Abstract
Thirty-six novel threoninamide carbamate derivatives were designed and synthesised using active fragment-based pharmacophore model. Antifungal activities of these compounds were tested against Oomycete fungi Phytophthora capsici in vitro and in vivo. Interestingly, compound I-1, I-2, I-3, I-6 and I-7 exhibited moderate control effect (>50%) against Pseudoperonospora cubensis in greenhouse at 6.25 μg/mL, which is better than that of control. Meanwhile most of these compounds exhibited significant inhibitory against P. capsici. The other nine fungi were also tested. More importantly, some compounds exhibited remarkably high activities against Sclerotinia sclerotiorum, P. piricola and R. solan in vitro with EC50 values of 3.74–9.76 μg/mL. It is possible that the model is reliabile and this method can be used to discover lead compounds for the development of fungicides.
Introduction
Oomycete fungi can cause several destructive diseases in crops, vegetables and fruits, such as Phytophthora infestans, Peronospora hyoscyami, Phytophthora capsici and Pseudoperonospora cubensisCitation1. The cell walls of the Oomycetes are different from other fungi, which contain cellulose, not chitin. So cellulose synthase represents a potential target for discovering new Oomycete inhibitorsCitation2, which can inhibit different stages in the life cycle of Oomycetes including mycelial growth, sporangium production, zoospore release and cystospore germination. Since dimethomorphCitation3 was discovered by Shell company, seven carboxylic acid amide (CAA) fungicidesCitation4,Citation5 including flumorphCitation6, pyrimorphCitation7, benthiavalicarbCitation8, benthiavalicarb-isopropylCitation9, iprovalicarbCitation10, valiphenalCitation11, and mandipropamidCitation12 were developed, which were divided as three different sub-classes by FRAC (www.frac.info) due to their common cross resistance pattern for the vast majority of Oomycetes. Since the dimethomorph was discovered as first CAA fungicide in 1988, only seven CAA fungicides are marketed until now.
However, it was still unknown that the structure of cellulose synthases in the Oomycete plant pathogen. Blum et al.Citation13,Citation14 reported that both mutations in the PiCesA3 gene of P. infestans result in a change to the same amino acid (glycine-1105) in the protein and the mutations in PiCesA3 were responsible for the mandipropamid insensitivity phenotype. The resistance mutants of some pathogens to CAA fungicides has been elucidated in recent reportsCitation15,Citation16. However, purified protein of Oomycete of cellulose synthase is not available. In recent reference, her groupCitation17 built a modelling of the P. capsici cellulose synthase 3. In our previous workCitation18–35, many bioactive compounds were designed and synthesised. In this paper, based on the structure of seven commercialised CAA fungicides, we found that they have similar structural fragments: amide bond, para-substituted phenyl, 3,4-dialkyloxy substituted phenyl. Only valinamide carbamates have two result fragments, so we established a pharmacophore model. The dialkyloxybenzene substructures were introduced into threoninamidecarbamates and designed the title compounds.
Results and discussion
Active-fragment-based pharmacophore model
The key technical challenge for this approach was the detection of fragment hits. Traditionally, fragment hits were often found by conventional bioassay-based methods and biophysical methods (X-ray, NMR and surface plasmon resonance). However, in our previous workCitation36–39, we find that three sub-classes fungicides have nearly identical structural fragments: including amide, halobenzene (or methylbenzene) and/or dialkoxyl benzene. The three fragments are exactly what we are looking for fragments with a good match with a target binding site, because any optimisation of the three fragments could lead to reduced antifungal activity. Furthermore, we noticed that valinamide carbamates only have two of three active structural fragments. In order to validate our idea, a new valinamide carbamate with three fragments was designed and synthesised (). The compound was found to display higher in vitro antifungal activities against P. capsici (EC50 0.15 μg/mL) than iprovalicarb (EC90 0.27 μg/mL)Citation38. This result prompted us to develop a new active-fragment-based drug discovery, which is especially suited if no purified protein and no structural information on the binding site are available.
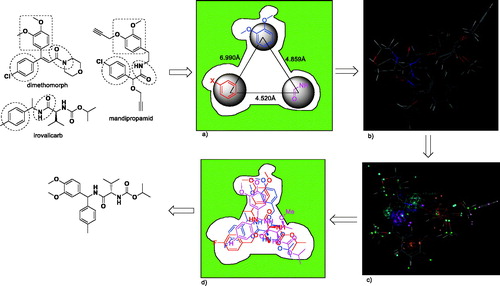
After a careful analysis of the seven structures of CAA fungicides, it was found that the skeleton structure of cinnamic acid amides molecules is rigid, and the skeleton structure of the other two kind of molecules are flexible. So it is possible that the active fragments of valinamide carbamates and mandelic acid amides bind to the same pocket sites as that of cinnamic acid amides. From these fungicides, the distance between these active fragments is easily identified ().
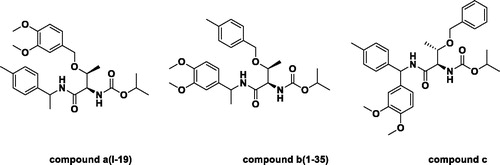
The three representative compounds (dimethomorph, iprovalicarb and mandipropamid) were performed using MOE. The 3D structures of the compounds were built by using the Builder option and geometry-optimized by using MMFF94x Forcefield and calculate forcefield partial charges. The three compounds were used successively for energy minimisation until the gradient value was smaller than 0.001 kcal/mol. The lowest-energy conformations of the three compounds were generated and the conformation of dimethomorph was served as templates in the study. Then the three compounds were aligned. The results are shown in . Through three sub-types of molecular alignment, we built a pharmacophore model using SYBYL 6.9, which is shown in . The results evaluated using pharmacophore scores. Threonine is an essential amino acid, which cannot be synthesised in humans. It’s structure is similar to valine. So a set of three threonine derivatives were designed and prepared using the above-described procedure for test case.
The compounds a and b showed good antifungal activity, their EC50 value were 3.49 and 3.10 μg/mL, respectively. Compound c bearing three benzene ring showed weaker (8.88 μg/mL) antifungal activity (). From the structural analysis, it is possible that the compound c have three benzene rings, which make the crowded space and affect the activity. In order to find higher active compounds, another 33 threonine derivatives were synthesised and screened for antifungal against P. capsici, and their results are listed in . The antifungal activity of compound a, b and c further indicated the model is reliable.
Synthesis and spectra
The synthesis procedures for compounds designed were shown in Scheme 1.
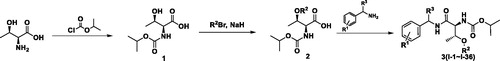
The intermediate amine was synthesised easily according to R. Lecchart reaction by using formamide and ketone as starting materials (Scheme 2). HCOONH4 was used as reactant under the solvent-free condition in the R. Lecchart reaction. When the reaction temperature heated to 160 °C, the reaction is violent and the yield is moderate (66%). In this article, the HCOONH4 was replaced by HCONH4 solution (5 quiv H2O), the reaction is mild and the yield increased (75%).
Isopropyl chloroformate is treated with L-threonine in aqueous sodium hydroxide solution to give isopropyloxycarbonyl-L-threonine 1. The intermediate 1 was readily alkylated by sodium hydride in THF to alkoxy-substituted compounds 2. And then, the carboxylic acid group of compounds 2 is activated by treating it with a equivalent of ethyl chloroformate under base conditions using tetrahydrofuran as a solvent. Finally, the mixed anhydride, which cannot be isolated, was treated with an auxiliary base and the amines intermediate in THF solution, as part of the same reaction step to form the title compounds 3 with the elimination of carbon dioxide and isopropanol in 60–88% yield. When R3 was methyl, designed compounds 3 could not be obtained by using the above-mentioned method. So L-threonine amides 4 were first synthesised by using the above-mentioned method. The free hydroxyl group of compounds 4 were then alkylated with iodomethane in the presence of silver oxide to obtain methyl-substituted compounds designed 3.
Antifungal activity and SAR
In our previous workCitation38, valinamide carbamate compounds exhibited excellent antifungal activity against P. capsici, so the title threonine compounds were screened in vitro against P. capsici directly. The EC50 values for compounds were determined and the results are listed in . From , compounds I-7, I-10, I-11, I-12, I-13, I-22, I-23, I-24, I-25, I-26 displayed excellent antifungal activity (EC50 < 1 μg/mL) against P. capsici. Especially compound I-24, the activity (EC50 = 0.14 μg/mL) is higher than that of control dimethomorph (EC50 = 0.37 μg/mL). The structure-activity relationship results showed that when R3 is Me group, R1 is p-Cl, p-Me, p-MeO, p-Pgoxy and 3-MeO-4-Pgoxy, R2 is Bn, p-FBn and p-ClBn, the compounds exhibited excellent activity, such as I-3, I-7, I-10, I-22, I-23, I-24, I-25, I-26. Among them, when R1 is p-Pgoxy or p-MeO and R2 is p-FBn, they exhibited highest antifungal activity, such as compound I-10, I-23, I-24, I-25, I-26. When the R3 of these compounds was replaced by phenyl group or substituted phenyl group, the activity decreased. These compounds exhibited weak antifungal activity, such as compound I-13, I-14, I-15, I-16, I-17.
Table 1. The EC50 values of threonine derivatives against P. capsici.
