Abstract
A series of bio-organometallic-hydrazones of the general formula [{(η5-C5H4)-C(R)=N–N(H)-C6H4-4-SO2NH2}]MLn(MLn = Re(CO)3, Mn(CO)3, FeCp; R=H, CH3) were prepared by reaction of formyl/acetyl organometallic precursors with 4-hydrazino-benzenesulphonamide. All compounds were characterized by conventional spectroscopic techniques (infra-red, 1H and 13C NMR, mass spectrometry and elemental analysis). Biological evaluation as carbonic anhydrase (CA, EC 4.2.1.1) inhibitors agents was carried out using four human/h) isoforms, hCA I, II, IX and XII. The cytosolic isoforms hCA I and II were effectively inhibited by almost all derivatives with inhibition constants of 1.7–22.4 nM. Similar effects were observed for the tumour-associated transmembrane isoform hCA XII (KIs of 1.9–24.4 nM). hCA IX was less sensitive to inhibition with these compounds. The presence of bio-organometallic or metallo-carbonyl moieties in the molecules of these CAIs makes them amenable for interesting pharmacologic applications, for example for compounds with CO donating properties.
1. Introduction
The carbonic anhydrases (CAs, EC 4.2.1.1) constitute a superfamily of enzymes in all living organism, their essential role being that of catalysing the reversible hydration of CO2 to bicarbonate and protonsCitation1–5. In this way, from two neutral molecules (carbon dioxide and water), a weak base (bicarbonate) and a strong acid (H+ ions) are generated with a huge efficacyCitation5–12. This is actually the main mechanism used by most living organism for pH regulationCitation1–4. CAs are second only to superoxide dismutase for their catalytic efficiency, with some members of the superfamily showing kcat/KM values close to the limit of the diffusion-controlled processes, in the range of 108 M−1 × s−1.Citation3–8 The crucial role played by the above mentioned reaction in organisms all over the phylogenetic tree explains why at least eight CA genetic families are known to date: the α-, β-, γ-, δ-, ζ-, η-, θ- and ι-CA classesCitation4–13. All of them are metalloenzymes and for a long period it has been considered that zinc is the essential metal ion in all CAs. However, recent studies demonstrated that the γ-CAs are probably Fe(II) enzymes, the ζ-CAs are Cd(II) enzymes and the ι-CAs Mn(II) proteinsCitation2–13. However, in all of them, a divalent metal hydroxide is the nucleophilic species converting the substrate CO2 to bicarbonate in a ping pong type of mechanism. In most CAs, the rate determining step of the catalytic cycle is the proton transfer reaction from a water molecule coordinated to the metal ion to the reaction medium with generation of the nucleophilic enzyme speciesCitation2–7. In α-CAs, this step is generally assisted by a His residue situated in the middle part of the active site, named proton shuttling residueCitation2–7, whereas for other CA genetic families the nature and positioning of the proton shuttle residue is less well understoodCitation8–13.
Inhibition of these enzymes with various classes of CA inhibitors (CAIs) has an extended range of pharmacologic applications, starting with diuretics and antiglaucoma agentsCitation14, to antiepilepticsCitation15, antiobesityCitation16, or antitumor drugsCitation17 and ending with anti-neuropathic painCitation18 anti-ischemiaCitation19 and anti-arthritis drugsCitation20. This is only possible because different hCA isoforms of the 15 known to date are involved in very diverse pathologies. There are currently 5 CA inhibition mechanisms, but detailed structural data is available only for the first four of themCitation4,Citation5. These are (i) zinc binders; (ii) inhibitors which anchor to the zinc coordinated water/hydroxide ion; (iii) inhibitors which occlude the entrance to the active site; (iv) inhibitors which bind out of the active site, and (v) compounds with unknown inhibition mechanismCitation4,Citation5. However, many of the first- and second-generation inhibitors do not show significant isoform selectivity and this is the main reason why such inhibitors used as drugs show a considerable number of side effectsCitation4,Citation5,Citation14–16. For this reason, the drug design of novel types of CAIs is constantly being pursued by many research groups. Here, we report bio-organometallic-hydrazones of the general formula [{(η5-C5H4)-C(R)=N–N(H)-C6H4-4-SO2NH2}]MLn(MLn = Re(CO)3, Mn(CO)3, FeCp; R=H, CH3) which were designed in order to obtain organometallic compounds with CA inhibitory properties.
