?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Lipopeptides have been reported to exhibit anti-obesity effects. In this study, we obtained a Bacillus velezensis strain FJAT-52631 that could coproduce iturins, fengycins, and surfactins. Results showed that the FJAT-52631 crude lipopeptide, purified fengycin, iturin, and surfactin standards exhibited strong inhibition activities against lipase with dose-dependence manners (half maximal inhibitory concentration (IC50) = 0.011, 0.005, 0.056, and 0.005 mg/mL, respectively). Moreover, fengycin and surfactin had the comparable activities with orlistat, but iturin not. It was revealed that the inhibition mechanism and type of the lipopeptides were reversible and competitive. The quenching mechanism of lipase was static and only one binding site between lipase and lipopoeptide was inferred from the fluorescence analysis. The docking analysis displayed that fengycin and surfactin could directly interact with the active amino acid residues (Ser or Asp) of lipase, but not with iturin. Our work suggests that the B. velezensis lipopeptides would have great potential to act as lipase inhibitors.
1. Introduction
The incidence of obesity has increased with astounding rapidity worldwide, rendering obesity a serious public health concern of the 21st century. Obesity is a complex, multifactorial disease that arises from the interaction of excessive caloric intake, sedentary lifestyle, metabolic disorder, and genetic predispositionCitation1. Indeed, obesity is the risk factor responsible for various chronic metabolic diseases or syndromes including diabetes mellitus, hypertension, hyperlipidaemia, osteoarthritis, hepatic steatosis, and so onCitation2,Citation3.
Dietary and lifestyle modifications such as calorie restriction and physical exercises are the common strategies adopted to control body weight; further, these methods have limited anti-obesity effectsCitation4. It has previously been reported that lipase inhibition is a potential strategy for counteracting obesity. Digestive lipase hydrolyses non-absorbable dietary triglycerides to smaller absorbable molecules of monoglycerides and free fatty acids, which are absorbed by the intestine. Inhibiting digestive lipase can reduce intestinal fat absorptionCitation5–7. Human pancreatic lipase is the main enzyme in intestinal digestion of dietary fats in the human digestive system. To date, a wide variety of natural products have been used as pancreatic lipase inhibitors, which originate from plants and metabolites of microorganisms. These include lipstatin, panclicins, saponins, polyphenols, flavonoids, caffeine, chitin, chitosan, etcCitation5. The lipase inhibitor orlistat is the only one obesity-treatment drug currently available in the market, which reduces intestinal fat absorption via inhibition of pancreatic lipase; however, it has been reported to cause certain side effects, e.g. oily stools, oily spotting, and flatulenceCitation1,Citation8. Some polyphenol compounds have been reported to have potential adverse effects on microorganisms and animal at high concentrationsCitation9,Citation10. Thus, there is still a need to explore safe and effective anti-obesity drugs.
Interestingly, surfactants were found to produce inhibitory effects on the lipolytic efficiency of lipase by generating inactive aqueous enzyme-surfactant complexes or by blocking the congregation of enzymes at the lipid/water interfaceCitation11. In fact, it has been reported that bacterial cyclic lipopeptides are the most popular amphiphilic molecules that are excellent surface active compoundsCitation12. The genus Bacillus is described as an efficient source of lipopeptide biosurfactants. The Bacillus lipopeptides are divided into three different families, including iturins, surfactins, and fengycins, consisting of a cyclic lipoheptapeptide or decapeptide with a long hydrophobic fatty acid moietyCitation13. Surfactin is a well-known surfactant consisting of a peptide ring of seven amino acids with a β-hydroxy-fatty-acid chain that can lower the surface tension of water from 72 to 27 mN/mCitation14. In contrast to surfactin, iturin contains a β-amino fatty acid linked to a peptide ring with seven amino acid residues, while fengycin is a cycle lipopeptide with 10 amino acid residues. It has been reported that the lipopeptide biosurfactants exhibit numerous bioactivities, such as antimicrobial, antiadhesive, antitumoral, antiviral, and hypoglycaemic activitiesCitation15–17. Additionally, the Bacillus lipopeptides possess high biodegradability, biocompatibility, and high stability towards extreme environments. These remarkable properties make lipopeptides potent candidate drugs for therapeutic medical applicationsCitation18.