Based on results of the in vitro antifungal activity against P. capsici, compounds I-1, I-2, I-3, I-6, I-7, I-10, I-18 and I-23 were selected for further in vivo antifungal activity against P. capsici and P. cubensis. The results are listed in . From , compounds I-1, I-3 and I-6 exhibited good control effect (100%) against P. capsici at 50 μg/mL, which is the same as the control dimethomorph (100%). Unluckily, the compound I-18 (65.2%) and I-23 (83.6%) exhibited moderate in vivo antifungal activity, although the two compounds exhibited good in vitro antifungal activity. Interestingly, most of these compounds also exhibited good control effect (>80%) against P. cubensis at 50 μg/mL, which is better than the control dimethomorph (74.2%). Among them, only compound I-23 (66.9%) exhibited moderate control effect against P. cubensis at 50 μg/mL. An extremely obvious phenomenon, the control effect decreased while the concentration dropped from 50 to 6.25 μg/mL. For example, most of these compounds exhibited moderate control effect against P. capsici at 25 μg/mL, except compound I-1 (100%). But the control effect against P. capsici is lower than the control dimethomorph(100%) at 12.5 μg/mL, even at lower concentration. Notably, only compound I-6 exhibited good control effect with the inhibitory of 66.0 ± 3.1 at 6.25 g/mL, which was weaker than that of the control dimethomorph (87.0%). But when the concentration was reduced to 3.125 μg/mL, the control effect is lower than that of control dimethomorph (62.5%). For the other fungal P. cubensis, most of tested compounds exhibited good control effect (>70%) at 50 μg/mL, which is the same as the control dimethomorph (74.2%). For instance, compounds I-2 (96.7%), I-6 (90.1%) displayed excellent control effect (>90%), which is much better than that of control dimethomorph (74.2%). The control effect of dimethomorph was 46.9% at 6.25 μg/mL, while the control effect of compounds I-2, I-3 and I-6 were all higher than 50%.
Table 2. The in vivo fungicidal activity against two fungi of some compounds.
In vitro other antifungal activity
The in vitro antifungal activity of title 16 compounds against Fusarium oxysporum (FO), Cercospora arachidicola (CA), Physalospora piricola (PP),Alternaria solani (AS), Gibberella zeae (GZ), Phytophthora infestans (PI), Sclerotinia sclerotiorum (SS), Botrytis cinerea (BC) and Rhizoctonia solani (RS) at 50 μg/mL was tested and listed in . Chlorothalonil and carbendazimwere used as the control.
Table 3. The in vitro fungicidal activity of compound at 50 μg/mL.
As shown in , most of the title compounds were found to exhibit certain antifungal activity. Among them, lots of compounds exhibited good fungicidal activity against P. piricola, S. sclerotiorum and R. solani. Among these compounds, I-23 (100, 79.5 and 84.3%), I-33 (92.0, 90.3 and 77.6%) also exhibited good activity against P. piricola, S. sclerotiorum and R. Solani, respectively. For the F. oxysporum, the compounds displayed moderate or low activity. Compounds I-23 and I-33 possessed good activity (76.9 and 76.9%) respectively, which is better than control Carbendazim (8.3%). All the compounds exhibited moderate inhibition against A. solani, (>70%), meanwhile, the control chlorothalonil and carbendazim also exhibited moderate activity (73.9 and 43.5%) against A. solani. For G. zeae and P. infestans, the control Carbendazim (100%) can kill them, but these compounds exhibited moderate activity (∼50%) against G. zeae and P. infestans. Compounds I-10 (80.4%) and I-23 (81.4%) possessed good antifungal activity against B. Cinerea, which is the same as the control Chlorothalonil (96.1%).
On the basis of the preliminary fungicidal activity results, compound I-10, I-18, I-21, I-23, I-33and Chlorothalonil were selected for further EC50 bioassays (> 90% inhibitory) and the results are shown in . From , compounds I-21 and I-33 exhibited good activity against P. piricola, which is better than that of control Chlorothalonil. For the S. sclerotiorum, only compound I-10 displayed good activity, which is better than that of control Chlorothalonil. Among them, when the R1 is MeO or p-Pgoxy, R3 is Me, they exhibited best fungicidal activity for the three fungi respectively, such as compounds I-33 and I-10.
Table 4. The EC50 of some compounds against three fungals (μg/mL).
Experimental
Instrument
All the chemical reagents were analytical grade or prepared in our lab. Melting points were measured using an X-4 apparatus (Taike, Beijing, China) and were uncorrected. 1H NMR and 13C NMR spectra were recorded on BRUKER Advance 400 MHz spectrometer using CDCl3 as solvent. HRMS was determined on a FTMS 7.0 instrument.
Synthesis
(2S, 3 R)-2-carbamate isopropyl-3-hydroxybutyric acid 1
L-threonine (35.8 g, 0.3 mol) was added into 2 N NaOH solution and stirred for 1 h, and isopropyl chloroformate (40.6 g, 0.36 mol) was added at 0 °C. The reaction was stopped by stirring at room temperature for 1 h, washed by ether (100 ml), then water phase was adjusted to pH 2–3 with 1 M dilute HCl, extracted by ether (80 ml*4), and dried by anhydrous MgSO4 to give 1, white solid (48 g, yield 78.0%). m.p. 112–114 °C, 1H NMR (400 MHz, CDCl3) δ 5.04–4.84 (m, 1H, (CH3)2CHO), 4.43 (s, 1H, NHCHCOOH), 4.34 (s, 1H, CH3CHOH), 1.28 (d, J = 6.1 Hz, 9H, CH3CH).
3-(benzyloxy)-2-((isopropoxycarbonyl)amino)butanoic acid
(2S, 3 R)−2-carbamate isopropyl-3-hydroxybutyric acid (5 g, 24.39 mmol) was dissolved in DMF (50 ml), then 60% NaH (2.93 g, 73.17 mmol) was added in batches at 0 °C. After stirring for 2 h, (chloromethyl)benzene (4.62g, 36.59 mmol) was added dropwise. The reaction was quenched by stirring for 5 h at room temperature. Then the mixture was poured into water (250 ml) and washed by ether (100 ml) once. the water phase was adjusted to pH 2–3 with 1 M dilute HCl, ether was extracted (80 ml*4), and dried by anhydrous MgSO4 to give 2a, yellow oil, yield 52.1%, 1H NMR (400 MHz, CDCl3) δ 7.38–7.11 (m, 5H, Ar-H), 4.96 (s, 1H, NHCHCOOH), 4.40 (s, 1H, (CH3)2CHO), 4.13 (s, 1H, CH3CHOCH2), 3.09 (d, J = 6.9 Hz, 2H, Ar-CH2O), 1.52–0.99 (m, 9H, CH3). 3-((4-fluorobenzyl)oxy)−2-((isopropoxycarbonyl)amino)butanoic acid 2b: yellow oil, yield 85.2%, 1H NMR (400 MHz, CDCl3) δ 9.41 (s, 1H, NHCHCOOH), 7.22 (dd, J = 8.4, 5.6 Hz, 2H, Ar-H), 6.98 (dd, J = 15.3, 6.6 Hz, 2H, Ar-H), 5.40 (d, J = 9.3 Hz, 1H, NHCHCOOH), 4.91 (td, J = 12.4, 6.2 Hz, 1H, NHCHCOOH), 4.59–4.34 (m, 2H, Ar-CH2O), 4.24–4.13 (m, 1H, (CH3)2CHO), 3.12–2.79 (m, 1H, CH3CHOCH2), 1.52–1.07 (m, 9H, CH3). 3-((4-chlorobenzyl)oxy)−2-((isopropoxycarbonyl)amino)butanoic acid 2c: yellow solid, mp 125–126 °C, yield 74.3%, 1H NMR (400 MHz, CDCl3) δ 9.11 (s, 1H, NHCHCOOH), 7.38–7.29 (m, 2H, Ar-H), 7.18 (d, J = 7.9 Hz, 2H, Ar-H), 5.40 (d, J = 9.0 Hz, 1H, NHCHCOOH), 4.55 (d, J = 11.8 Hz, 1H, NHCHCOOH), 4.40 (dd, J = 16.4, 10.7 Hz, 2H, Ar-CH2O), 4.25–4.10 (m, 1H, (CH3)2CHO), 3.49 (d, J = 6.8 Hz, 1H, CH3CHOCH2), 1.23 (dd, J = 19.8, 6.5 Hz, 9H, CH3). 2-((isopropoxycarbonyl)amino)−3-((4-methylbenzyl)oxy)butanoic acid 2d: yellow solid, mp 122–124 °C, yield 65.3%, 1H NMR (400 MHz, CDCl3) δ 10.04 (s, 1H, NHCHCOOH), 7.13 (d, J = 9.2 Hz, 4H, Ar-H), 5.49 (d, J = 8.9 Hz, 1H, NHCHCOOH), 4.95 (t, J = 12.7 Hz, 1H, NHCHCOOH), 4.54 (s, 2H, Ar-CH2O), 4.19 (septet, 1H, (CH3)2CHO), 3.58 (m, 1H, CH3CHOCH2), 2.33 (d, J = 9.1 Hz, 3H, Ar-CH3), 1.29 (m, 9H, CH3). 2-((isopropoxycarbonyl)amino)−3-((4-methoxybenzyl)oxy)butanoic acid 2e: yellow oil, yield 69.6%, 1H NMR (400 MHz, CDCl3) δ 7.16 (t, J = 18.9 Hz, 2H, Ar-H), 6.86 (t, J = 8.7 Hz, 2H, Ar-H), 5.43 (d, J = 8.8 Hz, 1H, NHCHCOOH), 4.69–4.32 (m, 2H, Ar-CH2O), 4.26–4.07 (m, 1H, NHCHCOOH), 3.79 (d, J = 6.0 Hz, 3H, Ar-OCH3), 3.53 (m, 1H, (CH3)2CHO), 2.95 (d, J = 33.4 Hz, 1H, CH3CHOCH2), 1.23 (m, 9H, CH3). 3-((3,4-dimethoxybenzyl)oxy)−2-((isopropoxycarbonyl)amino)butanoic acid 2f: yellow oil, yield 61.2%, 1H NMR (400 MHz, CDCl3) δ 6.99–6.58 (m, 3H, Ar-H), 5.45 (t, J = 29.5 Hz, 1H, NHCHCOOH), 4.90 (s, 1H, NHCHCOOH), 4.63–4.32 (m, 2H, Ar-CH2O), 4.17 (s, 1H, (CH3)2CHO), 3.96–3.74 (m, 6H, Ar-OCH3), 3.55–3.40 (m, 1H, CH3CHOCH2), 1.46–1.05 (m, 9H, CH3).
