 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A series of 16 novel benzenesulfonamides incorporating 1,3,5-triazine moieties substituted with aromatic amines, dimethylamine, morpholine and piperidine were investigated. These compounds were assayed for antioxidant properties by using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, 2,2`-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical decolarisation assay and metal chelating methods. They were also investigated as inhibitors of acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and tyrosinase, which are associated with several diseases such as Alzheimer, Parkinson and pigmentation disorders. These benzenesulfonamides showed moderate DPPH radical scavenging and metal chelating activity, and low ABTS cation radical scavenging activity. Compounds 2 b, 3d and 3 h showed inhibitory potency against AChE with % inhibition values of >90. BChE was also effectively inhibited by most of the synthesised compounds with >90% inhibition potency. Tyrosinase was less inhibited by these compounds.
1. Introduction
The 1,3,5-triazine scaffold, also known as s-triazine, and its derivatives have a wide range of applications due to broad biological activities including antiviral, antibacterial, anti-inflammatory, anti-HIV and more recently anti-cancer activityCitation1–4. Specifically, sulphonamides incorporating 1,3,5-triazine moieties were extensively studied as a potent and selective carbonic anhydrase inhibitorsCitation5–10. Some of these compounds showed the best selectivity ratio for tumour over-expressed membrane-bound carbonic anhydrase isoform IX (hCA IX) over the off-target isoform II (hCA II), between 166 to 706 foldCitation5–7. More recently, ureido benzenesulfonamides incorporating 1,3,5-triazine motifs were investigated as a potent class of inhibitors of the cancer related isoform human carbonic anhydrase IX (hCA IX) by our groupCitation11,Citation12.
Alzheimer’s disease (AD), a progressive neurodegenerative disorder of the brain, is characterised by cognitive dysfunction, memory decrease, speech impairment and dementia, which is the leading cause of disability in elderly people in the worldCitation13–15. It affects about 50 million people worldwide and this number might triplicate to 152 million by 2050 (World Alzheimer report)Citation16, as life expectancy is increasing worldwide. Acetylcholine (ACh), a neurotransmitter responsible for the conduction of electrical impulses from one nerve to another, is one of the main biochemical alteration in the brain of patients with AlzheimerCitation16–18. The level of ACh decreases due to its hydrolysis by acetylcholinesterase (AChE), which is a terminator enzyme of nerve impulse transmission. Butyrylcholinesterase (BChE) is a non-specific cholinesterase enzyme that hydrolyses many different choline-based estersCitation19,Citation20. Thus, AChE and BChE inhibition are being considered as a one of the most possible approaches for the treatment of ADCitation21,Citation22. There are several cholinesterase inhibitors used clinically for the treatment of AD such as, donepezil, tacrine, rivastigmine and galanthamine. However, these drugs have a limited efficacy, are toxic and have unfavourable side effects such as dizziness, hepatotoxicity, diarrhoea, vomiting and nauseaCitation21,Citation22. For these reasons, there is an urgent need for more potent and highly efficient cholinesterase inhibitors for the management of AD.
It has recently been documented that 1,3,5-triazine derivatives, specifically sulphonamide substituted ones, may show interesting biological properties as enzyme inhibitorsCitation5–12. To the best of our knowledge, there are no literature reports on the antioxidant, anticholinesterase and tyrosinase activities of 1,3,5-triazines containing sulphonamides. Therefore, in this study, we aimed to evaluate the antioxidant, anticholinesterase and tyrosinase activities of novel sulphonamides incorporating 1,3,5-triazine moieties.
2. Materials and methods
2.1. Chemistry
All chemicals and anhydrous solvents were purchased from Sigma-Aldrich, Merck, Alfa Aesar and TCI and used without further purification. FT-IR spectra were obtained by using Perkin Elmer Spectrum 100 FT-IR spectrometer. Nuclear Magnetic Resonance (1H-NMR and 13 C-NMR) spectra of compounds were recorded using a Bruker Advance III 300 MHz spectrometer in DMSO-d6 and TMS as an internal standard operating at 300 MHz for 1H-NMR and 75 MHz for 13 C-NMR. Thin-layer chromatography (TLC) was carried out on Merck silica gel 60 F254 plates.
2.1.1. General procedure for the synthesis of compounds 2(a–d)
At 0–5 °C, a 10 mmol solution of R1 (aromatic amine derivatives, -4 F, -4MeO, -3,4diCl, -3NO2) was added to 5 mmol of compound 1 in DMF under stirring. After complete addition, the mixture was allowed to warm to room temperature for 1 h, after that the reaction mixture was heated to 30–40 °C for 6–8 h. Then, the product was filtered off, washed with water and dried under vacuum at 40 °C. The obtained final pure products were fully characterised by FT-IR, 1H-NMR, 13 C-NMR, and melting points.