It had been reported that lipopeptides of Bacillus subtilis SPB1 could significantly reduce the body weight of obese rats and relieve hyperlipidaemia without apparent side effectsCitation18,Citation19. The anti-obesity effects are mediated by lipopeptides through inhibiting the serum pancreatic lipase activity to modulate dietary triglyceride digestionCitation18,Citation19. However, the molecular mechanism of lipopeptide interaction with lipase needs further exploration. The B. subtilis SPB1 lipopeptides consist of iturins, surfactins, fengycins, and other lipopeptide isoforms. Moreover, the surfactins were speculated to be the major contributor to the anti-obesity effects of B. subtilis SPB1 lipopeptide. However, it is still unknown whether all the types of lipopeptides display the inhibition effect on the lipase. Considering the structural differences between different lipopeptide families, the comparative studies of lipase inhibition activities of surfactin, iturin, and fengycin would be important for the application of lipopeptide as lipase inhibitor. The aim of this article is to report a new lipopeptide-produced Bacillus velezensis strain FJAT-52631 that could coproduce iturin, fengycin, and surfactin and to evaluate the inhibition activity of each type of lipopeptide. Furthermore, the action modes of lipopeptide on lipase catalysis was carried out.
2. Materials and methods
2.1. Chemicals and strains
Lyophilised powder of Mucor miehei lipase (EC3.1.1.3), 4-Nitrophenyl palmitate (4-NPP), iturin, and surfactin were purchased from Sigma-Aldrich (St. Louis, MO). Acetonitrile, hydrochloric acid (HCl) and Tris were purchased from Sinopharm (Shanghai, China).
The strain FJAT-52631 (CCTCC No. M 2019760) was isolated from a soil sample from Wuyi Mountain, Fujian Province, China and it was identified through whole genome sequence analyses.
2.2. Lipopeptide extraction and preparation
A single clone of the strain FJAT-52631 was inoculated in a 25-ml sterile tube with 5 ml liquid culture media (beef Extract 3 g/L, peptone 5 g/L, and glucose 10 g/L) and incubated for 25 h at 30 °C and 170 rpm. The pre-culture was inoculated (1%) into 250-mL flasks with a 50 mL potato dextrose broth culture medium and then cultivated for 48 h in a rotary shaker at 30 °C, 170 rpm. After fermentation, the cells were removed by centrifugation (6000 g for 5 min) and the lipopeptide in the culture supernatant was precipitated by adding 3 N HCl to achieve a final pH of 2. The precipitates were dissolved in a phosphate buffer and then lyophilised for anti-lipase activity tests and liquid chromatography quadrupole time-of-flight tandem mass spectrometry (LC-QTOF-MS/MS) analyses.
2.3. Lipopeptide identification and separation
The qualitative and quantitative analyses of lipopeptides produced from the FJAT-52631 were carried out using the LC-QTOF-MS/MS method described in our previous studiesCitation20. Then, the lipopeptides were purified using the C18 solid phase extraction method with methanol/water (v/v) as an elution solvent. Each elution fraction was evaporated at a reduced pressure (−50 psig, 50 °C), dissolved in water and then lyophilised.
2.4. Measurement of lipase activity
The lipase inhibition was determined according to the method described by Liu et al.Citation21 10 µg/mL lipase in water and 7.5 mmol/L 4-NPP in acetonitrile solutions were prepared. The crude lipopeptide and purified fengycin were dissolved in water, while the iturin and surfactin standards were dissolved in methanol, and then all were diluted to their appropriate concentrations. The 1 mL reaction mixture contained 0.75 mM 4-NPP, 0.4 µg/mL lipase, and different concentrations of inhibitor in Tris-HCl buffer (pH 7.8). The reaction was carried out at 37 °C and the detection wavelength was set at 405 nm.
The inhibition mechanisms were studied by fixing the concentration of substrate and changing the lipopeptides and enzymes to monitor enzymatic reaction. The inhibition types were determined based on the Lineweaver-Burk plotCitation22; this reaction system contained different concentrations of substrate and lipopeptide and 100 µL of lipase in Tris-HCl buffer. Then, the inhibition constant was calculated from a secondary plot of 1/Vm versus the inhibitor concentration.