Isopropyl ((2S,3R)-1-oxo-1-((1-phenylethyl)amino)-3-(prop-2-yn-1-yloxy)butan-2-yl)carbamate 3
Intermediate 2 (4.12 mmol) was dissolved in THF (50 ml), then triethylamine (0.50 g, 4.94 mmol) and ethyl chloroformate (0.45 g, 4.12 mmol) were added at 0 °C. The mixture was stirred for 1 h under this condition. The solution of substituted acetophenone amine (4.94 mmol) in THF (15 ml) was dropwised into the reaction solution. The mixture was further stirred for 5 h at room temperature. Remove the solvent, the residue was dissolved in ether, washed by dilute hydrochloric acid, saturated NaHCO3 washing, dried by anhydrous MgSO4, column chromatography separation (petroleum ether: ethyl acetate = 5:1) to obtain the target compound 3(I-1∼I-36). The detailed data can be found in supporting information.
Data for I-1. white solid, mp 95 − 97 °C. 1H NMR (400 MHz, CDCl3) δ 7.40–7.28 (m, 5H, Ar-H), 7.25–7.16 (m, 5H, Ar-H), 6.80 (s, 1H, Ar-CHNH), 5.63 (s, 1H, CHNHCO), 5.10 (s, 1H, Ar-CHCH3), 4.94–4.85 (m, 1H, (CH3)2CHO), 4.66–4.47 (m, 2H, Ar-CH2O), 4.32 (s, 1H, COCHNH), 4.17 (d, J = 17.1 Hz, 1H, CH3CHCH), 1.44 (dd, J = 12.6, 6.9 Hz, 3H, Ar-CHCH3), 1.25 (s, 6H, (CH3)2CHO), 1.06 (d, J = 6.0 Hz, 3H, CH3CHCH). HRMS calcd for C23H30N2O4 ([M + Na]): 421.2098; Found: 421.2098.
Data for I-2. white solid, mp 117 − 119 °C. 1H NMR (400 MHz, CDCl3) δ 7.42–7.26 (m, 5H, Ar-H), 7.18 (ddd, J = 24.3, 8.4, 5.5 Hz, 2H, Ar-H), 6.94 (dt, J = 23.6, 8.5 Hz, 2H, Ar-H), 6.75 (d, J = 8.7 Hz, 1H, Ar-CHNH), 5.61 (s, 1H, CHNHCO), 5.18–5.01 (m, 1H, Ar-CHCH3), 4.89 (dt, J = 12.2, 6.0 Hz, 1H, (CH3)2CHO), 4.56 (dt, J = 21.1, 11.2 Hz, 2H, Ar-CH2O), 4.32 (s, 1H, COCHNH), 4.15 (ddd, J = 9.2, 8.2, 4.9 Hz, 1H, CH3CHCH), 1.41 (dd, J = 12.1, 6.9 Hz, 3H, Ar-CHCH3), 1.24 (dd, J = 6.2, 3.9 Hz, 6H, (CH3)2CHO), 1.05 (d, J = 6.1 Hz, 3H, CH3CHCH). HRMS calcd for C23H29FN2O4 ([M + Na]): 439.2004; Found: 439.2005.
Data for I-3. white solid, mp 110 − 112 °C. 1H NMR (400 MHz, CDCl3) δ 7.41–7.26 (m, 5H, Ar-H), 7.25–7.16 (m, 1H, Ar-H), 7.07–6.89 (m, 3H, Ar-H), 6.78 (d, J = 9.9 Hz, 1H, Ar-CHNH), 5.62 (s, 1H, CHNHCO), 5.15–5.04 (m, 1H, Ar-CHCH3), 5.01–4.83 (m, 1H, (CH3)2CHO), 4.58 (dt, J = 24.2, 11.4 Hz, 2H, Ar-CH2O), 4.33 (s, 1H, COCHNH), 4.25–4.11 (m, 1H, CH3CHCH), 1.41 (dd, J = 15.0, 6.9 Hz, 3H, Ar-CHCH3), 1.28–1.21 (m, 6H, (CH3)2CHO), 1.13 (dd, J = 49.1, 6.2 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 170.82, 164.18, 161.73, 156.69, 145.67, 137.87, 130.18, 121.78, 114.21, 112.94, 74.96, 74.69, 71.63, 68.86, 57.51, 48.58, 22.02, 15.11. HRMS calcd for: C23H29ClN2O4 ([M + Na]+): 455.1708; Found: 455.1712.
Data for I-4. white solid, mp 130 − 132 °C. 1H NMR (400 MHz, CDCl3) δ 7.35 (dd, J = 15.7, 6.2 Hz, 5H, Ar-H), 7.27 (s, 2H, Ar-H), 7.20 (s, 1H, Ar-CHNH), 7.04 (d, J = 5.6 Hz, 2H, Ar-H), 5.65 (s, 1H, CHNHCO), 5.31 (dd, J = 14.4, 7.3 Hz, 1H, Ar-CHCH3), 4.99–4.87 (m, 1H, (CH3)2CHO), 4.60 (dt, J = 18.5, 11.5 Hz, 2H, Ar-CH2O), 4.35 (s, 1H, COCHNH), 4.23–4.08 (m, 1H, CH3CHCH), 1.48 (dd, J = 12.1, 7.0 Hz, 3H, Ar-CHCH3), 1.27 (dd, J = 6.0, 2.6 Hz, 6H, (CH3)2CHO), 1.15 (dd, J = 54.2, 6.1 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.56, 163.41, 160.14, 157.14, 138.92, 129.94, 128.96, 128.49, 127.75, 124.30, 115.98, 115.75, 74.68, 71.62, 68.73, 57.54, 45.25, 22.08, 21.52, 15.37. HRMS calcd for C23H29FN2O4 ([M + Na]): 439.2004; Found: 439.2010.
Data for I-5. white solid, mp 158 − 160 °C. 1H NMR (400 MHz, CDCl3) δ 7.38–7.30 (m, 3H, Ar-H), 7.30–7.22 (m, 3H, Ar-H), 7.17 (d, J = 8.3 Hz, 2H, Ar-H), 7.11 (d, J = 8.4 Hz, 1H, Ar-H), 6.77 (s, 1H, Ar-CHNH), 5.60 (s, 1H, CHNHCO), 5.05 (s, 1H, Ar-CHCH3), 4.91 (dd, J = 18.0, 10.7 Hz, 1H, (CH3)2CHO), 4.60 (t, J = 12.4 Hz, 1H, COCHNH), 4.32 (s, 2H, Ar-CH2O), 4.24–4.09 (m, 1H, CH3CHCH), 1.40 (dd, J = 13.0, 6.9 Hz, 3H, Ar-CHCH3), 1.27–1.21 (m, 6H, (CH3)2CHO), 1.20–1.01 (m, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 169.38, 168.72, 156.14, 141.57, 137.88, 135.11, 132.98, 128.95, 74.86, 72.96, 71.64, 68.78, 62.09, 57.41, 48.42, 47.16, 23.74, 14.12. HRMS calcd for C23H29ClN2O4 ([M + Na]): 455.1708; Found: 455.1712.
Data for I-6. white solid, mp 135 − 137 °C. 1H NMR (400 MHz, CDCl3) δ 7.42–7.22 (m, 5H, Ar-H), 7.18–7.01 (m, 4H, Ar-H), 6.77 (d, J = 6.8 Hz, 1H, Ar-CHNH), 5.61 (d, J = 7.0 Hz, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.90 (dd, J = 11.2, 5.2 Hz, 1H, (CH3)2CHO), 4.67–4.47 (m, 2H, Ar-CH2O), 4.31 (s, 1H, COCHNH), 4.22–4.09 (m, 1H, CH3CHCH), 2.32 (d, J = 4.9 Hz, 3H, Ar-CH3), 1.42 (dd, J = 12.5, 6.9 Hz, 3H, Ar-CHCH3), 1.27–1.20 (m, 6H, (CH3)2CHO), 1.13 (dd, J = 49.0, 6.3 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.44, 157.62, 139.96, 136.97, 129.32, 128.47, 127.78, 126.03, 75.03, 74.77, 71.61, 68.76, 57.51, 48.77, 30.97, 22.04, 21.08, 15.28. HRMS calcd for C24H32N2O4 ([M + Na]): 435.2254; Found: 435.2250.
