortorelli G, et al. Triazene compounds: mechanism of action and related DNA repair systems. Pharmacol Res 2007;56:275–87.
- Francisco AP, Mendes E, Santos AR, Perry MJ. Anticancer triazenes: from bioprecursors to hybrid molecules. Curr Pharm Des 2019;25:1623–42.
- Supuran CT, Scozzafava A, Jurca BC, Ilies MA. Carbonic anhydrase inhibitors-Part 49: synthesis of substituted ureido and thioureido derivatives of aromatic/heterocyclic sulfonamides with increased affinities for isozyme I. Eur J Med Chem 1998;33:83–93.
- Lolak N, Akocak S, Bua S, et al. Design and synthesis of novel 1, 3-diaryltriazene-substituted sulfonamides as potent and selective carbonic anhydrase II inhibitors. Bioorg Chem 2018;77:542–7.
- Bilginer S, Unluer E, Gul HI, et al. Carbonic anhydrase inhibitors. Phenols incorporating 2-or 3-pyridyl-ethenylcarbonyl and tertiary amine moieties strongly inhibit Saccharomyces cerevisiae β-carbonic anhydrase. J Enzyme Inhibit Med Chem 2014;29:495–9.
- Gul HI, Mete E, Eren SE, et al. Designing, synthesis and bioactivities of 4-[3-(4-hydroxyphenyl)-5-aryl-4, 5-dihydro-pyrazol-1-yl] benzenesulfonamides. J Enzyme Inhibit Med Chem 2017;32:169–75.
- Gul HI, Mete E, Taslimi P, et al. Synthesis, carbonic anhydrase I and II inhibition studies of the 1, 3, 5-trisubstituted-pyrazolines. J Enzyme Inhibit Med Chem 2017;32:189–92.
- Gul HI, Tugrak M, Gul M, et al. New phenolic Mannich bases with piperazines and their bioactivities. Bioorg Chem 2019;90:103057.
- Gul HI, Yamali C, Bulbuller M, et al. Anticancer effects of new dibenzenesulfonamides by inducing apoptosis and autophagy pathways and their carbonic anhydrase inhibitory effects on hCA I, hCA II, hCA IX, hCA XII isoenzymes. Bioorg Chem 2018;78:290–7.
- Gul HI, Yamali C, Yesilyurt F, et al. Microwave-assisted synthesis and bioevaluation of new sulfonamides. J Enzyme Inhibit Med Chem 2017;32:369–74.
- Ozgun DO, Yamali C, Gul HI, et al. Inhibitory effects of isatin Mannich bases on carbonic anhydrases, acetylcholinesterase, and butyrylcholinesterase. J Enzyme Inhibit Med Chem 2016;31:1498–501.
- Yamali C, Gul HI, Ece A, et al. Synthesis, molecular modeling, and biological evaluation of 4-[5-aryl-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazol-1-yl] benzenesulfonamides toward acetylcholinesterase, carbonic anhydrase I and II enzymes. Chem Biol Drug Des 2018;91:854–66.
- Yamali C, Tugrak M, Gul HI, Tanc M, et al. The inhibitory effects of phenolic Mannich bases on carbonic anhydrase I and II isoenzymes. J Enzyme Inhibit Med Chem 2016;31:1678–81.
- Bilginer S, Gul HI, Erdal FS, et al. Synthesis, cytotoxicities, and carbonic anhydrase inhibition potential of 6-(3-aryl-2-propenoyl)-2 (3H)-benzoxazolones. J Enzyme Inhibit Med Chem 2019;34:1722–9.
- Canakci D, Koyuncu I, Lolak N, et al. Synthesis and cytotoxic activities of novel copper and silver complexes of 1,3-diaryltriazene-substituted sulfonamides. J Enzyme Inhib Med Chem 2019;34:110–6.
- Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300.
- De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- Supuran CT. Carbon-versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J Enzyme Inhib Med Chem 2018;33:485–95.
- Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors: perfluoroalkyl/aryl-substituted derivatives of aromatic/heterocyclic sulfonamides as topical intraocular pressure-lowering agents with prolonged duration of action. J Med Chem 2000;43:4542–51.
- Scozzafava A, Briganti F, Mincione G, et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–700.
- Supuran CT, Clare BW. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50.
- Supuran CT, Nicolae A, Popescu A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: the first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur J Med Chem 1996;31:431–8.
- Sarikaya SBÖ, Topal F, Şentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.
- Sentürk M, Gülçin I, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Supuran CT, Ilies MA, Scozzafava A. Carbonic anhydrase inhibitors. Part 29. Interaction of isozymes I, II and IV with benzolamide-like derivatives. Eur J Med Chem 1998;33:739–52.