2.5. Spectrum analysis
Ultraviolet (UV) wavelength scanning spectra of the 4-NPP hydrolysis product was measured in the absence and in the presence of lipopeptide using a model 1510 spectrophotometer (Thermo Fischer Scientific, Waltham, MA). The 1 mL reaction mixture contained 0.75 mmol/L 4-NPP and 0.015 mg/mL of lipopeptide in Tris-HCl buffer (pH 7.8). The final concentration of the lipase was 0.4 µg/mL.
Fluorescence quenching spectra of the lipase and the lipopeptide were carried out according to the method described by Liu et al.Citation21, using a Cary Eclipse spectrophotometer (Agilent, Santa Clara, CA). The Stern-Volmer quenching constant (Ksv) was calculated according to EquationEquation (1)(1)
(1) :
(1)
(1)
where F and F0 are the fluorescence intensities with and without lipopeptide, respectively; further, [I] is the concentration of the lipopeptide. The binding constant (KA) and the binding affinity (n) were calculated according to the EquationEquation (2)
(1)
(1) :
(2)
(2)
2.6. In silico simulating molecular docking model
The interactions between lipopeptide and lipase were modelled using molecular operation environment software (MOE). The energy between the lipase and the ligand was minimised before docking. The docking parameters were set according to a previous studyCitation21.
3. Results and discussion
3.1. Identification of the strain FJAT-52631
A lipopeptide-produced strain FJAT-52631 was isolated from the soil sample, which was further accurately identified through the whole genome sequence analyses. The genome sequence of strain FJAT-52631 contains 3929791 bp (GenBank accession number: CP045186). The G + C content of the chromosomal DNA for strains FJAT-52631 was 46.5 mol %. The genome-based similarity calculated based on the OrthoANIu between strain FJAT-52631 and the type strain Bacillus velezensis CBMB205T was 99.95%. This value was above the threshold ANI value of 95–96% used for delineating prokaryotic species, suggesting that strain FJAT-52631 is a strain of the species B. velezensisCitation23.
3.2. Identification and separation of lipopeptides produced by the strain B. velezensis FJAT-52631
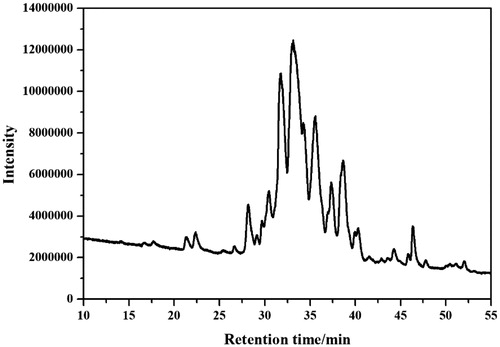
The lipopeptides produced from the strain FJAT-52631 were identified using the LC-QTOF-MS/MS method. Results revealed that three sets of homologue molecules with retention times in the range of 12.2–21.5, 27.0–38.7, and 45.2–54.0 min could be categorised as iturins, fengycins, and surfactins, respectively. Peptide sequences of each lipopeptide group were determined based on previous literature reportsCitation24–26. The retention times, MS and MS2 spectral data, and identification results were summarised, as shown in ; the iturins consisted of C14–C16 iturin A; the surfactins consisted of C12–C16 surfactin A, and C16 surfactin A derivative; further, the fengycins consisted of C16/C18 fengycin A, C16 fengycin A2/B2, C16–C17 fengycin B, and C15 fengycin A/B derivatives. The iturin, fengycin, and surfactin content in the supernatant were calculated as 2.66 ± 1.50, 86.95 ± 4.08, and 35.93 ± 2.28 mg/L, respectively. The results demonstrated that fengycin was the most abundant lipopeptide family that was produced by the strain FJAT-52631.The lipopeptide production from Bacillus spp. firstly depends on itself. For example, the B. subtilis SPB1 strain have the ability to coproduce iturins, fengycins, and surfactins and highest content of surfactin in lipopeptide mixture was observedCitation15.
Table 1. Identification of lipopeptides produced by B. velezensis FJAT-52631 using LC-QTOF-MS/MS.