Data for I-7. white solid, mp 125 − 127 °C. 1H NMR (400 MHz, CDCl3) δ 7.39–7.22 (m, 5H, Ar-H), 7.15 (dd, J = 21.5, 8.6 Hz, 2H, Ar-H), 6.83 (d, J = 8.7 Hz, 1H, Ar-CHNH), 6.77 (d, J = 8.5 Hz, 2H, Ar-H), 5.75–5.53 (m, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.95–4.85 (m, 1H, (CH3)2CHO), 4.67–4.49 (m, 2H, Ar-CH2O), 4.31 (s, 1H, COCHNH), 4.14 (dd, J = 13.5, 10.6 Hz, 1H, CH3CHCH), 3.78 (d, J = 7.6 Hz, 3H, Ar-OCH3), 1.42 (dd, J = 12.0, 6.9 Hz, 3H, Ar-CHCH3), 1.24–1.21 (m, 6H, (CH3)2CHO), 1.13 (dd, J = 53.8, 7.4 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 169.67, 158.77, 135.07, 128.48, 127.84, 127.71, 127.24, 113.97, 75.02, 74.79, 71.60, 68.78, 57.86, 55.29, 48.43, 32.10, 29.72, 22.10, 15.56. HRMS calcd for C24H32N2O5 ([M + Na]): 451.2203; Found: 451.2200.
Data for I-8. white solid, mp 90 − 92 °C. 1H NMR (400 MHz, CDCl3) δ 7.46–7.08 (m, 5H, Ar-H), 6.81 (dt, J = 12.1, 7.4 Hz, 4H, Ar-H), 7.09 (d, J = 8.0 Hz, 1H, Ar-CHNH), 5.65 (s, 1H, CHNHCO), 5.18–5.02 (m, 1H, Ar-CHCH3), 4.89 (dd, J = 12.3, 6.1 Hz, 1H, (CH3)2CHO), 4.70–4.49 (m, 2H, Ar-CH2O), 4.33 (s, 1H, COCHNH), 4.23–4.08 (m, 1H, CH3CHCH), 3.76 (d, J = 7.8 Hz,3H, Ar-OCH3), 1.49–1.37 (m, 3H, Ar-CHCH3), 1.28–1.22 (m, 6H, (CH3)2CHO), 1.16–1.02 (m, 3H, CH3CHCH);13C NMR (101 MHz, CDCl3) δ 171.58, 159.80, 144.60, 129.74, 128.49, 127.88, 118.30, 112.50, 75.48, 74.73, 71.63, 68.95, 58.12, 55.17, 49.02, 31.94, 29.71, 22.71, 22.07. HRMS calcd for C24H32N2O5 ([M + Na]): 451.2203; Found: 451.2209.
Data for I-9. white solid, mp 99 − 101 °C. 1H NMR (400 MHz, CDCl3) δ 7.38–7.29 (m, 5H, Ar-H), 7.26–7.15 (m, 3H, Ar-H), 6.94–6.90 (m, 1H, Ar-H), 6.87 (d, J = 8.0 Hz, 1H, Ar-CHNH),5.66 (d, J = 6.5 Hz, 1H, CHNHCO), 5.34 − 5.21 (m, 1H, Ar-CHCH3), 5.00–4.85 (m, 1H, (CH3)2CHO), 4.63–4.50 (m, 2H, Ar-CH2O), 4.31 (s, 1H, COCHNH), 4.20 (dd, J = 15.2, 8.2 Hz, 1H, CH3CHCH), 3.78–3.57 (m, 3H, Ar-OCH3), 1.48–1.38 (m, 3H, Ar-CHCH3), 1.24 (d, J = 7.6 Hz, 6H, (CH3)2CHO), 1.21–1.02 (m, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.54, 156.97, 135.15, 130.47, 128.96, 128.48, 128.40, 128.23, 127.88, 127.73, 120.81, 110.88, 71.65, 68.67, 62.53, 57.63, 55.08, 47.17, 29.72, 22.09, 14.13. HRMS calcd for C24H32N2O5 ([M + Na]): 451.2203; Found: 451.2206.
Data for I-10. white solid, mp 136 − 137 °C.1HNMR (400 MHz, CDCl3) δ 7.39–7.23 (m, 5H, Ar-H), 7.16 (dd, J = 22.4, 8.6 Hz, 2H, Ar-H), 6.88 (dd, J = 22.1, 8.6 Hz, 2H, Ar-H), 6.74 (s, 1H, Ar-CHNH), 5.61 (s, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.89 (dt, J = 12.3, 6.0 Hz, 1H, (CH3)2CHO), 4.66 (dd, J = 8.3, 2.3 Hz, 2H, Ar-CH2O), 4.63–4.47 (m, 2H, CHCCH2O), 4.31 (s, 1H, COCHNH), 4.14 (dd, J = 12.6, 9.8 Hz, 1H, CH3CHCH), 2.51 (dd, J = 5.2, 2.5 Hz, 1H, CHCCH2O), 1.42 (dd, J = 12.0, 6.9 Hz, 3H, Ar-CHCH3), 1.28–1.20 (m, 6H, (CH3)2CHO), 1.13 (dd, J = 52.4, 6.4 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.45, 156.72, 139.25, 136.01, 128.50, 127.86, 127.26, 114.94, 78.55, 75.58, 74.77, 71.59, 68.78, 57.57, 55.80, 48.36, 22.10, 21.88, 15.27. HRMS calcd for C26H32N2O5 ([M + Na]): 475.2203; Found: 475.2207.
Data for I-11. white solid, mp 122 − 124 °C. 1H NMR (400 MHz, CDCl3) δ 7.30 (dd, J = 20.8, 9.5 Hz, 5H, Ar-H), 6.93 (dd, J = 24.2, 8.1 Hz, 1H, Ar-CHNH), 6.78 (d, J = 20.3 Hz, 3H, Ar-H), 5.62 (s, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.96–4.84 (m, 1H, (CH3)2CHO), 4.73 (d, J = 4.4 Hz, 2H, CHCH2O), 4.59 (t, J = 16.6 Hz, 2H, Ar-CH2O), 4.32 (s, 1H, COCHNH), 4.15 (s, 1H, CH3CHCH), 3.78 (d, J = 13.3 Hz, 3H), 2.49 (s, 1H, CHCH2O), 1.43 (dd, J = 13.5, 6.9 Hz, 3H, Ar-CHCH3), 1.23 (d, J = 5.9 Hz, 6H, (CH3)2CHO), 1.08 (d, J = 5.6 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.51, 156.26, 149.64, 145.98, 137.88, 136.99, 128.50, 127.87, 127.67, 117.81, 114.25, 110.18, 78.60, 75.84, 74.79, 71.57, 68.80, 57.49, 56.73, 55.83, 48.64, 22.10, 21.84, 15.25. HRMS calcd for C27H34N2O6 ([M + Na]): 505.2309; Found: 505.2314.
Data for I-12. white solid, mp 117 − 119 °C. 1H NMR (400 MHz, CDCl3) δ 7.39–7.20 (m, 5H, Ar-H), 6.98–6.85 (m, 1H, Ar-CHNH), 6.83–6.72 (m, 3H, Ar-H), 5.63 (s, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.88 (dd, J = 12.1, 6.2 Hz, 1H, (CH3)2CHO), 4.59 (t, J = 17.2 Hz, 2H, Ar-CH2O), 4.32 (s, 1H, COCHNH), 4.14 (s, 1H, CH3CHCH), 3.91–3.72 (m, 6H, Ar-OCH3), 1.43 (dd, J = 13.7, 6.9 Hz, 3H, Ar-CHCH3), 1.23 (d, J = 6.1 Hz, 6H, (CH3)2CHO), 1.07 (d, J = 6.1 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.58, 157.23, 148.95, 148.21, 137.90, 135.55, 128.47, 127.88, 127.64, 118.02, 111.13, 109.66, 74.89, 71.63, 68.75, 57.44, 55.83, 48.69, 22.06, 21.89, 15.23. HRMS calcd for C27H34N2O6 ([M + Na]): 505.2309; Found: 482.2417.
Data for I-13. white solid, mp 132 − 135 °C. 1H NMR (400 MHz, CDCl3) δ 7.32–7.24 (m, 5H, Ar-H), 7.22–7.10 (m, 5H, Ar-H), 6.72 (ddd, J = 27.2, 16.5, 10.8 Hz, 3H, Ar-H), 6.18 (d, J = 7.7 Hz, 1H, Ar-CHNH), 5.65 (s, 1H, Ar-CH-Ar), 4.98–4.84 (m, 1H, (CH3)2CHO), 4.66–4.49 (m, 2H, Ar-CH2O), 4.41 (s, 1H, COCHNH), 4.18 (dd, J = 6.3, 2.9 Hz, 1H, CH3CHCH), 3.79 (dd, J = 46.1, 9.3 Hz, 6H, Ar-OCH3), 1.27–1.18 (m, 6H, (CH3)2CHO), 1.15 (d, J = 6.2 Hz, 3H, CH3CHCH). HRMS calcd for C30H36N2O6 ([M + Na]): 543.2466; Found: 543.2471.