2. Experimental
2.1. Materials
All manipulations were conducted under an N2 atmosphere using Schlenk techniques. The compounds (η5-C5H4CHO)Re(CO)3Citation21, (η5-C5H4COCH3)Re(CO)3Citation22, (η5-C5H4CHO)Mn(CO)3Citation23 and 4-hydrazinyl-benzenesulphonamideCitation24 were prepared according to published procedures. Ferrocene carboxaldehyde (98%), acetyl ferrocene (95%), acetylcymantrene (98%) and sulphanilamide (99%) were obtained from Sigma-Aldrich (Chicago, IL) and used without additional purification. Solvents such as CH2Cl2, hexane, acetone, EtOH, DMSO and THF were obtained commercially and purified using standard methods. Infra-red spectra were recorded in solid state (KBr pellet) on a Jasco FT-IR 4600 spectrophotometer. 1H NMR spectra were measured on a Bruker spectrometer model ASCEND TM 400 MHz. All NMR spectra are reported in parts per million (ppm, δ) relative to tetramethylsilane (Me4Si), with the residual solvent proton resonances used as internal standards. Coupling constants (J) are reported in Hertz (Hz), and integrations are reported as number of protons. The following abbreviations were used to describe the peak patterns: s = singlet, d = doublet, t = triplet and m = multiplet. Mass spectra were obtained on a Shimadzu model QP5050A GC-MS at the Laboratorio de Servicios Analíticos, Pontificia Universidad Católica de Valparaíso. Elemental analyses were measured on a Perkin Elmer CHN Analyser 2400.
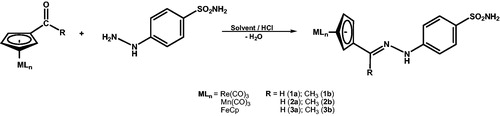
2.2. Synthesis of organometallic-hydrazones [{(η5-C5H4)-C(H)=N–N(H)-C6H4-4-SO2NH2}]MLn(1a, 2a, 3a)
To a stirred suspension of 4-hydrazinyl-benzenesulphonamide (1 eq.) in water (12 ml) and three drops of HCl 32%, the formyl-organometallic precursor (1 eq.) was added. The resulting mixture was stirred for 18 h at room temperature. The precipitate obtainedwas washed with water (2 × 10 ml), diethyl ether (2 × 10 ml) and dried in vacuum for 2 h. The hydrazone derivatives (1a, 2a, 3a) were recrystallized from acetone/hexane (1:5) at −18 °C.
2.2.1. [{(η5-C5H4)-CH=N–N(H)-C6H4-4-SO2NH2}]re(CO)3 (1a)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Re(CO)3 (91 mg, 0.25 mmol) and 4-hydrazinyl-benzenesulphonamide (47 mg, 0.25 mmol). Brown-yellow solid, yield 48% (64 mg, 0.12 mmol). IR (KBr, cm−1): 3373–3262 (νNH/NH2); 2022 (νRe-CO); 1922 (νRe-CO); 1590 (νC=N); 1323 (νS-O); 1152 (νS-O). 1H NMR (acetone-d6): δ 5.65 (t, 2H, J = 2.0 Hz, C5H4); 6.10 (t, 2H, J = 2.0 Hz, C5H4); 6.32 (s, 2H, NH2); 7.15 (d, 2H, J = 8.8 Hz, C6H4); 7.69 (d, 2H, J = 8.8 Hz, C6H4); 7.72 (s, 1H, CH=N); 9.89 (s, 1H, NH). 13C NMR (acetone-d6): δ 83.6 (C5H4); 84.9 (C5H4); 102.6 (C5H4ipso); 111.6 (C6H4); 127.8 (C6H4); 131.4 (CH=N); 134.4 (C6H4); 147.7 (C6H4); 194.4 (Re-CO). Mass spectrum (based on 187Re) (m/z): 533 [M+]; 449 [M+ – 3CO]. Anal. (%) Calc. for C15H12N3O5SRe: C, 33.83; H, 2.27 and N, 7.89; found: C, 33.63; H, 2.27 and N, 7.78.
2.2.2. [{(η5-C5H4)-CH=N–N(H)-C6H4-4-SO2NH2}]Mn(CO)3 (2a)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)Mn(CO)3 (58 mg, 0.25 mmol) and 4-hydrazinyl-benzenesulphonamide (47 mg, 0.25 mmol). Brown solid, yield 52% (52 mg, 0.13 mmol). IR (KBr, cm−1): 3365–3250 (νNH/NH2); 2020 (νMn-CO); 1931 (νMn-CO); 1597 (νC=N); 1325 (νS-O); 1147 (νS-O). 1H NMR (acetone-d6): δ 5.05 (t, 2H, J = 2.0 Hz, C5H4); 5.50 (t, 2H, J = 2.0 Hz, C5H4); 6.31 (s, 2H, NH2); 7.18 (d, 2H, J = 8.8 Hz, C6H4); 7.64 (s, 1H, CH=N); 7.74 (d, 2H, J = 8.8 Hz, C6H4); 9.90 (s, 1H, NH). 13C NMR (acetone-d6): δ 82.5(C5H4); 83.0 (C5H4); 98.3 (C5H4ipso); 111.6 (C6H4); 127.8 (C6H4); 132.5 (CH=N); 134.4 (C6H4); 148.2 (C6H4); Mn–CO (not observed). Mass spectrum (m/z): 401 [M+]; 317 [M+ – 3CO]. Anal. (%) Calc. for C15H12N3O5SMn: C, 44.90; H, 3.01 and N, 10.47; found: C, 44.09; H, 3.27 and N, 10.66.