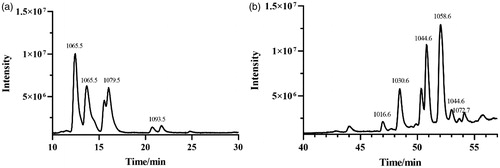
The crude lipopeptide from strain FJAT-52631 was further purified by solid-phase extraction method, and characterised by LC-TOF-MS analysis. Results show that the purified fengycin in the crude lipopeptide was obtained by elution with 80% methanol (), while the iturin and surfactin in the crude lipopeptide were not successfully purified using the above method. LC-QTOF-MS analysis revealed that the composition of iturin and surfactin in the crude lipopeptide yield from FJAT-52631 were identical to the iturin and surfactin standards (). Thus, iturin and surfactin standards were used to substitute the corresponding substances in further study.
3.3. Inhibitory activity of lipopeptide acting on lipase
As reported in literatures, surfactants could inhibit lipase activity at certain concentrations due to their special propertyCitation14. Lipopeptides from the Bacillus group have been proved to be excellent surfactants and could reduce lipase activity in plasma from alloxan-induced diabetic ratsCitation18. In spite of this, the manner of lipopeptide interaction with lipase and the mechanism of enzyme inhibition are still unknown. In the present study, we report the effects of crude lipopeptide (), purified fengycin (), iturin (), and surfactin () standards on the lipase. Both the lipase from M. miehei and human pancreatic lipase are serine proteases, which possess the same active site and catalytic modeCitation27. Moreover, the lipase from M. miehei exhibits much higher enzyme activity, better purity, and more mature processes than the human pancreatic lipaseCitation28. Thus, the M. miehei lipase was selected for use in the present study.
Figure 3. Lipase inhibitory activity of (a) crude lipopeptide, (b) purified fengycin, (c) iturin, and (d) surfactin.
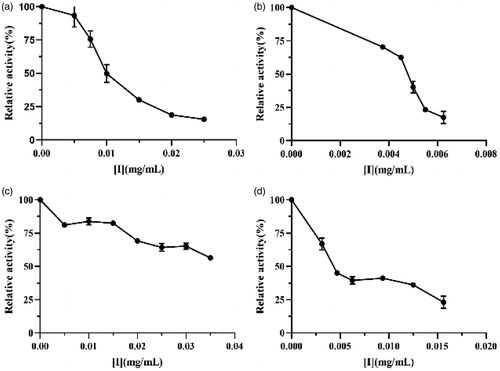
Results showed that lipase inhibition was dose dependent. After adding these four inhibitors, the relative enzyme activity decreased significantly with increasing concentration of effectors. The half maximal inhibitory concentration (IC50) of crude lipopeptide, purified fengycin, iturin, and surfactin standards were 0.011, 0.005, 0.056, and 0.005 mg/mL, respectively. We have previously reported that furoic acid and oxalic acid inhibited lipase with IC50 of 0.242 and 1.425 mg/mL, respectivelyCitation21. Moreover, we found that the inhibitory effects of the lipopeptides were within one order of magnitude of orlistat (IC50 = 0.004 mg/mL)Citation7. These results suggest that lipopeptides have strong lipase inhibition activity and could act as lipase inhibitors to prevent obesity.
The anti-lipase activities of natural compounds depend on their structure characteristics, including number and position of hydroxyl groups, degree of polymerisation, elimination of glycosylation, and the size of the moleculesCitation6. Buchholz et al.Citation6have reported that a high number of phenolic hydroxyl groups in active flavonoids increases their inhibitory effect. In present study, it was found that the fengycin and surfactin exhibit much stronger inhibition activities on lipase (about 10-fold) than that of iturin, which possibly be attributed to the fengycins and surfactins composing of β-hydroxy fatty acids, whereas the iturins carrying a β-amino fatty acid modificationCitation14,Citation15. The crude lipopeptide produced by FJAT-52631 was composed of 2.7% iturin, 73.9% fengycin, and 23.4% surfactin, which indicated that the inhibition effect of crude lipopeptide produced by FJAT-52631 was mainly attribute to the fengycin. To our knowledge, this is the first report of the strong inhibitory activity of lipopeptide from B. velezensis against lipase in vitro.