4-((4-chloro-6-((4-fluorophenyl)amino)-1,3,5-triazin-2-yl)amino)benzenesulfonamide (2a)
Yield: 75%; Colour: white solid; m.p.: 262–265 °C; FT-IR (cm−1): 3418, 3309, 3248, 1617, 1496 (asymmetric), 1322, 1157 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.92 (d, 2H, J = 6.3, Ar-H), 7.85 (d, 2H, J = 6.3, Ar-H), 7.76 (d, 2H, J = 6.6, Ar-H), 7. 55 (d, 2H, J = 6.3, Ar-H), 7.38 (s, 2H, -SO2NH2): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.8 (C-Cl), 167.2, 165.5 (Ctriaz-N), 157.4 (C-F), 143.7, 135.4, 130.9, 130.1, 120.7, 116.4, 113.7;
4-((4-chloro-6-((4-methoxyphenyl)amino)-1,3,5-triazin-2-yl)amino)benzenesulfonamide (2 b)
Yield: 68%; Colour: white solid; FT-IR (cm−1): 3447, 3316, 3255, 1623, 1505 (asymmetric), 1329, 1162 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.89 (d, 2H, J = 6.3, Ar-H), 7.81 (d, 2H, J = 6.3, Ar-H), 7.74 (d, 2H, J = 6.6, Ar-H), 7. 45 (d, 2H, J = 6.3, Ar-H), 7.33 (s, 2H, -SO2NH2), 3.85 (s, 3H, -OCH3): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.5 (C-Cl), 167.1, 165.2 (Ctriaz-N), 156.3, 143.2, 135.8, 131.4, 130.3, 120.4, 116.2, 113.3, 56.5;
4-((4-chloro-6-((3,4-dichlorophenyl)amino)-1,3,5-triazin-2-yl)amino)benzenesulfonamide (2c)
Yield: 55%; Colour: white solid; m.p.: 193–196 °C; FT-IR (cm−1): 3430, 3306, 3262, 1620, 1508 (asymmetric), 1336, 1160 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.06 (d, 2H, J = 6.3, Ar-H), 7.92 (d, 2H, J = 6.3, Ar-H), 7.60 (s, H, Ar-H), 7. 58 (d, 2H, J = 6.3, Ar-H), 7.45 (s, 2H, -SO2NH2): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 170.2, 167.5, 165.7, 164.1, 157.2, 150.4, 142.9, 136.3, 130.6, 129.9, 126.4, 120.6, 118.1;
4-((4-chloro-6-((3-nitrophenyl)amino)-1,3,5-triazin-2-yl)amino)benzenesulfonamide (2d)
Yield: 68%; Colour: white solid; FT-IR (cm−1): 3337, 3272, 3132, 1614, 1490 (asymmetric), 1350, 1156 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.95 (d, 2H, J = 6.0, Ar-H), 7.89 (d, 2H, J = 6.0, Ar-H), 7.58 (s, H, Ar-H), 7. 56 (m, 3H, Ar-H), 7.42 (s, 2H, -SO2NH2): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.8, 167.2, 165.1, 163.8, 157.0, 150.8, 142.3, 135.9, 130.2, 129.5, 125.8, 120.1, 117.5;
2.1.2. General procedure for the synthesis of compounds 3(a-l)
Under stirring, a 2 mmol solution of R2-H (dimethyl amine, morpholine and piperidine) was added to 1 mmol of 2(a-d) in DMF at room temperature for 1 h. Then, the reaction temperature was raised to 90 °C for 5 h. After cooling to room temperature, the mixture was filtered and the precipitate was washed with water and dried at 50 °C. The obtained final pure products 3(a-o) were fully characterised by FT-IR, 1H-NMR, 13 C-NMR, and melting points.