2.2.3. [{(η5-C5H4)-CH=N–N(H)-C6H4-4-SO2NH2}]FeCp (3a)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4CHO)FeCp (54 mg, 0.25 mmol) and 4-hydrazinyl-benzenesulphonamide (47 mg, 0.25 mmol). Red solid, yield 56% (53 mg, 0.14 mmol). IR (KBr, cm−1): 3407–3302 (νNH/NH2); 1603 (νC=N); 1325 (νS-O); 1153 (νS-O). 1H NMR (acetone-d6): δ 4.17 (s, 5H, C5H5); 4.34 (st, 2H, C5H4); 4.62 (st, 2H, C5H4); 6.28 (s, 2H, NH2); 7.11 (d, 2H, J = 8.8 Hz, C6H4); 7.70 (d, 2H, J = 8.8 Hz, C6H4); 7.80 (s, 1H, CH=N); 9.56 (s, 1H, NH). 13C NMR (acetone-d6): δ 66.9 (C5H4); 68.9 (C5H5); 69.4 (C5H4); 80.9 (C5H4ipso); 111.0 (C6H4); 127.8 (C6H4); 133.1 (C6H4); 139.7 (CH=N); 148.6 (C6H4). Mass spectrum (m/z): 383 [M+]. Anal. (%) Calc. for C17H17N3O2SFe: C, 53.28; H, 4.47 and N, 10.96; found: C, 53.55; H, 4.36 and N, 10.82.
2.3. Synthesis of organometallic-hydrazones [{(η5-C5H4)-C(CH3)=N–N(H)-C6H4-4-SO2NH2}]MLn(1b, 2b, 3b)
4-Hydrazinyl-benzenesulphonamide (1 eq.) was charged in a two neck round bottomed flask with dried ethanol (10 ml) and a magnetic stir bar. The solution was refluxed under nitrogen atmosphere to obtain a clear solution. To the reaction mixture, acetyl-organometallic precursor (1 eq.) and four drops of HCl were added under stirring condition and the reaction was refluxed for 6 h. After this time, the reaction mixture was filtered and the precipitate was washed with cold hexane (3 × 10 ml) and dried under vacuum for 2 h. The solid obtained was purified using slow diffusion crystallization from THF/hexane (1:5) at −18 °C.
2.3.1. [{(η5-C5H4)-C(CH3)=N–N(H)-C6H4-4-SO2NH2}]re(CO)3 (1b)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4COCH3)Re(CO)3 (60 mg, 0.16 mmol) and 4-hydrazinyl-benzenesulphonamide (30 mg, 0.16 mmol). Brown solid, yield 50% (43 mg, 0.08 mmol). IR (KBr, cm−1): 3374–3256 (νNH/NH2); 2025 (νRe-CO); 1900 (νRe-CO); 1560 (νC=N); 1328 (νS-O); 1155 (νS-O).1H NMR (acetone-d6): δ 2.02 (s, 3H, CH3); 5.74 (t, 2H, J = 2.0 Hz, C5H4); 6.31 (t, 2H, J = 2.0 Hz, C5H4); 6.43 (s, 2H, NH2); 7.81 (d, 2H, J = 8.8 Hz, C6H4); 8.13 (d, 2H, J = 8.8 Hz, C6H4); 9.10 (s, 1H, NH). 13C NMR (acetone-d6): δ 21.4 (CH3); 85.9 (C5H4); 86.3 (C5H4); 103.6 (C5H4ipso); 128.9 (C6H4); 130.8 (C6H4); 138.4 (C6H4); 140.3 (C=N); 147.8 (C6H4); 194.9 (Re-CO). Mass spectrum (based on 187Re) (m/z): 547 [M+]; 463 [M+ – 3CO]. Anal. (%) Calc. for C16H14N3O5SRe: C, 35.16; H, 2.58 and N, 7.69; found: C, 35.26; H, 2.60 and N, 7.68.
2.3.2. [{(η5-C5H4)-C(CH3)=N–N(H)-C6H4-4-SO2NH2}]Mn(CO)3 (2b)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4COCH3)Mn(CO)3 (62 mg, 0.25 mmol) and 4-hydrazinyl-benzenesulphonamide (47 mg, 0.25 mmol). Brown solid, yield 48% (50 mg, 0.12 mmol). IR (KBr, cm−1): 3388–3255 (νNH/NH2); 2020 (νMn-CO); 1921 (νMn-CO); 1597 (νC=N); 1326 (νS-O); 1154 (νS-O). 1H NMR (acetone-d6): δ 2.10 (s, 3H, CH3); 4.92 (s, 2H, C5H4); 5.43 (t, 2H, C5H4); 6.90 (s, 2H, NH2); 7.85 (d, 2H, J = 8.8 Hz, C6H4); 8.16 (d, 2H, J = 8.8 Hz, C6H4); 9.15 (s, 1H, NH). 13C NMR (acetone-d6): δ21.4 (CH3); 83.0 (C5H4); 84.1 (C5H4); 99.3 (C5H4ipso); 128.0 (C6H4); 130.0 (C6H4); 136.5 (C6H4); 141.4(C=N); 148.2 (C6H4); Mn–CO (not observed). Mass spectrum (m/z): 415 [M+]; 331 [M+ – 3CO]. Anal. (%) Calc. for C16H14N3O5SMn: C, 46.27; H, 3.40 and N, 10.12; found: C, 45.99; H, 3.39 and N, 10.09.