3.4. Inhibition mechanism and type of lipopeptide on lipase activity
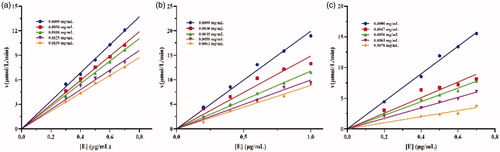
The mechanism of enzyme inhibition by drugs can be either reversible or irreversibleCitation29. The inhibition mechanisms of crude lipopeptide, fengycin, and surfactin on lipase catalysis were studied. As shown in , all the plots of the remaining enzyme activity versus the enzyme concentrations generated a series of lines intersecting at the origin, and whose slopes decreased with increasing lipopeptide content. These results demonstrated that the above catalytic action is reversible, which means that the lipopeptide declined the catalytic activity of lipase but did not lead to enzyme deactivation. Studies showed that most common mechanism of the inhibitor observed is reversible, such as furoic acid and oxalic acid, others like orlistat display irreversible effectsCitation21,Citation29.
Figure 4. Inhibition mechanisms of (a) crude lipopeptide, (b) fengycin, and (c) surfactin on lipase.
Four inhibition methods have been proposed for enzyme inhibitors, including competitive, non-competitive, uncompetitive, and mixed typeCitation30. The inhibition type could be related to the properties of inhibitors, such as structure, molecular weight, etc.Citation21. To determine the inhibition type of crude lipopeptide, fengycin, and surfactin on lipase catalysis, plots of 1/v versus 1/[S] of lipopeptide on lipase shown in were drawn, where v is the reaction rate and S is the substrate concentration. The inhibition constants KI of different lipopeptides were calculated and summarised in . Our results demonstrated that all the tested lipopeptides (mixture, fengycin, or surfactin) inhibit lipase in a competitive manner with respect to substrate concentration, which is subvert the explanation reported by Zouari et al.Citation18, who deduced that B. subtilis SPB1 lipopeptide biosurfactant exhibits an uncompetitive inhibition mode action on lipase activity. Our above finding suggested that the lipopeptide binds to the free enzyme at the substrate-binding site.
Figure 5. Inhibition types of (a), crude lipopeptide, line 5-1: mean 0, 0.0013, 0.002, 0.0025, 0.003 mg/mL, respectively; (b) fengycin, line 5-1: mean 0, 0.0013, 0.002, 0.0025, 0.003 mg/mL, respectively; and (c) surfactin, line 5-1: mean 0, 0.0008, 0.0009, 0.0011, 0.0013 mg/mL, respectively on lipase.
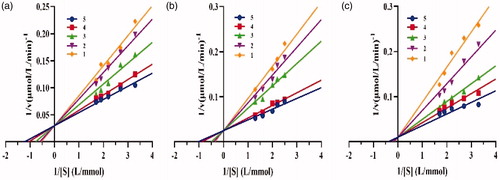
Table 2. Inhibition effects of lipopeptides for lipase.
3.5. Spectrum analysis of product in the presence and absence of lipopeptide
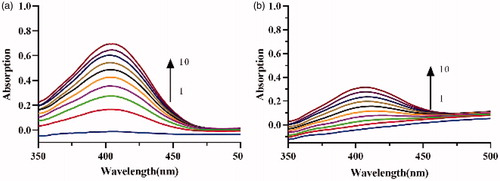
The UV spectra of lipase catalysis in the absence () and presence () of the crude lipopeptide were measured. Results show that the typical peak absorbent intensity of the product at 405 nm increased along with an extension of the catalytic reaction time. After 10 min, in the presence of 0.015 mg/mL lipopeptide, the peak absorbance was reduced to 54.5%, which indicates that the crude lipopeptide produced from FJAT-52631 was a stronger inhibitor. This result was consistent with the enzyme activity assay.
Figure 6. Consecutive spectra obtained during the lipase catalysis in the (a) absence and (b) presence of crude lipopeptide. Curves 1–10 depict the addition of the enzyme in 10 min.