4-((4-(dimethylamino)-6-((4-fluorophenyl)amino)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3a)
Yield: 77%; Colour: white solid; m.p.: 222–225 °C; FT-IR (cm−1): 3366, 3325, 3262, 1625, 1485 (asymmetric), 1337, 1159 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.05 (d, 2H, J = 6.3 Hz, Ar-H), 7.91 (d, 2H, J = 6.3 Hz, Ar-H), 7.72 (d, 2H, J = 6.6 Hz, Ar-H), 7. 58 (d, 2H, J = 6.6 Hz, Ar-H), 7.49 (s, 2H, -SO2NH2), 3.05 (s, 6H, -CH3): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 167.2, 165.6, 164.5, 157.2, 143.5, 136.4, 130.8, 129.3, 120.9, 117.2, 36.8;
4-((4-((4-fluorophenyl)amino)-6-morpholino-1,3,5-triazin-2-yl)amino)benzenesulfonamide (3 b)
Yield: 83%; Colour: white solid; m.p.: 249–252 °C; FT-IR (cm−1): 3334, 3263, 3202, 1605, 1484 (asymmetric), 1356, 1152 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.01 (d, 2H, J = 6.6 Hz, Ar-H), 7.89 (d, 2H, J = 6.3 Hz, Ar-H), 7.70 (d, 2H, J = 6.6 Hz, Ar-H), 7. 55 (d, 2H, J = 6.6 Hz, Ar-H), 7.46 (s, 2H, -SO2NH2), 3.80–3.42 (m, 8H, morpholine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 166.9, 165.3, 164.2, 157.5, 143.1, 136.6, 130.3, 129.5, 120.7, 117.6, 65.4, 42.5;
4-((4-((4-fluorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3c)
Yield: 85%; Colour: white solid; m.p.: 228–230 °C; FT-IR (cm−1): 3319, 3260, 1603, 1494 (asymmetric), 1336, 1151 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.04 (d, 2H, J = 6.6 Hz, Ar-H), 7.88 (d, 2H, J = 6.3 Hz, Ar-H), 7.72 (d, 2H, J = 6.6 Hz, Ar-H), 7. 56 (d, 2H, J = 6.6 Hz, Ar-H), 7.47 (s, 2H, -SO2NH2), 3.43–3.21 (m, 4H, piperidine), 1.75–1.45 (m, 6H, piperidine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 166.7, 165.4, 164.1, 157.6, 143.0, 136.8, 130.5, 129.4, 120.8, 117.3, 42.7, 25.4, 24.2;
4-((4-(dimethylamino)-6-((4-methoxyphenyl)amino)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3d)
Yield: 77%; Colour: cream solid; FT-IR (cm−1): 3447, 3310, 3265, 1613, 1495 (asymmetric), 1325, 1160 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.87 (d, 2H, J = 6.6, Ar-H), 7.79 (d, 2H, J = 6.3, Ar-H), 7.70 (d, 2H, J = 6.6, Ar-H), 7. 46 (d, 2H, J = 6.3, Ar-H), 7.38 (s, 2H, -SO2NH2), 3.87 (s, 3H, -OCH3), 3.03 (s, 6H, -CH3): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.3, 167.0, 165.4, 156.9, 143.8, 136.2, 131.2, 129.9, 120.6, 116.9, 56.8, 36.9;
4-((4-((4-methoxyphenyl)amino)-6-morpholino-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3e)
Yield: 80%; Colour: cream solid, m.p.: 209–212 °C; FT-IR (cm−1): 3437, 3305, 3255, 1610, 1502 (asymmetric), 1328, 1159 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.90 (d, 2H, J = 6.3, Ar-H), 7.82 (d, 2H, J = 6.0, Ar-H), 7.73 (d, 2H, J = 6.6, Ar-H), 7. 49 (d, 2H, J = 6.3, Ar-H), 7.39 (s, 2H, -SO2NH2), 3.86 (s, 3H, -OCH3), 3.79–3.43 (m, 8H, morpholine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.7, 167.3, 165.2, 157.3, 143.2, 136.4, 131.0, 129.8, 120.2, 116.6, 65.3, 56.7, 42.2;
4-((4-((4-methoxyphenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3f)
Yield: 82%; Colour: cream solid, m.p.: 252–254 °C; FT-IR (cm−1): 3425, 3312, 3248, 1605, 1501 (asymmetric), 1332, 1160 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.88 (d, 2H, J = 6.3, Ar-H), 7.80 (d, 2H, J = 6.0, Ar-H), 7.71 (d, 2H, J = 6.6, Ar-H), 7. 47 (d, 2H, J = 6.3, Ar-H), 7.40 (s, 2H, -SO2NH2), 3.88 (s, 3H, -OCH3), 3.45–3.28 (m, 4H, piperidine), 1.77–1.46 (m, 6H, piperidine):): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.1, 167.7, 165.1, 157.8, 143.6, 136.2, 130.6, 129.7, 120.1, 116.2, 56.8, 42.4, 25.1, 24.4;
4-((4-((3,4-dichlorophenyl)amino)-6-(dimethylamino)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3 g)
Yield: 75%; Colour: white solid; m.p.: 254–257 °C; FT-IR (cm−1): 3353, 3270, 3262, 1600, 1502 (asymmetric), 1327, 1150 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.04 (d, 2H, J = 6.6, Ar-H), 7.94 (d, 2H, J = 6.3, Ar-H), 7.58 (s, H, Ar-H), 7. 53 (d, 2H, J = 6.3, Ar-H), 7.42 (s, 2H, -SO2NH2), 3.08 (s, 6H, -CH3): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.8, 167.3, 165.6, 164.4, 157.5, 150.2, 141.7, 136.2, 130.3, 129.5, 126.6, 120.8, 118.3, 36.6;
4-((4-((3,4-dichlorophenyl)amino)-6-morpholino-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3 h)