Data for I-14. white solid, mp 115 − 117 °C. 1H NMR (400 MHz, CDCl3) δ 7.26 (d, J = 35.2 Hz, 5H, Ar-H), 7.14 (d, J = 25.0 Hz, 2H, Ar-H), 6.98 (dd, J = 18.0, 9.1 Hz, 2H, Ar-H), 6.73 (dd, J = 49.0, 17.0 Hz, 3H, Ar-H), 6.18 (s, 1H, Ar-CHNH), 5.66 (s, 1H, Ar-CH-Ar), 4.93 (s, 1H, (CH3)2CHO), 4.59 (dd, J = 30.3, 10.8 Hz, 2H, Ar-CH2O), 4.45 (d, J = 18.8 Hz, 1H, COCHNH), 4.20 (s, 1H, CH3CHCH), 3.94–3.67 (m, 6H, Ar-OCH3), 1.25 (s, 6H, (CH3)2CHO), 1.17 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 170.11, 164.25, 161.50, 157.19 149.12, 148.80, 137.22, 133.65, 128.77, 128.50, 127.66, 119.58, 115.55, 115.35, 111.07, 110.61, 75.00, 71.73, 68.88, 57.61, 56.12, 55.91, 22.06, 15.17. HRMS calcd for C30H35FN2O6 ([M + Na]): 561.2371; Found: 561.2373.
Data for I-15. white solid, mp 118 − 120 °C. 1H NMR (400 MHz, CDCl3) δ 7.30 (dd, J = 14.9, 7.0 Hz, 5H, Ar-H), 7.21 (d, J = 3.7 Hz, 2H, Ar-H), 7.17–7.05 (m, 2H, Ar-H), 6.89–6.57 (m, 3H, Ar-H), 6.16 (d, J = 7.8 Hz, 1H, Ar-CHNH), 5.67 (d, J = 6.1 Hz, 1H, CHNHCO), 5.00–4.90 (m, 1H, Ar-CH-Ar), 4.83 (s, 1H, (CH3)2CHO), 4.70–4.47 (m, 2H, Ar-CH2O), 4.45 (s, 1H, COCHNH), 4.21 (dd, J = 6.3, 3.0 Hz, 1H, CH3CHCH), 3.86 (dd, J = 12.2, 6.5 Hz, 3H, Ar-OCH3), 3.77 (t, J = 8.5 Hz, 3H, Ar-OCH3), 1.26 (dd, J = 11.8, 7.7 Hz, 6H, (CH3)2CHO), 1.18 (d, J = 5.8 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 170.70, 157.35, 149.16, 148.54, 139.97, 138.44, 133.36, 128.72, 128.54, 127.97, 127.68, 119.65, 111.11, 110.66, 75.01, 71.75, 70.27, 68.91, 59.38, 55.87, 29.72, 22.07. HRMS calcd for C30H35ClN2O6 ([M + Na]): 577.2076; Found: 577.2081.
Data for I-16. white solid, mp 130 − 132 °C.1H NMR (400 MHz, CDCl3) δ 7.39 (ddd, J = 22.2, 14.3, 7.9 Hz, 2H, Ar-H), 7.32–7.27 (m, 3H, Ar-H), 7.22–7.15 (m, 2H, Ar-H), 7.03 (dd, J = 26.1, 8.3 Hz, 2H, Ar-H), 6.81–6.54 (m, 3H, Ar-H), 6.11 (d, J = 7.7 Hz, 1H, Ar-CHNH), 5.63 (d, J = 6.1 Hz, 1H, CHNHCO), 4.96–4.84 (m, 1H, Ar-CH-Ar), 4.80 (s, 1H, (CH3)2CHO), 4.55 (dd, J = 46.2, 23.3 Hz, 2H, Ar-CH2O), 4.40 (s, 1H, COCHNH), 4.24–4.11 (m, 1H, CH3CHCH), 3.83 (dd, J = 12.1, 6.4 Hz, 3H, Ar-OCH3), 3.74 (t, J = 8.6 Hz, 3H, Ar-OCH3), 1.27–1.19 (m, 6H, (CH3)2CHO), 1.15 (d, J = 5.9 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 169.77, 157.19, 149.17, 148.56, 140.49, 138.07, 133.26, 131.68, 129.01, 128.88, 128.51, 121.31, 119.66, 111.13, 75.00, 71.74, 68.90, 57.64, 56.28, 55.87, 22.06, 15.73. HRMS calcd for C30H35BrN2O6 ([M + Na]): 621.1571; Found: 621.1574.
Data for I-17. white solid, mp 144 − 146 °C. 1H NMR (400 MHz, CDCl3) δ 7.31–7.27 (m, 5H, Ar-H), 7.20 (dd, J = 6.5, 2.9 Hz, 2H, Ar-H), 7.08 (t, J = 6.5 Hz, 2H, Ar-H), 6.77–6.62 (m, 3H, Ar-H), 6.13 (t, J = 7.1 Hz, 1H, Ar-CHNH), 5.65 (d, J = 6.1 Hz, 1H, CHNHCO), 4.95–4.86 (m, 1H, Ar-CH-Ar), 4.64–4.51 (m, 2H, Ar-CH2O), 4.40 (s, 1H, COCHNH), 4.18 (dd, J = 6.4, 2.9 Hz, 1H, COCHNH), 3.85 (t, J = 4.6 Hz, 3H, Ar-OCH3), 3.82 (d, J = 3.8 Hz, 1H, CH3CHCH), 3.73 (d, J = 11.8 Hz, 3H, Ar-OCH3), 2.32 (d, J = 3.2 Hz, 6H, (CH3)2CHO), 1.26–1.20 (m, 3H, CH3CHCH), 1.15 (d, J = 5.5 Hz, 3H, Ar-CH3). 13 C NMR (101 MHz, CDCl3) δ 169.57, 156.84, 149.00, 148.28, 139.34, 137.95, 137.18, 134.08, 129.31, 128.45, 127.80, 127.71, 127.24, 127.13, 119.49, 110.98, 110.55, 75.00, 71.64, 68.80, 57.52, 56.67, 55.89, 22.05, 21.09, 15.51. HRMS calcd for C31H28N2O6 ([M + Na]): 557.2622; Found: 557.2628.
Data for I-18. white solid, mp 104 − 106 °C. 1H NMR (400 MHz, CDCl3) δ 7.23–7.10 (m, 2H, Ar-H), 7.04–6.87 (m, 2H, Ar-H), 6.82 (dd, J = 16.8, 9.4 Hz, 3H, Ar-H), 5.60 (s, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.89 (d, J = 5.4 Hz, 1H, (CH3)2CHO), 4.63–4.41 (m, 2H, Ar-CH2O), 4.31 (s, 1H, COCHNH), 4.20–4.08 (m, 1H, CH3CHCH), 3.86 (t, J = 13.4 Hz, 6H, CH3O), 1.40 (dd, J = 14.0, 6.9 Hz, 3H, Ar-CHCH3), 1.23 (dd, J = 10.5, 5.7 Hz, 6H, (CH3)2CHO), 1.02 (d, J = 6.0 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.56, 164.21, 160.78, 157.54, 148.90, 138.81, 130.41, 128.26, 120.48, 115.27, 111.34, 110.95, 74.44, 71.56, 68.73, 57.29, 55.88, 55.82, 48.32, 22.08, 15.09. HRMS calcd for C25H33FN2O6 ([M + Na]): 499.2215; Found:499.2216.
Data for I-19. white solid, mp 124 − 126 °C. 1H NMR (400 MHz, CDCl3) δ 7.08 (dd, J = 25.5, 11.8 Hz, 4H, Ar-H), 6.82 (dd, J = 16.0, 7.3 Hz, 3H, Ar-H), 5.63 (s, 1H, CHNHCO), 5.05 (s, 1H, Ar-CHCH3), 4.89 (s, 1H, (CH3)2CHO), 4.52 (dd, J = 25.8, 10.1 Hz, 2H, Ar-CH2O), 4.31 (s, 1H, COCHNH), 4.13 (s, 1H, CH3CHCH), 3.83 (d, J = 32.1 Hz, 6H, CH3O), 2.31 (s, 3H, Ar-CH3), 1.52 − 1.29 (m, 3H, Ar-CHCH3), 1.22 (s, 6H, (CH3)2CHO), 1.11 (d, J = 48.5 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.41, 156.73, 149.00, 148.78, 141.90, 139.99, 136.87, 130.52, 129.31, 125.94, 120.45, 110.97, 74.40, 71.57, 68.72, 57.31, 55.78, 49.96, 48.74, 22.10, 21.05. HRMS calcd for C26H36N2O6 ([M + Na]): 495.2466; Found:495.2467.
Data for I-20. white solid, mp 110– 112 °C. 1H NMR (400 MHz, CDCl3) δ 7.11 (s, 2H, Ar-H), 6.80 (d, J = 13.7 Hz, 5H, Ar-H), 5.62 (s, 1H, CHNHCO), 5.04 (s, 1H, Ar-CHCH3), 4.88 (s, 1H, (CH3)2CHO), 4.64 (s, 2H, Ar-CH2O), 4.49 (d, J = 28.3 Hz, 2H, CHCCH2O), 4.29 (s, 1H, COCHNH), 4.13 (s, 1H, CH3CHCH), 3.82 (d, J = 29.3 Hz, 6H, CH3O), 2.50 (s, 1H, CHCCH2O), 1.41 (s, 3H, Ar-CHCH3), 1.22 (s, 6H, (CH3)2CHO), 1.04 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.41, 156.70, 156.24, 148.94, 135.96, 130.35, 127.28, 127.18, 120.50, 114.90, 111.32, 110.95, 78.53, 75.60, 74.42, 71.53, 68.73, 57.32, 55.92, 55.79, 48.32, 22.09, 21.96, 15.31. HRMS calcd for C28H36N2O7 ([M + Na]): 535.2415; Found:535.2413.