The fluorescence emission spectra of crude lipopeptides on lipase are shown in . Results showed that the fluorescence intensities of the emission peak at 338 nm gradually declined with increasing lipopeptide content (); this indicated that the lipopeptide and the lipase form a complexCitation21,Citation31. The effects of different concentrations of lipopeptides on lipase did not lead the endogenous fluorescence peak to red shift or blue shift; this suggests that there has not been a complete change of the composition of the enzyme molecule and that the enzyme is still activeCitation32. The plot of F0/F versus [I] was drawn and a linear regression equation of F0/F = 1 + 0.525[I] was obtained (). The value of Stern-Volmer quenching constant Ksv was found to be 0.525 L/g according to the Stern-Volmer equation. This value is larger than the maximum dynamic quenching constant of biomacromolecule (100 L/mol); this indicated that the fluorescence quenching mechanism of lipase is static when the lipopeptide is presentCitation33. Further, a linear regression equation lg[(F0−F)/F] = 1.429lg[I] − 0.108 (R2=0.9255) was obtained from . The values of the binding constant KA and binding site n from the intercept of the above equation and the slope were calculated as 1.429 L/g and 0.78, respectively. This result indicates that the interaction between lipase and lipopeptides is quite intensive and there is only one binding site between themCitation33.
Figure 7. Effect of lipopeptides on the emission spectrum of lipase. (a) Emission spectra of lipase with crude lipopeptide concentration of 0, 0.194, 0.265, 0.324, 0.375, 0.419, and 0.457 mg/mL, respectively (1–7, respectively); (b) Florescence intensity changes with lipopeptide; (c) Plot of F0−F against [I] for lipopeptide; (d) Plot of lg[(F0−F)/F] versus lg[I] for lipopeptide.
![Figure 7. Effect of lipopeptides on the emission spectrum of lipase. (a) Emission spectra of lipase with crude lipopeptide concentration of 0, 0.194, 0.265, 0.324, 0.375, 0.419, and 0.457 mg/mL, respectively (1–7, respectively); (b) Florescence intensity changes with lipopeptide; (c) Plot of F0−F against [I] for lipopeptide; (d) Plot of lg[(F0−F)/F] versus lg[I] for lipopeptide.](/cms/asset/50182915-5e08-44c2-83e9-20f7cee7a467/ienz_a_1734798_f0007_c.jpg)
3.6. Molecular docking analysis
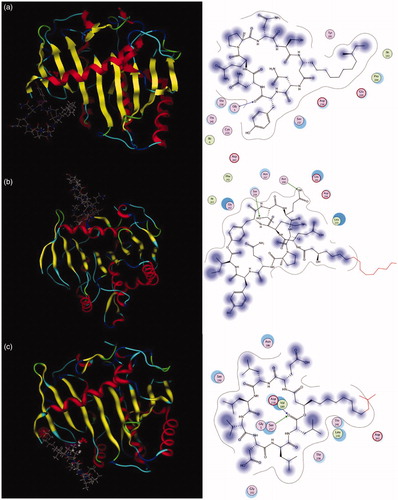
The M. miehei lipase have three active site residues Ser, His, and Asp that forming an oxygen negative ion hole, which are covered by an “alpha-screw lid”Citation34,Citation35. The lipase was composed of hydrophilic groups surrounded by exposed hydrophobic and its activity depends upon the conformation of lid (in open or in closed)Citation35. The conformation of lipase would be altered when the active site of lipase interacted with an inhibitor, leading to the reduced catalytic activity of lipaseCitation36. Previous studies have shown that the ring structure and the carbonyl groups of the inhibitor is important for their correct binding in the active site of lipaseCitation37. The molecular docking analysis was carried out to explore the possible binding action mode between lipopeptide and lipase. Results demonstrated that the carbonyl group on the Tyr residue in iturin A might directly act on Gly 5 of lipase (); the carbonyl group on the Leu residue in surfactin A might directly act on Asp 238 and Ser 237 of lipase (); and the oxygen atoms of the hydroxyl group on the Thr and Glu residues in fengycin might directly act on Ser 259 and Asn 100 of lipase (), respectively. The hydroxyl and carbonyl groups on the lipopeptides formed hydrogen bonds with the amino residues of lipase to stabilise the interactions between the lipopeptide and the lipase. This manner could block substrate access to the catalytic active site of the lipase enzyme. Our results displayed that the fengycin and surfactin could be in direct contact with the active amino acids of lipase, and not those of iturin. The interactions of oxygen with the catalytic site might enhance the lipase inhibition activity. Moreover, the active site residue Ser is essential for the hydrolytic activity of the enzymeCitation6. This could explain in part the weaker potency of iturin. The above modelling results support the data derived from the enzymology studies.