Yield: 78%; Colour: white solid; m.p.: 199–201 °C; FT-IR (cm−1): 3329, 3211, 1625, 1490 (asymmetric), 1337, 1153 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.06 (d, 2H, J = 6.6, Ar-H), 7.92 (d, 2H, J = 6.3, Ar-H), 7.57 (s, H, Ar-H), 7. 52 (d, 2H, J = 6.3, Ar-H), 7.44 (s, 2H, -SO2NH2), 3.81–3.46 (m, 8H, morpholine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.4, 167.1, 165.8, 164.3, 157.2, 150.0, 141.9, 136.6, 130.8, 129.2, 126.5, 120.2, 118.6, 65.6, 42.2;
4-((4-((3,4-dichlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3i)
Yield: 81%; Colour: white solid; m.p.: 245–248 °C; FT-IR (cm−1): 3340, 3211, 2935, 2854, 1603, 1496 (asymmetric), 1361, 1155 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 8.02 (d, 2H, J = 6.3, Ar-H), 7.89 (d, 2H, J = 6.0, Ar-H), 7.54 (s, H, Ar-H), 7. 50 (d, 2H, J = 6.0, Ar-H), 7.41 (s, 2H, -SO2NH2), 3.44–3.27 (m, 4H, piperidine), 1.72–1.41 (m, 6H, piperidine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 168.2, 166.9, 165.2, 164.1, 157.8, 150.3, 141.5, 136.4, 130.3, 129.6, 126.2, 120.4, 118.7, 42.5, 25.1, 24.4;
4-((4-(dimethylamino)-6-((3-nitrophenyl)amino)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3j)
Yield: 78%; Colour: light yellow solid; m.p.: 233–235 °C; FT-IR (cm−1): 3345, 3282, 3135, 1612, 1498 (asymmetric), 1345, 1158 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.97 (d, 2H, J = 6.3, Ar-H), 7.87 (d, 2H, J = 6.3, Ar-H), 7.55 (s, H, Ar-H), 7. 51 (m, 3H, Ar-H), 7.42 (s, 2H, -SO2NH2), 3.05 (s, 6H, -CH3): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.6, 167.5, 164.9, 163.5, 157.2, 150.5, 142.1, 135.4, 130.7, 129.8, 125.4, 120.8, 117.2, 36.8;
4-((4-morpholino-6-((3-nitrophenyl)amino)-1,3,5-triazin-2-yl)amino)benzenesulfonamide (3k)
Yield: 86%; Colour: light yellow solid; m.p.: 215–218 °C; FT-IR (cm−1): 3338, 3280, 3145, 1615, 1502 (asymmetric), 1348, 1159 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.99 (d, 2H, J = 6.3, Ar-H), 7.85 (d, 2H, J = 6.3, Ar-H), 7.59 (s, H, Ar-H), 7. 52 (m, 3H, Ar-H), 7.44 (s, 2H, -SO2NH2), 3.83–3.48 (m, 8H, morpholine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 169.0, 167.8, 164.5, 163.2, 157.8, 150.3, 142.4, 135.8, 130.1, 129.3, 125.6, 120.5, 117.8, 65.5, 42.4;
4-((4-((3-nitrophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)amino) benzenesulfonamide (3 l)
Yield: 84%; Colour: light yellow solid; m.p.: 181–184 °C; FT-IR (cm−1): 3341, 3215, 3125, 1602, 1502 (asymmetric), 1350, 1154 (symmetric) (S = O); 1H-NMR (DMSO-d6, 300 MHz, δ ppm): 7.96 (d, 2H, J = 6.0, Ar-H), 7.82 (d, 2H, J = 6.3, Ar-H), 7.58 (s, H, Ar-H), 7. 50 (m, 3H, Ar-H), 7.41 (s, 2H, -SO2NH2), 3.46–3.29 (m, 4H, piperidine), 1.74–1.45 (m, 6H, piperidine): 13 C-NMR (DMSO-d6, 75 MHz, δ ppm): 168.8, 167.3, 164.6, 162.7, 157.4, 150.6, 142.1, 135.4, 130.7, 129.2, 125.1, 120.0, 117.2, 42.3, 25.0, 24.2;
2.2. Determination of the antioxidant, anticholinesterase and tyrosinase activity of benzenesulfonamides 2(a-d) and 3(a-l)
2.2.1. DPPH radical scavenging assay
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of the synthesised compounds was determined by spectrophotometric method based on the reduction of an ethanol solution of DPPHCitation23,Citation24. 2, 5, 10, 20 µL of 1 mM stock solution of each compound was completed to 40 µL with the DMSO and mixed with 160 µL of 0.1 mM of DPPH free radical solution. The mixture was led to stand for 30 min in the dark and the absorbance was then measured at 517 nm against a blank. Inhibition of free radical, DPPH, in percent (I %) was calculated as follows:
where Acontrol is the absorbance of the control reaction (containing all reagents except for the tested compounds), and Asample is the absorbance of the test compounds. Tests were carried out in triplicate. BHA, BHT and α-Tocopherol were used as positive controlCitation25–28.
2.2.2. ABTS cation radical decolorisation assay
The percent inhibition of decolorisation of ABTS (2,2`-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) cation radical is obtained as a function of time and concentration, and evaluated by comparison with the BHT, BHA and α-Tocopherol compounds used as standardCitation29,Citation30. The tested compounds at different concentrations are added to each well and 160 µL of 7 mM ABTS solution is added. After 6 min at room temperature, the absorbances were measured at 734 nm. ABTS cation radical decolorisation activities were determined by
where A is the absorbance. Tests were carried out in triplicate. BHA, BHT and α-Tocopherol were used as positive control.