Data for I-21. white solid, mp 139 − 140 °C. 1H NMR (400 MHz, CDCl3) δ 7.17 (d, J = 8.6 Hz, 1H, Ar-H), 7.11 (d, J = 8.4 Hz, 1H, Ar-H), 6.87–6.72 (m, 5H, Ar-H), 5.62 (d, J = 5.9 Hz, 1H, CHNHCO), 5.04 (d, J = 4.9 Hz, 1H, Ar-CHCH3), 4.95–4.82 (m, 1H, (CH3)2CHO), 4.60–4.41 (m, 2H, Ar-CH2O), 4.30 (s, 1H, COCHNH), 4.14 (dd, J = 12.1, 5.6 Hz, 1H, CH3CHCH), 3.90–3.86 (m, 6H, Ar-OCH3), 3.78 (d, J = 6.1 Hz, 3H, Ar-OCH3), 1.41 (dd, J = 13.4, 6.8 Hz, 3H, Ar-CHCH3), 1.26–1.20 (m, 6H, (CH3)2CHO), 1.10 (dd, J = 50.9, 6.2 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 169.74, 160.35, 149.21, 148.88, 137.18, 130.81, 127.27, 127.16, 120.44, 113.94, 111.34, 110.99, 74.61, 71.55, 69.02, 57.38, 55.90, 55.82, 55.25, 48.86, 22.10, 22.00, 15.56. HRMS calcd for C28H36N2O7 ([M + Na]): 535.2415; Found:535.2418.
Data for I-22. white solid, mp 152 − 154 °C. 1H NMR (400 MHz, CDCl3) δ 7.17 (dd, J = 43.4, 23.8 Hz, 4H, Ar-H), 6.99 (d, J = 21.6 Hz, 4H, Ar-H), 6.74 (s, 1H, Ar-CHNH), 5.60 (s, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.91 (s, 1H, (CH3)2CHO), 4.59–4.39 (m, 2H, Ar-CH2O), 4.30 (s, 1H, COCHNH), 4.18 (s, 1H, CH3CHCH), 2.32 (s, 3H, Ar-CH3), 1.44 (d, J = 6.2 Hz, 3H, Ar-CHCH3), 1.23 (s, 6H, (CH3)2CHO), 1.13 (d, J = 47.3 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.44, 163.60, 161.26, 156.31, 139.90, 137.08, 133.65, 129.45, 126.03, 115.30, 74.66, 70.84, 68.85, 57.62, 48.77, 22.00, 21.01, 15.36. HRMS calcd for C24H31FN2O4 ([M + Na]): 453.2160; Found:453.2160.
Data for I-23. white solid, mp 130 − 131 °C. 1H NMR (400 MHz, CDCl3) δ 7.34–7.27 (m, 2H, Ar-H), 7.22–7.00 (m, 4H, Ar-H), 6.80 (t, J = 8.5 Hz, 2H, Ar-H), 6.75 (s, 1H, Ar-CHNH), 5.62 (s, 1H, CHNHCO), 5.05 (s, 1H, Ar-CHCH3), 4.98–4.81 (m, 1H, (CH3)2CHO), 4.63 (t, J = 12.2 Hz, 2H, Ar-CH2O), 4.32 (s, 1H, COCHNH), 4.24–4.07 (m, 1H, CH3CHCH), 3.77 (d, J = 1.7 Hz, 3H, Ar-OCH3), 1.43 (t, J = 6.3 Hz, 3H, Ar-CHCH3), 1.30–1.21 (m, 6H, (CH3)2CHO), 1.13 (dd, J = 54.8, 6.3 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.30, 163.19, 158.75, 135.17, 129.83, 127.26, 125.53, 124.20, 115.26, 113.91, 75.11, 68.72, 65.58, 55.25, 48.50, 22.10, 21.81, 15.01. HRMS calcd for C24H31FN2O5 ([M + Na]): 469.2109; Found:469.2115.
Data for I-24. white solid, mp 145 − 147 °C. 1H NMR (400 MHz, CDCl3) δ 7.17 (dd, J = 17.9, 7.6 Hz, 4H, Ar-H), 7.06–6.80 (m, 4H, Ar-H), 6.70 (s, 1H, Ar-CHNH), 5.59 (d, J = 10.7 Hz, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.90 (s, 1H, (CH3)2CHO), 4.67 (s, 2H, Ar-CH2O), 4.50 (d, J = 28.3 Hz, 2H, CHCCH2O), 4.29 (s, 1H, COCHNH), 4.14 (d, J = 20.9 Hz, 1H, CH3CHCH), 2.51 (s, 1H, CHCCH2O), 1.46–1.38 (m, 3H, Ar-CHCH3), 1.23 (s, 6H, (CH3)2CHO), 1.13 (d, J = 48.8 Hz, 3H, CH3CHCH). HRMS calcd for C26H31FN2O5 ([M + Na]): 493.2109; Found:493.2114.
Data for I-25. white solid, mp 135 − 137 °C. 1H NMR (400 MHz, CDCl3) δ 7.18 (s, 2H, Ar-H), 6.96 (s, 2H, Ar-H), 6.84 − 6.68 (m, 3H, Ar-H), 5.59 (s, 1H, CHNHCO), 5.04 (s, 1H, Ar-CHCH3), 4.89 (s, 1H, (CH3)2CHO), 4.50 (d, J = 25.1 Hz, 2H, Ar-CH2O), 4.29 (s, 1H, COCHNH), 4.15 (s, 1H, CH3CHCH), 3.89–3.74 (m, 6H, Ar-OCH3), 1.44 (d, J = 6.3 Hz, 3H, Ar-CHCH3), 1.23 (s, 6H, (CH3)2CHO), 1.13 (d, J = 42.4 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.41, 165.59, 161.97, 156.81, 149.57, 148.31, 135.47, 134.44, 129.47, 117.85, 115.19, 109.73, 74.66, 70.82, 68.84, 57.56, 55.86, 48.72, 29.70, 21.92, 15.30. HRMS calcd for C25H33FN2O6 ([M + Na]): 499.2215; Found:499.2215.
Data for I-26. white solid, mp 133 − 135 °C. 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 1H, Ar-H), 6.98 (s, 3H, Ar-H), 6.78 (s, 2H, Ar-H), 5.60 (s, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.89 (s, 1H, (CH3)2CHO), 4.74 (s, 2H, Ar-CH2O), 4.53 (s, 2H, CHCCH2O), 4.30 (s, 1H, COCHNH), 4.15 (s, 1H, CH3CHCH), 3.80 (s, 3H, Ar-OCH3), 2.50 (s, 1H, CHCCH2O), 1.44 (s, 3H, Ar-CHCH3), 1.23 (s, 6H, (CH3)2CHO), 1.09 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.46, 164.55, 162.77, 156.89, 149.65, 147.12, 136.88, 134.98, 129.47, 117.69, 114.17, 110.28, 78.53, 75.87, 74.64, 70.82, 68.85, 57.58, 56.69, 55.84, 48.75, 22.07, 21.79, 15.30. HRMS calcd for C27H33FN2O6 ([M + Na]): 523.2215; Found:523.2217.
Data for I-27. white solid, mp 125 − 127 °C. 1H NMR (400 MHz, CDCl3) δ 7.29 (s, 2H, Ar-H), 7.18 (s, 2H, Ar-H), 6.96 (dd, J = 22.6, 7.8 Hz, 1H, Ar-CHNH), 6.89–6.63 (m, 3H, Ar-H), 5.61 (s, 1H, CHNHCO), 5.09 (d, J = 5.4 Hz, 1H, Ar-CHCH3), 4.92 (d, J = 6.0 Hz, 1H, (CH3)2CHO), 4.77 (s, 2H, Ar-CH2O), 4.54 (d, J = 25.5 Hz, 2H, CHCCH2O), 4.32 (s, 1H, COCHNH), 4.18 (s, 1H, CH3CHCH), 3.83 (d, J = 11.6 Hz, 3H, Ar-OCH3), 2.53 (s, 1H, CHCCH2O), 1.62–1.39 (m, 3H, Ar-CHCH3), 1.26 (s,6H, (CH3)2CHO), 1.12 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 170.95, 157.95, 149.66, 147.10, 136.85, 134.41, 128.81, 117.78, 114.13, 110.28, 101.68, 78.55, 75.88, 74.78, 70.78, 69.49, 58.03, 56.70, 55.87, 48.64, 22.09, 21.82, 15.67. HRMS calcd for C27H33ClN2O6 ([M + Na]): 539.1919; Found:539.1921.
Data for I-28. white solid, mp 137 − 139 °C. 1H NMR (400 MHz, CDCl3) δ 7.50–7.15 (m, 5H, Ar-H), 7.00–6.71 (m, 3H, Ar-H), 5.76 (d, J = 28.0 Hz, 1H, CHNHCO), 5.15 (s, 1H, Ar-CHCH3), 4.99 (s, 1H, (CH3)2CHO), 4.54 (dd, J = 31.5, 18.0 Hz, 2H, Ar-CH2O), 4.41 (s, 1H, COCHNH), 4.23 (d, J = 30.6 Hz, 1H, CH3CHCH), 3.87 (s, 3H, Ar-OCH3), 1.52 (s, 3H, Ar-CHCH3), 1.32 (s, 6H, (CH3)2CHO), 1.16 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 175.24, 168.55, 158.85, 156.28, 136.45, 134.87, 133.54, 128.69, 127.30, 113.97, 74.95, 70.48, 68.84, 55.26, 48.52, 21.92, 21.79, 16.24. HRMS calcd for C24H31ClN2O5 ([M + Na]): 485.1814; Found:485.1813.