2.3.3. [{(η5-C5H4)-C(CH3)=N-N(H)-C6H4-4-SO2NH2}]FeCp (3b)
This compound was prepared according to the general procedure described above, using in this case: (η5-C5H4COCH3)FeCp (57 mg, 0.25 mmol) and 4-hydrazinyl-benzenesulphonamide (47 mg, 0.25 mmol). Red solid, yield 56% (56 mg, 0.14 mmol). IR (KBr, cm−1): 3330–3202 (νNH/NH2); 1599 (νC=N); 1330 (νS-O); 1158 (νS-O). 1H NMR (acetone-d6): δ 2.11 (s, 3H, CH3); 3.99 (s, 5H, C5H5); 4.30 (s, 2H, C5H4); 4.82 (s, 2H, C5H4); 6.76 (s, 2H, NH2); 7.43 (d, 2H, J = 8.8 Hz, C6H4); 7.85 (d, 2H, J = 8.8 Hz, C6H4); 8.77 (s, 1H, NH). 13C NMR (acetone-d6): δ20.6 (CH3); 67.5 (C5H4); 68.9 (C5H5); 69.9 (C5H4); 80.9 (C5H4ipso); 128.1 (C6H4); 129.8 (C6H4); 137.1 (C6H4); 142.2 (C=N); 155.0 (C6H4). Mass spectrum (m/z): 397 [M+]. Anal. (%) Calc. for C18H19N3O2SFe: C, 54.42; H, 4.82 and N, 10.58; found: C, 54.46; H, 4.88 and N, 10.55.
2.4. CA inhibition studies
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activityCitation25. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10−100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5−10% of the reaction have been used for determining the initial velocity. The uncatalysed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled−deionized water, and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 6 h at room temperature prior to assay to allow for the formation of the E−I complex. The inhibition constants were obtained by nonlinear least-squares methods using PRISM 3 and the Cheng−Prusoff equation, as reported earlierCitation26–28, and represent the mean from at least three different determinations. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation26,Citation29.
3. Results and discussion
3.1. Synthesis and characterisation of bioorganometallic-hydrazones derivatives from sulphanilamide
Continuing our interest in organometallic compounds with enzyme inhibitory properties, we decided to prepare such derivatives by using the imine bond formation between 4-hydrazino-benzenesulphonamide and organometallic derivatives incorporating aldehyde or methylketone functionalities. The bio-organometallic-hydrazones of the general formula [{(η5-C5H4)-C(R)=N-N(H)-C6H4-4-SO2NH2}]MLn(MLn = Re(CO)3, Mn(CO)3, FeCp; R=H, CH3) were thus prepared by the reaction between formyl or acetyl organometallic precursors and4-hydrazino-benzenesulphonamidein H2O (1a, 2a, 3a) or anhydrous ethanol (1b, 2b, 3b) in the presence of HCl (Scheme 1). The new compounds were isolated with moderate yields (48–56%) as brown (1a–b, 2a-b) or red (3a–b) solids after crystallization from acetone/hexane or THF/hexane, and exhibited good solubility in most polar organic solvents.
All compounds were characterised by electron impact (EI) mass spectrometry, elemental analysis, infra-red and NMR spectroscopies (Supporting Information). A strong molecular ion was shown in the mass spectrum of each organometallic-hydrazone, in addition to the detection of notable successive losses of CO ligands for the cyrhetrenyl (1a–b) and cymantrenyl (2a–b) derivatives (Supplementary Figure S1†). The elemental analysis data determined for all compounds is in agreement with their proposed formulas.
The FT-IR spectra (KBr disk) of all compounds showed the expected absorption bands for the νN–H, νC=N and νSO2 stretches in the ranges of 3407–3202 cm−1, 1603–1560 cm−1 and 1330–1147 cm−1, respectively (Supplementary Figure S2†). Similar frequency values have been reported for others organicCitation30,Citation31 and organometallic-hydrazonesCitation32–34. In addition, IR analysis of compounds 1a–b and 2a–b revealed the presence of two intense bands in the region of 2025–1900 cm−1, that are characteristic of the asymmetric (νas) and symmetric (νs) stretchings of the terminal CO ligands of cyrhetrene and cymantrene derivativesCitation35–37 (Supplementary Figure S3†).