2.2.3. Metal chelating activity
The chelating ability of synthesised compounds was examined according to the method of Dinis et al.Citation31 The tested compounds at different concentrations were added to each well and 4 µL of 2 mM ferrous (II) chloride was added. Then 8 µL of 5 mM ferrozine was added and the reaction was started. After 10 min at room temperature, the absorbance was measured at 562 nm against blank. The results were expressed as percentage of inhibition of the ferrozine-Fe2+ complex formation. The percentage inhibition of the ferrozine -Fe2+ complex formation was calculated as follows:
where A is the absorbance. Tests were carried out in triplicate. EDTA was used as a positive controlCitation32–34.
2.2.4. Anti-cholinesterase assay
The inhibitory effect of novel benzenesulfoanmides incorporating 1,3,5-triazine moieties 2(a-d) and 3(a-l) on AChE and BChE activities was determined according to the slightly modified spectrophotometric method of Ellman et al.Citation35. All compounds were dissolved in DMSO to prepare stock solutions at 4 mM concentration. Aliquots of 150 µL of 100 mM sodium phosphate buffer (pH 8.0), 10 µL of sample solution and 20 µL AChE (or BChE) solution were mixed and incubated for 15 min at 25 °C, and DTNB (5,5’-Dithio-bis(2-nitro-benzoic)acid) (10 µL) is added. The reaction was then initiated by the addition of acetylthiocholine iodide (or butyrylthiocholine iodide) (10 µL). The final concentration of the tested compounds’ solution was 200 µM.
where A is the absorbance. Tests were carried out in triplicate. Galantamine was used as positive control.
2.2.5. Anti-tyrosinase activity
Anti-tyrosinase activity of the compounds was performed according to the method designed by Hearing and JimenezCitation36. The inhibition of the diaphanous function of the compounds was evaluated with L-DOPA as substrate. Tyrosinase from mushroom (E.C. 1.14.18.1) (30 U, 28 nM) was dissolved in Na-phosphate buffer (pH = 6.8, 50 nM) and the compounds were added to the solution for pre-incubation at room temperature for ten minutes. After incubation, 0.5 mM L-DOPA was added to the mixture and the change in absorbance was measured at 475 nm at 37 °C. For the positive control, kojic acid was used as an inhibitor. The following formula was used to calculate the percentage of all enzyme inhibitions:
where A is absorbance.
2.3. Statistical analysis
The results of the antioxidant, anticholinesterase and tyrosinase activity assays are expressed as the mean ± SD of three parallel measurements. The statistical significance was estimated using a Student’s t-test, where p values < 0.05 were considered significant.
3. Result and discussion
3.1. Chemistry
The rationale for designing this novel benzenesulfonamides incorporating 1,3,5-triazine structural motifs presented in this work are based on our previous work which showed efficient carbonic anhydrase IX (tumour over-expressed isozyme) inhibition potency associated with such derivativesCitation5–12. A number of structurally diverse benzenesulfonamides incorporating 1,3,5-triazine moieties were synthesised according to the general synthetic route depicted in Scheme 1. In order to generate chemical diversity, different substituted aromatic amines (-4 F, -4MeO, -3,4diCl and -3NO2 substituted anilines) were chosen and reacted at one side of the triazine moiety, whereas on the other side the derivatisation was achieved by using dimethlyamine, morpholine and piperidine functionalities.
Scheme 1. General synthetic route for the synthesis of benzenesulfonamides incorporating 1,3,5-triazine moieties. Reagents and conditions: (i) R1 (–4 F, –4MeO, –3,4diCl, and –3NO2), DMF, 0 to 5 °C, 1 h, then 30–40 °C, 8 h, (ii) R2H (dimethylamine, morpholine and piperidine), DMF, room temperature, 1 h, then 90 °C, 5 h.
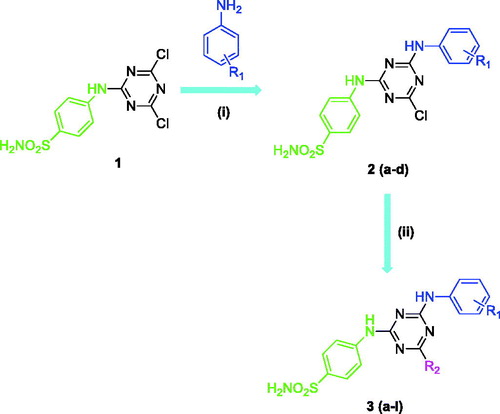
The synthesis of benzenesulfonamides incorporating 1,3,5-triazine moieties 2(a-d) and 3(a-l) was carried out according to the procedure described in our previous papersCitation11,Citation12. Briefly, the starting key intermediate compound 1 was coupled with substituted aromatic anilines (-4 F, -4MeO, -3,4diCl and -3NO2), leading to formation of compounds 2(a-d). After that, the third chloride atom of the starting material 1,3,5-triazine (cyanuric chloride) was derivatised with dimethylamine, morpholine and piperidine to produce compounds 3(a-l). The structures of benzenesulfonamides incorporating 1,3,5-triazine moieties 2(a-d) and 3(a-l) were confirmed by using several analytical and spectral data (FT-IR, 1H-NMR, 13 C-NMR, and melting points) as described in the experimental part.