Data for I-29. white solid, mp 149 − 151 °C.1H NMR (400 MHz, CDCl3) δ 7.25–7.16 (m, 3H, Ar-H), 7.09 (dt, J = 30.1, 10.4 Hz, 3H, Ar-H), 6.82 (dd, J = 18.1, 8.2 Hz, 2H, Ar-H), 6.62 (t, J = 7.8 Hz, 1H, Ar-CHNH), 5.60–5.46 (m, 1H, CHNHCO), 5.00 (s, 1H, Ar-CHCH3), 4.83 (dd, J = 12.1, 5.9 Hz, 1H, (CH3)2CHO), 4.61 (s, 2H, Ar-CH2O), 4.41 (dt, J = 26.8, 12.9 Hz, 2H, CHCCH2O), 4.22 (s, 1H, COCHNH), 4.15–4.01 (m, 1H, CH3CHCH), 2.45 (s, 1H, CHCCH2O), 1.36 (t, J = 7.5 Hz, 3H, Ar-CHCH3), 1.20–1.14 (m, 6H, (CH3)2CHO), 1.05 (dd, J = 46.7, 6.0 Hz, 3H, CH3CHCH). HRMS calcd for C26H31ClN2O5 ([M + Na]): 509.1814; Found:509.2062.
Data for I-30. white solid, mp 136 − 138 °C. 1H NMR (400 MHz, CDCl3) δ 7.17 (s, 4H, Ar-H), 6.91–6.75 (m, 4H, Ar-H), 5.68 (s, 1H, CHNHCO), 5.05 (s, 1H, Ar-CHCH3), 4.89 (s, 1H, (CH3)2CHO), 4.46 (dt, J = 31.3, 10.1 Hz, 2H, Ar-CH2O), 4.31 (s, 1H, COCHNH), 4.12 (d, J = 21.3 Hz, 1H, CH3CHCH), 3.78 (d, J = 7.1 Hz, 6H, Ar-CH2O), 1.40 (d, J = 6.5 Hz, 3H, Ar-CHCH3), 1.22 (s, 6H, (CH3)2CHO), 1.04 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 173.77, 168.61, 159.05, 156.40, 135.05, 130.34, 128.62, 127.30, 113.89, 71.24, 68.69, 57.47, 55.24, 48.47, 22.07, 21.88, 16.35. HRMS calcd for C26H31ClN2O5 ([M + Na]): 509.1814; Found:509.1808.
Data for I-31. white solid, mp 119 − 120 °C. 1H NMR (400 MHz, CDCl3) δ 7.18 (dd, J = 21.1, 8.9 Hz, 4H, Ar-H), 6.88 (dt, J = 34.8, 17.3 Hz, 4H, Ar-H), 5.67 (s, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.91 (d, J = 5.2 Hz, 1H, (CH3)2CHO), 4.67 (d, J = 3.7 Hz, 2H, Ar-CH2O), 4.61–4.37 (m, 2H, CHCCH2O), 4.32 (s, 1H, COCHNH), 4.14 (d, J = 17.6 Hz, 1H, CH3CHCH), 3.82 (s, 3H, Ar-OCH3), 2.54 (s, 1H, CHCCH2O), 1.47–1.39 (m, 3H, Ar-CHCH3), 1.25 (s, 6H, (CH3)2CHO), 1.13 (dd, J = 50.9, 5.2 Hz, 3H, CH3CHCH). HRMS calcd for C27H34N2O6 ([M + Na]): 505.2309; Found:505.2304.
Data for I-32. white solid, mp 95 − 96 °C. 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 2H, Ar-H), 6.81 (d, J = 15.1 Hz, 5H, Ar-H), 5.65 (s, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.91 (s, 1H, (CH3)2CHO), 4.75 (s, 2H, Ar-CH2O), 4.52 (d, J = 20.3 Hz, 2H, CHCCH2O), 4.33 (s, 1H, COCHNH), 4.15 (s, 1H, CH3CHCH), 3.82 (s, 6H, Ar-OCH3), 2.52 (s, 1H, CHCCH2O), 1.46 (s, 3H, Ar-CHCH3), 1.25 (s, 6H, (CH3)2CHO), 1.09 (s, 3H, CH3CHCH). HRMS calcd for C28H36N2O7 ([M + Na]): 535.2415; Found: 535.2413.
Data for I-33. white solid, mp 142 − 144 °C. 1H NMR (400 MHz, CDCl3) δ 7.35–7.08 (m, 6H, Ar-H), 6.92–6.76 (m, 2H, Ar-H), 6.74 (d, J = 7.1 Hz, 1H, Ar-CHNH), 5.62 (s, 1H, CHNHCO), 5.17–4.98 (m, 1H, Ar-CHCH3), 4.90 (dd, J = 12.1, 5.9 Hz, 1H, (CH3)2CHO), 4.54 (dd, J = 23.7, 11.9 Hz, 2H, Ar-CH2O), 4.30 (s, 1H, COCHNH), 4.14 (d, J = 7.8 Hz, 1H, CH3CHCH), 3.79 (t, J = 5.5 Hz, 3H, Ar-OCH3), 2.35 (d, J = 1.1 Hz, 3H, Ar-CH3), 1.42 (dd, J = 10.4, 6.9 Hz, 3H, Ar-CHCH3), 1.27–1.19 (m, 6H, (CH3)2CHO), 1.11 (dd, J = 52.3, 6.2 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 168.45, 158.76, 156.88, 137.57, 135.04, 129.17, 127.30, 126.68, 113.91, 71.48, 68.67, 57.39, 55.27, 48.46, 22.04, 21.22, 15.00. HRMS calcd for C25H34N2O5 ([M + Na]): 465.2360; Found:465.2363.
Data for I-34. white solid, mp 126 − 128 °C. 1H NMR (400 MHz, CDCl3) δ 7.19 (s, 3H, Ar-H), 6.81 (d, J = 15.1 Hz, 5H, Ar-H), 5.65 (s, 1H, CHNHCO), 5.07 (s, 1H, Ar-CHCH3), 4.91 (s, 1H, (CH3)2CHO), 4.75 (s, 2H, Ar-CH2O), 4.52 (d, J = 20.3 Hz, 2H, CHCCH2O), 4.33 (s, 1H, COCHNH), 4.15 (s, 1H, CH3CHCH), 3.82 (s, 3H), 2.52 (s, 1H, CHCCH2O), 1.46 (s, 3H, Ar-CHCH3), 1.25 (s, 6H, (CH3)2CHO), 1.09 (s, 3H, CH3CHCH). HRMS calcd for C27H34N2O5 ([M + Na]): 489.2360; Found:489.2365.
Data for I-35. white solid, mp 128 − 130 °C.1H NMR (400 MHz, CDCl3) δ 7.12 (s, 4H, Ar-H), 6.87 (d, J = 6.7 Hz, 1H, Ar-H), 6.77 (d, J = 18.2 Hz, 2H, Ar-H), 5.73 (s, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.92 (dd, J = 27.9, 13.9 Hz, 1H, (CH3)2CHO), 4.55 (dd, J = 13.9, 8.7 Hz, 2H, Ar-CH2O), 4.35 (s, 1H, COCHNH), 4.13 (d, J = 14.6 Hz, 1H, CH3CHCH), 3.81 (dd, J = 21.7, 8.6 Hz, 6H, Ar-OCH3), 2.32 (s, 3H, Ar-CH3), 1.53–1.32 (m, 3H, Ar-CHCH3), 1.21 (d, J = 15.2 Hz, 6H, (CH3)2CHO), 1.12 (d, J = 28.0 Hz, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 172.32, 169.02, 157.56, 156.39, 148.98, 148.29, 137.74, 135.75, 131.98, 118.01, 111.16, 109.73, 73.19, 68.96, 61.92, 55.84, 48.85, 46.60, 21.93, 21.10, 15.37. HRMS calcd for C26H36N2O6([M + Na]): 495.2466; Found:495.2463.
Data for I-36. white solid, mp 118 − 120 °C. 1H NMR (400 MHz, CDCl3) δ 7.13 (s, 4H, Ar-H), 6.81 (s, 3H, Ar-H), 5.70 (s, 1H, CHNHCO), 5.06 (s, 1H, Ar-CHCH3), 4.90 (s, 1H, (CH3)2CHO), 4.73 (s, 2H, Ar-CH2O), 4.55 (s, 2H, CHCCH2O), 4.34 (s, 1H, COCHNH), 4.12 (s, 1H, Ar-CHCH3), 3.77 (s, 3H, Ar-OCH3), 2.49 (s, 1H, CHCCH2O), 2.33 (s, 3H, Ar-CH3), 1.41 (d, J = 23.5 Hz, 3H, Ar-CHCH3), 1.23 (s, 6H, (CH3)2CHO), 1.09 (s, 3H, CH3CHCH). 13 C NMR (101 MHz, CDCl3) δ 172.03, 169.56, 156.64, 150.15, 146.40, 137.09, 135.58, 132.28, 117.78, 114.20, 110.19, 78.57, 73.11, 69.58, 61.87, 57.53, 56.71, 55.81, 48.73, 46.61, 22.06, 21.03, 15.32. HRMS calcd for C28H36N2O6([M + Na]): 519.2466; Found:519.2466.
Fungicidal activity
Detailed fungicidal activity test method were according to our previous workCitation40–43. According to statistical requirements, each fungicidal activity was repeated at least three times.