It is well-known that hydrazones may adopt two different forms (E- or Z-)Citation38 in the solid state and also in solution. 1H and 13C{1H} NMR spectra of all compounds showed only one set of resonances, thus suggesting that only one of the two isomers was present in acetone-d6 solution at room temperature. On this regard, 1H-NMR spectra of 1a, 2a and 3a showed exhibited a sharp singlet was observed in the ranges of 7.80–7.64 ppm, and it was assigned to the iminic proton. In addition, a signal due to the methyl protons of the –C(CH3)=N–fragment was also observed at approximately δ 2.1 for compounds 1b, 2b and 3b. These results are in agreement with the values reported for organicCitation39 and organometallic analoguesCitation40,Citation41. In addition, the resonances observed between 8.16 and 7.11 ppm were assigned to the hydrogen atoms of the C6H4 ring. As per literature reports, the broad singlet observed at 6.90–6.28 ppm was assigned to the hydrogen nuclei of the SO2NH2 groupCitation42,Citation43. Moreover, 1H NMR spectra for 1a-b and 2a-b showed sets of resonances in the region of 6.31–4.92 ppm, which are ascribed to the protons of the cyrhetrenyl and cymantrenyl moietiesCitation44,Citation45. On this regard, the ferrocenyl derivatives 3a–b exhibited resonances around δ 4.82–4.30 due to the non-equivalent alpha and beta protons containing in the substituted Cp ring and a singlet in the region of 4.17–3.99 ppm, which was assigned to the proton resonances of the unsubstituted cyclopentadienyl groupCitation46,Citation47. The presence of the NH group registered as a broad singlet in the range of 9.9–8.7 ppm. Similar δ have been reported for other organometallic to sylhydrazonesCitation48. It is important to note that the chemical shifts of the NH resonance showed a clear dependence on the presence of the organometallic moiety bound to the iminic entity. In fact, the downfield shift observed for the cyrhetrenyl (1a–b) and cymantrenyl (2a–b) tosyl HYDs (Δδ ∼ 0.30) compared with ferrocenyl analogues (3a–b) can be related to the electron-withdrawing properties of the (η5-C5H4)M(CO)3 moietiesCitation49, which produce a deshielding of the NH resonance, thus, suggesting that the nature of the organometallic framework modifies the degree of electronic delocalisation on the –C(R) = N–NH– unit. We have found similar results for ferrocenyl and cyrhetrenyl 1,3,4-thiadiazolesCitation50 and thiosemicarbazonesCitation51.
The 13C NMR data are also in agreement with the proposed structures, that is, all compounds showed the carbon nuclei of the organometallic fragments, C=N bridge and phenyl moiety. As expected, the resonances for the carbon atoms of the CH3 and C6H4 groups were observed at δ 21 and 155–110 and did not show any noticeable differences from those reported for the organicCitation52 and organometallic analoguesCitation48,Citation53. The most important feature of the 13C NMR spectra is the presence of a low field resonance (148–131 ppm), which was assigned to the iminyl carbon [C=N].
The carbon chemical shifts of this group for 1a, 2a and 3a also showed a clear dependence on the electronic properties of the organometallic moiety attached to it. The upfield shift observed for the cyrhetrenyl (1a) and cymantrenyl (2a) tosyl HYDs (∼132 ppm) compared with the ferrocenic analogue (3a) (140 ppm) can also be related to the opposite electronic effects of these organometallic moieties. This proposal is in agreement with the trend observed in the resonance of the NH proton mentioned above. We previously reported similar results for Schiff basesCitation54, thiosemicarbazonesCitation51 and hydrazonesCitation55 containing ferrocenyl and cyrhetrenyl moieties. This phenomenon is not observed in compounds 1b, 2b and 3b, in which the hydrazone fragment possess a methyl group attached to the C=N entity. In these cases, we believe that the inductive effect of the CH3 substituent could better stabilise any charge generated in the hydrazone entity; therefore, no difference in the iminyl carbon resonance would be evidenced. It is important to mention that all assignments were confirmed by 1H 13C NMRHSQC (Supplementary Figure S4†).
3.2. CA inhibition studies
The obtained sulphonamides (1–3)a,b were investigated for their CA inhibitory properties by using a stopped-flow CO2 hydrase assayCitation25 and four human CA isoforms (hCA I, II, IX and XII) known to be drug targetsCitation1–5 ().
Table 1. hCA I, II, IX and XII inhibition data with compounds (1–3)a,b and acetazolamide (AAZ) as standard drug, by a stopped-flow CO2 hydrase assay [Citation25].