3.2. Antioxidant activity
The benzenesulfonamides incorporating 1,3,5-triazine moieties were screened for their antioxidant activity by three methods, namely DPPH free radical scavenging, ABTS cation radical scavenging, and metal chelating activity. All of the compounds showed antioxidant activities in a dose-dependent manner and the results were summarised in , which demonstrates the IC50 values of the synthesised derivatives and standard compounds (BHA, BHT, α-tocopherol, and EDTA).
Table 1. Antioxidant activity of sulphonamides 2 and 3.
The results revealed that benzenesulfonamides incorporating 1,3,5-triazine moieties 2(a-d) and 3(a-l) shows, in general, moderate DPPH radical scavenging and metal chelating activity, and low ABTS cation radical scavenging activity. Specifically, three compounds from the synthesised derivatives (3 b, 3 h and 3j) indicates high DPPH radical scavenging activity with IC50 values of 73.25, 60.18, and 58.59 µM, respectively. These compounds have better antioxidant activity than standards BHA (IC50: 61.72 µM) and BHT (IC50: 232.11 µM). On the other hand, compounds 3a, 3c, 3e, and 3f showed any activity with IC50 values of >1000 µM. Furthermore, the ABTS cation radical scavenging activity of the compounds was also assayed and compared with standards BHT, BHA, and α-Tocopherol. All compounds showed weak activity with IC50 values of <1000 µM, except the compounds 3d, 3f and 3k exhibited moderate activity with IC50 values of 294.12, 408.44 and 481.21 µM, respectively (). The metal chelating activity of the synthesised compounds was also screened and compared with standard EDTA. None of the compounds showed better activity than standard. Moreover, several derivatives (3a, 3e and 3k) displayed close metal chelating activity to EDTA with IC50 values of 84.98, 84.32 and 88.42 µM, respectively. The remaining compounds were shown to have moderate metal chelating activity with IC50 values ranging from 98.84 to 488.29 µM.
In the present study, sulphonamides incorporating 1,3,5-triazine moieties 2(a-d) and 3(a-l) were also evaluated for their anticholinesterase (AChE and BChE) activities (). In general, all compounds showed better BChE inhibitory activity than AChE, except the compound 2 b, which displayed more AChE inhibitory activity (% inhibition 96.37) than BChE (91.99). Compounds 2 b, 3d and 3 h found to have most potent AChE inhibitors, having better % inhibition than standard drug galantamine, with % inhibition values of 96.37, 91.10 and 93.19, respectively. Some compounds from the series (2a, 2c, 3a, 3e, 3f, 3 g, 3i, 3j, and 3 l) showed any activity (NA) against AChE enzyme. The remaining compounds (2d, 3 b, 3c, and 3k) showed low activity to AChE enzyme with % inhibition values ranging from 14.18 to 36.55. On the other hand, BChE enzyme was effectively inhibited by most of the synthesised compounds. Specifically, compounds 2 b, 2d, 3d, 3f, 3 h, 3j, and 3 l showed >90% inhibition, which is higher than standard drug galantamine with a % inhibition value of 87.86. Among the remaining compounds, some of them (2c, 3 b, 3c, and 3i) showed close % inhibition scale to standard drug with % inhibition values of 87.44, 88.76, 89.21, and 88.48, respectively. Only one non active compound (3e) was observed against BChE enzyme, in which this compound also inactive against AChE enzyme, too (). Interestingly, three compounds from the current series (2 b, 3d and 3 h) displayed higher activity against both enzyme (AChE and BChE) together than standard drug galantamine, which is a one of the most important findings of the current study. These three compounds gave promising anticholinesterase activity and might be improved and used as powerful cholinesterase inhibitors.
Table 2. Anti-cholinesterase and anti-tyrosinase activity of compounds 2 and 3.
Tyrosinase is a bi-functional copper containing enzyme which is widely distributed in different organisms such as animals, plants, and microorganismsCitation37. This enzyme catalyses hydroxylation of monophenols to o-diphenols which generates melaninCitation37,Citation38. Tyrosinase inhibitors capable of inhibiting the biosynthesis of melanin are used for various applications in the foodCitation39, cosmeticsCitation40 and medicinal industriesCitation37,Citation38. In the present study, tyrosinase enzyme inhibition activity was also evaluated and moderate activity was observed against this enzyme (). In general, none of the compounds showed better inhibition potency than standard Kojic acid, which has % inhibition value of 95.26. Two compounds showed high activity, 3f and 3j, with % inhibition values of 86.35 and 77.30, respectively. The remaining compounds indicated moderate activity depends on the substitution on compounds with % inhibition values ranging from 6.29 to 58.59. Only one compound (2d) was none active against this enzyme.