Figure 8. Interactions between key amino acids in (a) lipase and iturin, (b) fengycin, or (c) surfactin were investigated by in silico modelling.
Studies showed that the small molecules are easily to enter the gap in the oxygen negative ion to interact with the catalytic site of the enzyme. For example, the furoic acid and oxalic acid can directly act on active amino acid of Ser, while the big molecular orlistat does not contact with the active amino acids of, Ser, His or AspCitation21. Lipopeptides are the famous surfactants that composed of both hydrophilic and hydrophobic groups. The structures of surfactants play an important role in protein–surfactant interactionsCitation35. This suggested that lipopeptides lead to strong inhibition of M. miehei lipase activity by direct interact with the active site, which are closely related to the amphiphilic molecular structures of lipopeptides.
4. Conclusion
We report here on the strong inhibitory activity and action modes of lipopeptide from B. velezensis against lipase in vitro. The lipopeptides produced from B. velezensis FJAT-52631 were categorised as iturin, fengycin, and surfactin. The crude lipopeptides, iturin, fengycin, and surfactin exhibited strong inhibition activities against lipase with dose-dependence. The lipopeptide inhibition catalytic reaction was reversible and the inhibition type was competitive. The fengycins and surfactins directly interact with the residues of the enzyme’s active centre, while iturins do not. Fengycins were the most abundant compounds among the crude lipopeptides, which were considered to contribute significantly to the inhibited activities against lipase. Our results suggest that these lipopeptides could be used directly as lipase inhibitors and the in vivo anti-obesity effects of lipopeptides will be explored in further studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- GBD. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27.
- Vecchié A, Dallegri F, Carbone F, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med 2018;48:6–17.
- Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res 2017;122:1–7.
- Wolfenden L, Barnes C, Jones J, et al. Strategies to improve the implementation of healthy eating, physical activity and obesity prevention policies, practices or programmes within childcare services. Cochrane Database Syst Rev 2020;2:CD011779.
- Mohamed GA, Ibrahim SRM, Elkhayat ES, Dine RSE. Natural anti-obesity agents. BFPC 2014;52:269–84.
- Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med 2015;81:771–83.
- Glisan SL, Grove KA, Yennawar NH, Lambert JD. Inhibition of pancreatic lipase by black tea theaflavins: comparative enzymology and in silico modeling studies. Food Chem 2017;216:296–300.
- Saunders KH, Umashanker D, Igel LI, et al. Obesity pharmacotherapy. Med Clin North Am 2018;102:135–48.
- Field JA, Lettinga G. Toxicity of tannic compounds to microorganisms. In: Hemingway RW, Laks PE, eds. Plant polyphenols: basic life sciences 59. Heidelberg: Springer Verlag; 1992: 673–692.
- Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs. risks. Chem Res Toxicol 2007;20:583–5.
- Delorme V, Dhouib R, Canaan S, et al. Effects of surfactants on lipase structure, activity, and inhibition. Pharm Res 2011;28:1831–42.
- Hentati D, Chebbi A, Hadrich F, et al. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol Environ Saf 2019;15:441–9.
- Chen Y, Liu SA, Mou H, et al. Characterization of lipopeptide biosurfactants produced by Bacillus licheniformis MB01 from marine sediments. Front Microbiol 2017;8:871–82.
- Jacques P. Surfactin and other lipopeptides from Bacillus spp. In: Soberón-Chávez G, ed. Biosurfactants: from genes to applications. Vol. 20. Berlin: Springer-Verlag; 2011:57–91.
- Sarwar S, Hassan MN, Imran M, et al. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol Res 2018;209:1–13.
- Meena KR, Kanwar SS. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed Res Int 2015;2015:473050.