The new organometallic derivatives exhibited effective inhibition of the cytosolic isoforms hCA I and II, with inhibition constants in the range of 1.7–22.4 nM, except for 2b which was a moderate-weak hCA I inhibitor (KI of 286.6 nM; ). Thus, apart this outlier compound 2b, the nature of the metal ion, the presence/absence of CO moieties or the aldehyde/ketone nature of the starting materials did not influence significantly the CA inhibitory properties. The ferrocenyl derivatives 3a and 3b were slightly more effective as CAIs compared to the structurally similar derivatives, but the differences were not impressive. Against hCA IX, a transmembrane, tumour-associated isoform, only 2a was a low nanomolar inhibitor, and 1b and 3b had KI values <50 nM. The remaining derivatives showed medium potency inhibitory action, with inhibition constants in the range of 221.3–579.8 nM. However, the second transmembrane, tumour-associated isoform hCA XII was effectively inhibited by all new compounds reported here, with KI values in the range of 1.9–24.4 nM. The structure–activity relationship (SAR) is thus quite flat, with a very modest range of variation in the inhibition constant values. Anyhow, the best CAI was 3b with an inhibition constant of 1.9 nM (ferrocenyl derivative) and the least effective one was the rhenium carbonyl derivative 1a with a KI of 24.4 nM.
4. Conclusion
A small number of organometallic benzenesulphonamide derivatives were obtained by reaction of aldehydes/ketones incorporating ferrocenyl or rhenium/manganese tricarbonyl moieties with 4-hydrazino-benzenesulphonamide. The new compounds were investigated as inhibitors of four pharmacologically relevant human CA isoforms, hCA I, II, IX and XII. The cytosolic isoforms hCA I and II were effectively inhibited by almost all derivatives with inhibition constants in the range of 1.7–22.4 nM. The same effect was observed for the tumour-associated transmembrane isoform hCA XII, for which KIs in the range of 1.9–24.4 nM were measured. hCA IX was on the other hand less sensitive to inhibition with these compounds, with only one derivative showing low nanomolar inhibitory action. The presence of metallo-organic or metallo-carbonyl moieties in the molecules of these CAIs makes them amenable for interesting pharmacologic applications, for example for compounds with CO donating properties.
Supplemental Material
Download PDF (283.7 KB)Acknowledgements
R. A. acknowledges FONDECYT-Chile (Project 1190327) and VRID-UdeC [Grant 216.021.034–1.0] for the funding of this study. J. B. acknowledges the internships of IUT, Lannion.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- (a) Nocentini A, Supuran CT. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin Drug Discov 2019;14:1175–97. (b) De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 2013;8:793–810.
- Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018;38:1799–836.
- (a) Berrino E, Supuran CT. Novel approaches for designing drugs that interfere with pH regulation. Expert Opin Drug Discov 2019;14:231–48. (b) Köhler K, HillebrechtA S, Wischeler J, et al. Saccharin inhibits carbonic anhydrases: possible explanation for its unpleasant metallic aftertaste. Angew Chem Int Ed Engl 2007;46:7697–9.
- Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70.
- Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12.
- Jensen EL, Clement R, Kosta A, et al. A new widespread subclass of carbonic anhydrase in marine phytoplankton. Isme J 2019;13:2094–106.
- (a) Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91. (b) Carta F, Supuran CT, Scozzafava A. Novel therapies for glaucoma: a patent review 2007–2011. Expert Opin Ther Pat 2012;22:79–88. (c) Masini E, Carta F, Scozzafava A, Supuran CT. Antiglaucoma carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat 2013;23:705–16.
- Aggarwal M, Kondeti B, McKenna R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: a patent review. Expert Opin Ther Pat 2013;23:717–24.
- (a) Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 2013;23:725–35. (b) Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin Ther Pat 2018;28:713–21. (c) Supuran CT. Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev Neurother 2015;15:851–6.
- (a) Supuran CT. Carbonic anhydrases and metabolism. Metabolites 2018;8:25. (b) Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:E48.
- (a) Carta F, Di Cesare Mannelli L, Pinard M, et al. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem 2015;23:1828–40. (b) Supuran CT. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev Neurother 2016;16:961–8.
- Di Cesare Mannelli L, Micheli L, Carta F, et al. Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. Enzyme Inhib Med Chem 2016;31:894–9.
- (a) Margheri F, Ceruso M, Carta F, et al. Overexpression of the transmembrane carbonic anhydrase isoforms IX and XII in the inflamed synovium. J Enzyme Inhib Med Chem 2016;31:60–3. (b) Bua S, Di Cesare Mannelli L, Vullo D, et al. Design and synthesis of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the treatment of rheumatoid arthritis. J Med Chem 2017;60:1159–70. (c) Akgul O, Di Cesare Mannelli L, Vullo D, et al. Discovery of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs-CAIs) for the management of rheumatoid arthritis. J Med Chem 2018;61:4961–77.
- Heldt JM, Fischer-Durand N, Salmain M, et al. Preparation and characterization of poly(amidoamine) dendrimers functionalized with a rhenium carbonyl complex and PEG as new IR probes for carbonyl metallo immunoassay. J Organomet Chem 2004;689:4775–82.
- Herrmann WA. Synthetic methods of organometallic and inorganic chemistry. Stuttgart: Thieme Medical Pub; 1997.