4. Conclusions
In the present study, we report a novel series of benzenesulfonamides incorporating 1,3,5-triazine moieties substituted with aromatic amine derivatives, dimethylamine, morpholine and piperidine. The novel compounds were investigated as an antioxidant and inhibitors of AChE, BChE and tyrosinase enzymes. The results revealed that benzenesulfonamides incorporating 1,3,5-triazine moieties 2(a-d) and 3(a-l) show, in general, moderate DPPH radical scavenging and metal chelating activity, and low ABTS cation radical scavenging activity. Compounds 2 b, 3d and 3 h displayed great inhibition potency against AChE with % inhibition values of 96.37, 91.10 and 93.19, respectively. BChE also effectively inhibited by most of the synthesised compounds having >90% inhibition potency which is a one of the most important findings from the current work. Several lead compounds were also obtained against tyrosinase enzyme and they might be improved and used as effective inhibitors of these enzymes.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zheng M, Xu C, Ma J, et al. Synthesis and antitumor evaluation of a novel series of triamino triazine derivatives. Bioorg Med Chem 2007;15:1815–27.
- Patel RV, Keum YS, Park SW. Medicinal chemistry discoveries among 1,3,5-triazines: recent advances (2000-2013) as antimicrobial, anti-TB, anti-HIV and antimalarials. Mini Rev Med Chem 2014;14:768–89.
- Liu B, Sun T, Zhou Z, Du L. A systematic review on antitumor agents with 1,3,5-triazines. Med Chem 2015;5:131–48.
- Cascioferro S, Parrino B, Spano V, et al. 1,3,5-Triazines: a promising scaffold, for anticancer drugs development. Eur J Med Chem 2017;142:523–49.
- Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg Med Chem Lett 2004;14:5427–33.
- a) Havrankova E, Csollei J, Vullo D, et al. Novel sulfonamide incorporating piperazine, aminoalcohol and 1,3,5-triazine structural motifs with carbonic anhydrase I, II and IX inhibitory action. Bioorg Chem 2018;77:25–37. b) Garaj V, Puccetti L, Fasolis G, et al. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II, and IX. Bioorg Med Chem Lett 2005;15:3102–8. c) Mikus P, Krajciova D, Mikulova M, et al. Novel sulfonamides incorporating 1,3,5-triazine and amino acid structural motifs as inhibitors of the physiological carbonic anhydrase isozymes I, II, and IV and tumor-associated isozyme IX. Bioorg Chem 2018;81:241–52.
- a) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:168–81; b) Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68; c) Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32; d) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- a) Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70; b) Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97; c) Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88; d) De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- a) Ceruso M, Vullo D, Scozzafava A, Supuran CT. Inhibition of human carbonic anhydrase isoforms I-XIV with sulfonamides incorporating fluorine and 1,3,5-triazine moieties. Bioorg Med Chem 2013;21:6929–36; b) Carta F, Garaj V, Maresca A, et al. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII, and XIV over cytosolic isoforms I and II: solution and X-ray crystallographic studies. Bioorg Med Chem 2011;19:3105–19; c) Oztürk Sarikaya SB, Topal F, Sentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.
- a) Supuran CT. Carbonic Anhydrases and Metabolism. Metabolites 2018;8:25; b) Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12; c) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21; d) Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77; e) Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med Res Rev 2018;38:1799–836.
- a) Lolak N, Akocak S, Bua S, Supuran CT. Design, synthesis and biological evaluation of ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as potent carbonic anhydrase IX inhibitors. Bioorg Chem 2019;82:117–22; b) Lolak N, Akocak S, Bua S, et al. Discovery of new ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as carbonic anhydrase I, II, IX and XII inhibitors. Bioorg Med Chem 2019;27:1588–94.
- a) Tars K, Vullo D, Kazaks A, et al. Sulfocoumarins (1,2-benzoxathiine 2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J Med Chem 2013;56:293–300; b) Köhler K, Hillebrecht A, Schulze Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–9; c) Nishimori I, Minakuchi T, Morimoto K, et al. Carbonic anhydrase inhibitors: DNA cloning and inhibition studies of the alpha-carbonic anhydrase from Helicobacter pylori, a new target for developing sulfonamide and sulfamate gastric drugs. J Med Chem 2006;49:2117–26.
- a) Bag S, Tulsan R, Sood A, et al. Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg Med Chem 2015;25:626–30; b) Gocer H, Akincioglu A, Goksu S, et al. Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem 2014;30:316–20; c) Scozzafava A, Kalin P, Supuran CT, et al. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J Enzyme Inhib Med Chem 2015;30:941–6; d) Gulcin I, Scozzafava A, Supuran CT, et al. The effect of caffeic acid phenethyl ester (CAPE) on metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione S-transferase, lactoperoxidase, and carbonic anhydrase isoenzymes I, II, IX, and XII. J Enzyme Inhib Med Chem 2016;31:1095–101.