- Morejón MC, Laub A, Kaluđerović GN, et al. A multicomponent macrocyclization strategy to natural product-like cyclic lipopeptides: synthesis and anticancer evaluation of surfactin and mycosubtilin analogues. Org Biomol Chem 2017;15:3628–37.
- Zouari R, Hamden K, Feki AE, et al. Protective and curative effects of Bacillus subtilis SPB1 biosurfactant on high-fat-high-fructose diet induced hyperlipidemia, hypertriglyceridemia and deterioration of liver function in rats. Biomed Pharmacother 2016;84:323–9.
- Zouari R, Abdallah-Kolsi RB, Hamden K, et al. Assessment of the antidiabetic and antilipidemic properties of Bacillus subtilis SPB1 biosurfactant in alloxan-induced diabetic rats. J Pept Sci 2015;104:764–74.
- Chen MC, Wang JP, Zhu YJ, et al. Antibacterial activity against Ralstonia solanacearum of the lipopeptides secreted from the Bacillus amyloliquefaciens strain FJAT-2349. J Appl Microbiol2019;126:1519–29.
- Liu TT, He XR, Xu RX, et al. Inhibitory mechanism and molecular analysis of furoic acid and oxalic acid on lipase. Int J Biol Macromol 2018;120:1925–34.
- Chai WM, Shi Y, Feng HL, et al. NMR, HPLC-ESI-MS, and MALDI-TOF MS analysis of condensed tannins from Delonix regia (Bojer ex Hook.) Rat and their bioactivities. J Agric Food Chem 2012;60:5013–22.
- Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci 2009;106:19126–31.
- Pecci Y, Rivardo F, Martinotti MG, Allegrone G. LC/ESI-MS/MS characterisation of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. J. Mass Spectrom 2010;45:772–8.
- Pathak KV, Keharia H, Gupta K, et al. Lipopeptides from the banyan endophyte, Bacillus subtilis K1: mass spectrometric characterization of a library of fengycins. J Am Soc Mass Spectrom 2012;23:1716–28.
- Jemil N, Manresa A, Rabanal F, et al. Structural characterization and identification of cyclic lipopeptides produced by Bacillus methylotrophicus DCS1 strain. J Chromatogr B 2017;1060:374–86.
- Uppenberg J, Ohrner N, Norin M, et al. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochem 1995;34:16838–51.
- Sharma R, Chist i Y, Banerjee UC. Production, purification, characterization, and applications of lipases. Biotechnol Adv 2001;19:627–62.
- Moini A, Kanani M, Kashani L, et al. Effect of orlistat on weight loss, hormonal and metabolic profiles in women with polycystic ovarian syndrome: a randomized double-blind placebo-controlled trial. Endocrine 2015;49:286–9.
- Yoshino M, Murakami K. A graphical method for determining inhibition constants. J Enzym Inhib Med Chem 2009;24:1288–90.
- Liu Y, Cao RX, Qin PF, Liu RT. Assessing the potential toxic effect of one persistent organic pollutant: non-covalent interaction of dicofol with the enzyme trypsin. Spectrochim Acta Part A 2012;89:210–5.
- Wang YQ, Zhang HM, Zhang GC, et al. Studies of the interaction between paraquat and bovine hemoglobin Int. J Biol Macromol 2007;41:243–50.
- Zhang YZ, Chen XX, Dai J, et al. Spectroscopic studies on the interaction of lanthanum(III) 2‐oxo‐propionic acid salicyloyl hydrazone complex with bovine serum albumin. Luminescence 2008;23:150–6.
- Tilbeurg HV, Sarda L, Verger R, Cambillau C. Structure of the pancreatic lipaseprocolipase complex. Nature 1992;359:159–62.
- Alam P, Rabbani G, Badr G, et al. The surfactant-induced conformational and activity alterations in Rhizopus niveus lipase. Cell Biochem Biophys 2015;71:1199–206.
- Sanchez J, Priego T, Palou M, et al. Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinol 2008;149:733–40.
- Bobcheva Z, Zhiryakova D, Guncheva M. Evaluation of the inhibitory potential of five squaric acid derivatives against pancreatic lipase. J Enzyme Inhib Med Chem 2011;26:587–91.