- Hromadová M, Salmain M, Sokolová R, et al. Novel redox label for proteins: electron transfer properties of (ƞ5-cyclopentadienyl)tricarbonyl manganese bound to bovine serum albumin. J Organomet Chem 2003;668:17–24.
- Winum JY, Dogné JM, Casini A, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/membrane associated carbonic anhydrase isozymes i, ii, and ix with sulphonamides incorporating hydrazino moieties. J Med Chem 2005;48:2121–5.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Vermelho AB, da Silva Cardoso V, Ricci Junior E, et al. Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J Enzyme Inhib Med Chem 2018;33:139–46. (b) Nocentini A, Carta F, Tanc M, et al. Deciphering the mechanism of human carbonic anhydrases inhibition with sulfocoumarins: computational and experimental studies. Chemistry 2018;24:7840–4. (c) Awadallah FM, Bua S, Mahmoud WR, et al. Inhibition studies on a panel of human carbonic anhydrases with N1-substituted secondary sulfonamides incorporating thiazolinone or imidazolone-indole tails. J Enzyme Inhib Med Chem 2018;33:629–38.
- (a) Bua S, Bozdag M, Del Prete S, et al. Mono- and di-thiocarbamate inhibition studies of the δ-carbonic anhydrase TweCAδ from the marine diatom Thalassiosira weissflogii. J Enzyme Inhib Med Chem 2018;33:707–13. (b) Ferraroni M, Gaspari R, Scozzafava A, et al. Dioxygen, an unexpected carbonic anhydrase ligand. J Enzyme Inhib Med Chem 2018;33:999–1005. (c) El-Gazzar MG, Nafie NH, Nocentini A, et al. Carbonic anhydrase inhibition with a series of novel benzenesulfonamide-triazole conjugates. J Enzyme Inhib Med Chem 2018;33:1565–74. (d) Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1,3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J Enzyme Inhib Med Chem 2018;33:1575–80.
- (a) Nocentini A, Bonardi A, Gratteri P, et al. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J Enzyme Inhib Med Chem 2018;33:1453–9. (b) Nocentini A, Trallori E, Singh S, et al. 4-Hydroxy-3-nitro-5-ureido-benzenesulfonamides selectively target the tumor-associated carbonic anhydrase isoforms IX and XII showing hypoxia-enhanced antiproliferative profiles. J Med Chem 2018;61:10860–74. (c) Chohan ZH, Munawar A, Supuran CT. Transition metal ion complexes of Schiff bases. Synthesis, characterization and antibacterial properties. Met Based Drugs 2001;8:137–43. (d) OztürkSarikaya SB, Topal F, Sentürk M, et al. In vitro inhibition of α-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–62.
- (a) Awadallah FM, Bua S, Mahmoud WR, et al. Inhibition studies on a panel of human carbonic anhydrases with N1-substituted secondary sulfonamides incorporating thiazolinone or imidazolone-indole tails. J Enzyme Inhib Med Chem 2018;33:629–38. (b) Supuran CT, Clare BW. Carbonic anhydrase inhibitors. Part 57. Quantum chemical QSAR of a group of 1,3,4-thiadiazole and 1,3,4-thiadiazoline disulfonamides with carbonic anhydrase inhibitory properties. Eur J Med Chem 1999;34:41–50. (c) Sentürk M, Gülçin I, Daştan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11.
- Demurtasa M, Baldisserotto A, Lampronti I, et al. Indole derivatives as multifunctional drugs: synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorg Chem 2019;85:568–76.
- Soares Coimbra E, Nora de SM, Sequetto Terrora M, et al. Synthesis, biological activity, and mechanism of action of new 2-pyrimidinyl hydrazone and N-acylhydrazone derivatives, a potent and new classes of antileishmanial agents. Eur J Med Chem 2019;184:111742.
- Manzur C, Baeza E, Millan L, et al. Organometallic iron (II) hydrazines and hydrazones – syntheses, characterisations and the X-ray crystal structures of [Fe(η5-Cp)(η6-C6H5NHNH2)]+PF6− and [Fe(η5-Cp)(η6-p-MeC6H4NHNCMe2)]+PF6. J Organomet Chem 2000;608:126–32.
- Trujillo A, Fuentealba M, Manzur C, et al. Synthesis and properties of new dinuclearorganoiron(II) hydrazones combining the potent electron-donating [(η5-C5H4)FeCp] fragment with [CpFe(η6-arene)]+-type acceptors. J Organomet Chem 2003;681:150–7.
- Huentupil Y, Peña L, Novoa N, et al. New sulfonamides containing organometallic-acylhydrazones: synthesis, characterisation and biological evaluation as inhibitors of human carbonic anhydrases. J Enzyme Inhib Med Chem 2019;34:451–8.
- Toro P, Acuña A, Mallea M, et al. Condensation and substitution products obtained in reactions of isomeric bromo-nitrofuraldehydes with ferrocenylamine: electrochemistry and anti-parasitic evaluation. J Organomet Chem 2019;901:120946.