Conclusions
In summary, a series of new CAA analogues were designed and synthesised using pharmacophore model. Their structures were determined by the spectra analysis, and their fungicidal activities were assayed in vitro and in vivo. From the bioassay results, some of target compounds exhibited more potent fungicidal activity against Oomycete fungi P. capsici in vitro. Interestingly, compound I-1, I-2, I-3, I-6 and I-7 exhibited moderate control effect (>50%) against P. cubensis in greenhouse at 6.25 μg/mL. Furthermore, further structure expeditions are undergoing using pharmacophore model and will be reported in the near future.
Supplemental Material
Download PDF (5.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Spencer-Phillips PTN, Gisi U, Lebeda A. Chemical control of downy mildews. In Advances in downy mildew research. Kluwer: Dordrecht; 2002:119–159.
- Li J-Q, Wang Z-P, Gao Y, et al. Design, synthesis and effect of the introduction of a propargyloxy group on the fungicidal activities of 1-substituted phenoxypropan-2-amino valinamide carbamate derivatives. RSC Adv 2016;6:82131–7.
- AlbertCurtze GJ, Drandarevski CA. Brighton crop protection conference pests and diseases; 1988:17.
- Jeschke P, Witschel M, Krämer W, et al. (Eds). Carboxylic acid amide fungicides in modern crop protection compounds, 3rd ed. Weinheim: Wiley-VCH; 2019:845–69.
- Lamberth C, Dinges J. (Eds) Carboxylic acid amide fungicides for the control of downy mildew diseases in bioactive carboxylic compound classes: pharmaceuticals and agrochemicals. Weinheim: Wiley-VCH; 2016:395–403.
- Liu CW, Liu CL. Novel fungicide flumorph(SYP-L190) with high activity. Pesticide 2001;41:8–11.
- Yan XJ, Qin WC, Sun LP, et al. Study of inhibitory effects and action mechanism of the novel fungicide Pyrimorph against Phytophthora capsici. J Agr Food Chem 2010;58:2715–20.
- Miyake Y, Sakai J, Shibata M, et al. Fungicidial activity of benthiavalicarb-isopropyl against Phytophthora infestans and its controlling activity against late blight diseases. J Pestic Sci 2005;30:390–6.
- Stenzel RPK, Seitz T, Tiemann AWR. Brighton crop protection conference pests and diseases; 1998:367.
- Agosteo G, Marsilii E, Pane A, et al. Strategie innovative di difesa nel settore ortoflorofrutticolo. International Society for Plant Pathology; 2010:61.
- Lamberth C, Jeanguenat A, Cederbaum F, et al. Multicomponent reactions in fungicide research: The discovery of mandipropamid. Biorg Med Chem 2008;15:1531–45.
- Blum M, Waldner M, Gisi U. A single point mutation in the novel PvCesA3 gene confers resistance to the carboxylic acid amide fungicide mandipropamid in Plasmopara viticola. Fungal Genet Biol 2010;47:499–510.
- Blum M, Boehler M, Randall E, et al. Mandipropamid targets the cellulose synthase-like PiCesA3 to inhibit cell wall biosynthesis in the oomycete plant pathogen, Phytophthora infestans. Mol Plant Pathol 2010;11:227–43.
- Blum M, Waldner M, Olaya G, et al. Resistance mechanism to carboxylic acid amide fungicides in the cucurbit downy mildew pathogen Pseudoperonospora cubensis. Pest Manage Sci 2011;67:1211–4.
- Aoki Y, Furuya S, Suzuki S. Method for rapid detection of the PvCesA3 gene allele conferring resistance to mandipropamid a carboxylic acid amide fungicide in Plasmopara viticola populations. Pest Manage Sci 2011;67:1557–61.
- Pang Z, Shao J, Chen L, et al. Resistance to the novel fungicide Pyrimorph in Phytophthora capsici: risk assessment and detection of point mutations in CesA3 that confer resistance. PLoS One 2013;8:e56513.
- Wu C, Zhao J, Li Z, et al. Modeling of the Phytophthora capsici cellulose synthase 3 and its inhibitors activity assay. Pest Manage Sci 2019;75:3024–30.
- Yan SL, Yang MY, Sun ZH, et al. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett Drug Des Discov 2014;11:940–3.
- Jin T, Zhai ZW, Han L, et al. Synthesis crystal structure docking and antifungal activity of a new pyrazole acylurea compound. Chin J Struct Chem 2018;37:1259–64.
- Wang H, Zhai ZW, Shi YX, et al. Novel trifluoromethyl pyrazole acyl urea derivatives: synthesis crystal structure fungicidal activity and docking study. J Mol Struct 2018;1171:631–8.
- Fang YM, Zhang RR, Shen ZH, et al. Synthesis and antifungal activity of some 6-tert-butyl-8-chloro-2 3-dimethylquinolin-4-ol derivatives against Pyricularia oryae. Lett Drug Des Discov 2018;15:1314–8.
- Fang YM, Zhang RR, Shen ZH, et al. Synthesis antifungal activity and SAR study of some new 6-perfluoropropanyl quinoline derivatives. J Heterocycl Chem 2018;55:240–5.
- Liu XH, Fang YM, Xie F, et al. Synthesis and in vivo fungicidal activity of some new quinoline derivatives against rice blast. Pest Manag Sci 2017;73:1900–7.
- Zhai ZW, Wang Q, Shen ZH, et al. Synthesis and biological activity of 124-triazole thioether derivatives containing pyrazole moiety. Chin J Org Chem 2017;37:232–6.
- Zhang LJ, Yang MY, Sun ZH, et al. Synthesis and antifungal activity of 134-thiadiazole derivatives containing pyridine group. Lett Drug Des Discov 2014;11:1107–11.
- Fu Q, Cai PP, Cheng L, et al. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag Sci 2019;76:868–79.
- Cheng L, Zhang RR, Wu HK, et al. The synthesis of 6-(tertbutyl)-8-fluoro-2,3-dimethylquinoline carbonate derivatives and their antifungal activity against Pyricularia oryzae. Front Chem Sci Eng 2019;13:369–76.
- Shen ZH, Sun ZH, Becnel JJ, et al. Synthesis and mosquiticidal activity of novel hydrazone containing pyrimidine derivatives against Aedes aegypti. Lett Drug Des Discov 2018;15:951–6.
- Liu XH, Qiao L, Zhai ZW, et al. Novel 4-pyrazole carboxamide derivatives containing flexible chain motif: design, synthesis and antifungal activity. Pest Manag Sci 2019;75:2892–900.
- Sun NB, Shen ZH, Zhai ZW, et al. Design, synthesis, fungicidal activity and docking study of acyl thiourea derivatives containing pyrazole moiety. Chin J Org Chem 2017;37:2705–10.
- Sun NB, Shen ZH, Shen ZH, et al. Synthesis, crystal structure and antifungal activity of N-((2,6-Difluorophenyl)carbamoyl)1,3-dimethyl-1H-pyrazole-4-carboxamide. Chin J Struct Chem 2017;36:1667–72.
- Shen ZH, Zhai ZW, Sun ZH, et al. Synthesis, crystal structure and biological activity of 2-chloro-5-(((5-(1-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-4-phenyl-4H-1,2,4-triazol-3-yl)thio)methyl)thiazole. Chin J Struct Chem 2017;36:1137–41.
- Liu XH, Wang Q, Sun ZH, et al. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag Sci 2017;73:953–9.
- Cai PP, Cheng L, Tan CX, et al. New quinoline carbonate derivatives with perfluoroisopropyl hybrid: design, synthesis, and fungicidal activity. Indian J Heterocycl Chem 2019;29:243–7.
- Liu XH, Zhao W, Shen ZH, et al. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur J Med Chem 2017;125:881–9.
- Su N, Wang ZJ, Wang LZ, et al. Synthesis and biological evaluation of isosteric analogs of mandipropamid for the control of oomycete pathogens. Chem Biol Drug Des 2011;78:101–11.
- Li S, Cui C, Wang MY, et al. Synthesis and fungicidal activity of new fluorine-containing mandelic acid amide compounds. J Flor Chem 2012;137:108–12.
- Du XJ, Bian Q, Wang HX, et al. Design synthesis and fungicidal activity of novel carboxylic acid amides represented by N-benzhydryl valinamode carbamates. Org Bioorg Chem 2014;12:5427–34.
- Yao HW, Cui C, Li YQ, et al. Synthesis and bioactivity of N-(2-Alkoxy-2-substituted phenyl-ethyl) phenyl Amide. Chem J Chin Univ 2012;33:1481–5.
- Wang Q, Shen ZH, Sun ZH, et al. Synthesis of two 1,3,4-thiadiazole compounds: crystal structure, theoretical and antifungal activity study. J Chem Soc Pak 2017;39:524–31.
- Sun NB, Zhai ZW, Tong JY, et al. Synthesis, crystal structure and fungicidal activity of 3-(difluoromethyl)-1-methyl-N-((2-(trifluoromethyl)phenyl) carbamoyl)-1H-pyrazole-4-carboxamide. Chin J Struct Chem 2019; 38:706–12.
- Min LJ, Zhai ZW, Shi YX, et al. Synthesis and biological activity of acyl thiourea containing difluoromethyl pyrazole motif. Phosphorus Sulfur Silicon Relat Elem 2020;195:22–8.
- Min LJ, Wang Q, Tan CX, et al. Synthesis, Crystal Structure and Fungicidal Activity of 2-Chloro-N-(o-tolylcarbamoyl)nicotinamide. Chin J Struct Chem 2020, DOI: 10.14102/j.cnki.0254-5861.2011-2459