- a) Rishton GM, Retz DM, Tempest PA, et al. Fencyhylamine sulfonamide inhibitors of amyloid beta peptide production by the gamma-secretase proteolytic pathway: potential small-molecule therapeutic agents for the treatment of Alzheimer’s disease. J Med Chem 2000;43:2297–9; b) Gocer H, Topal F, Topal M, et al. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J Enzyme Inhib Med Chem 2016;31:441–7; c) Ozgeris B, Goksu S, Kose LP, et al. Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg Med Chem 2016;24:2318–29; d) Akincioglu A, Akincioglu H, Durdagi S, et al. Discovery of potentcarbonic anhydrase and acetylcholine esterase inhibitors: Novel sulfamoyl carbamates and sulfamides derived from acetophenones. Bioorg Med Chem 2015;23:3592–602.
- a) Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease-insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 2017;13:612–23; b) Yilmaz S, Akbaba Y, Ozgeris B, et al. Synthesis and inhibitory properties of some carbamates on carbonic anhydrase and acetylcholine esterase. J Enzyme Inhib Med Chem 2016;31:1484–91; c) Ozgun DO, Yamali C, Gul HI, et al. Inhibitory effects of isatin Mannich bases on carbonic anhydrases, acetylcholinesterase, and butyrylcholinesterase. J Enzyme Inhib Med Chem 2016;31:1498–501.
- a) Alzheimer’s Disease International. World Alzheimer Report 2019: attitudes to dementia. London: Alzheimer’s Disease International. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf [last accessed 19 Dec 2019]; b) Swerdlow RH. Pathogenesis of Alzheimer's disease. Clin Interv Aging 2007;2:347–59.
- Dong S, Duan Y, Hu Y, Zhao Z. Advances in the pathogenesis of Alzheimer’s disease: a re-evaluation of amyloid cascade hypothesis. Transl Neurodegener 2012;1:1–18.
- Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer’s disease therapys. Int Psychogeriatr 2002;14:77–91.
- Klatte ET, Scharre DW, Nagaraja HN, et al. Combination therapy of donepezil and vitamin E in Alzheimer disease. Alzheimer Dis Assoc Disord 2003;17:113–6.
- Cai P, Fang SQ, Yang HL, et al. Donepezil-butylated hydroxytoluene (BHT) hybrids as Anti-Alzheimer’s disease agents with cholinergic, antioxidant, and neuroprotective properties. Eur J Med Chem 2018;157:161–76.
- Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res 2013;36:375–99.
- L.G. de Souza LG, Renno MN, Figueroa-Villar JD. Coumarins as cholinesterase inhibitors: a review. Chem Biol Interact 2016;254:11–23.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199–200.
- Akocak S, Boga M, Lolak N, et al. Design, synthesis and biological evaluation of 1,3-diaryltriazene-substituted sulfonamides as antioxidant, acetylcholinesterase, and butyrylcholinesterase inhibitors. J Turk Chem Soc Sect A: Chem 2019;6:63–70.
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006;217:213–20.
- Gulcin I. Antioxidant and antiradical activities of L-carnitine. Life Sciences 2006;78:803–11.
- Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37.
- Gulcin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2006;32:431–8.
- Pellegrini RRN, Proteggente A, Pannala A, et al. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Rad Bio Med 1999;26:1231–7.
- Akocak S, Lolak N, Tuneg M, Boga M. Antioxidant, acetylcholinesterase and butyrylcholinesterase inhibition profiles of histamine Schiff bases. J Turk Chem Soc Sect A: Chem 2019;6:157–64.
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid preoxidation and as preoxyl radical scavengers. Arc Biochem Biophy 1994;315:161–9.
- Gulcin I. Antioxidant activity of L-adrenaline: a structure-activity insight. Chem Biol Interact 2009;179:71–80.
- Gulcin I. Antioxidant properties of resveratrol: a structure-activity insight. Innovative Food Sci Emerg Tech 2010;11:210–8.
- Gulcin I, Bursal E, Sehitoglu MH, et al. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 2010;48:2227–38.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Hearing VJ, Jiménez M. Mammalian tyrosinase - the critical regulatory control point in melanocyte pigmentation. Int J Biochem 1987;19:1141–7.
- Zolghadri S, Bahrami A, Khan MTH, et al. comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem 2019;34:279–309.
- Sahu RK, Roy A, Dwivedi J, Jha AK. Promotion and computation of inhibitory effect on tyrosinase activity of herbal cream by incorporating indigenous medicinal plants. Pak J Biol Sci 2014;17:146–50.
- Sohretoglu D, Sari S, Barut B, Ozel A. Tyrosinase inhibition by some flavonoids: inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem 2018;81:168–74.
- Halaouli S, Asther M, Kruus K, et al. Characterization of new tyrosinase from Pycnoporus species with high potential for food technological applications. J Appl Microbiol 2005;98:332–43.