- Gómez J, Sierra D, Fuentealba M, et al. Homo- and heterobimetallicazines derived from ferrocene and cyrhetrene: synthesis, structural characterization and electrochemical studies. J Organomet Chem 2019;883:65–70.
- Mishra S, Tirkey V, Ghosh A, et al. Ferrocenyl–cymantrenyl hetero-bimetallic chalcones: synthesis, structure and biological properties. J Mol Struct 2015;1085:162–72.
- Barton D, Ollis WD, eds. Comprehensive organic chemistry. Oxford (UK): Pergamon; 1979.
- Mandewale M, Patil U, Shedge S, et al. A review on quinoline hydrazone derivatives as a new class of potent antitubercular and anticancer agents. Beni-Suef Univ J Basic Appl Sci 2017;6:354–61.
- Fuentealba M, Toupet L, Manzur C, et al. Pentamethylcyclopentadienylorganoiron(II) hydrazone complexes: synthesis, spectroscopic characterization, and second-order nonlinear optical properties. X-ray crystal structure of[(η5-C5Me5)Fe[(η6-C6H5)NHNH2]+ PF6. J Organomet Chem 2007;692:1099–109.
- Gómez J, Leiva N, Arancibia R, et al. Synthesis, characterization, crystal structures and computational studies on novel cyrhetrenylhydrazones. J Organomet Chem 2016;819:129–37.
- Krasavin M, Kalinin S, Zozulya S, et al. Screening of benzenesulfonamide in combination with chemically diversefragments against carbonic anhydrase by differential scanning fluorimetry. J Enzyme Inhib Med Chem 2020;35:306–10.
- Bilginer S, Gonder B, Gul HI, et al. Novel sulphonamides incorporating triazene moieties show powerful carbonic anhydrase I and II inhibitory properties. J Enzyme Inhib Med Chem 2020;34:325–9.
- Glans L, Hu W, Jöst C, et al. Synthesis and biological activity of cymantrene and cyrhetrene 4-aminoquinoline conjugates against malaria, leishmaniasis, and trypanosomiasis. Dalton Trans 2012;41:6443–50.
- Muñoz-Osses M, Godoy F, Fierro A, et al. New organometallicimines of rhenium (i) as potential ligands of GSK-3b: synthesis, characterization and biological studies. Dalton Trans 2018;47:1233–42.
- Dewangan S, Barik T, Parida R, et al. Solvent free synthesis of ferrocene based rhodamine – hydrazone molecular probe with improved bioaccumulation for sensing and imaging applications. J Organomet Chem 2019;904:120999.
- Almendras I, Huentupil Y, Novoa N, et al. Trinuclear Ni(II), Pd(II) and Cu(II) complexes containing the 2-hydroxy-benzaldehyde-ferrocenyl-sulfonylhydrazone ligand: synthesis, structural characterization and antiplasmodial evaluation. Inorg Chim Acta 2019;496:119050.
- Förster C, Veit P, Ksenofontov V, Heinze K. Diferrocenyltosylhydrazone with an ultrastrong NH⋯Fe hydrogen bond as double click switch. Chem Commun 2015;51:1514–6.
- Quintana C, Silva G, Klahn A, et al. New cyrhetrenyl and ferrocenyl sulfonamides: synthesis, characterization, X-ray crystallography, theoretical study and anti-Mycobacterium tuberculosis activity. Polyhedron 2017;134:166–72.
- Quintana C, Klahn AH, Artigas V, et al. Cyrhetrenyl and ferrocenyl1,3,4-thiadiazole derivatives: synthesis, characterization, crystal structures and in vitro antitubercular activity. Inorg Chem Commun 2015;55:48–50.
- Arancibia R, Klahn A, Lapier M, et al. Synthesis, characterization and in vitro anti-Trypanosoma cruzi and anti-Mycobacterium tuberculosis evaluations of cyrhetrenyl and ferrocenyl thiosemicarbazones. J Organomet Chem 2014;755:1–6.
- Aslan H, Karacan N, Aslan E. Synthesis, characterization and antimicrobial activity of a new aromatic sulfonyl hydrazone derivative and its transition metal complexes. J Chin Chem Soc 2013;60:212–7.
- Veit P, Prantl E, Förster C, Heinze K. Competitive NH···Ru/Fe hydrogen bonding in ferrocenyl ruthenocenyl tosyl hydrazone. Organometallics 2016;35:249–57.
- Arancibia R, Klahn AH, Buono-Core GE, et al. Synthesis, characterization and anti-Trypanosoma cruzi evaluation of ferrocenyl and cyrhetrenyl imines derived from 5-nitrofurane. J Organomet Chem 2011;696:3238–44.
- Concha C, Quintana C, Klahn AH, et al. Organometallic to sylhydrazones: synthesis, characterization, crystal structures and in vitro evaluation for anti-Mycobacterium tuberculosis and antiproliferative activities. Polyhedron 2017;131:40–5.